Temporal Variation and Hysteresis of Soil Respiration and Sap Flow of Pinus densiflora in a Cool Temperate Forest, Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Measurement of Meteorological and Soil Environments
2.3. Measurement of Soil Respiration
2.4. Measurement of Sap-Flow
2.5. Quality Control and Data Analysis
2.6. Statistical Analysis
3. Results
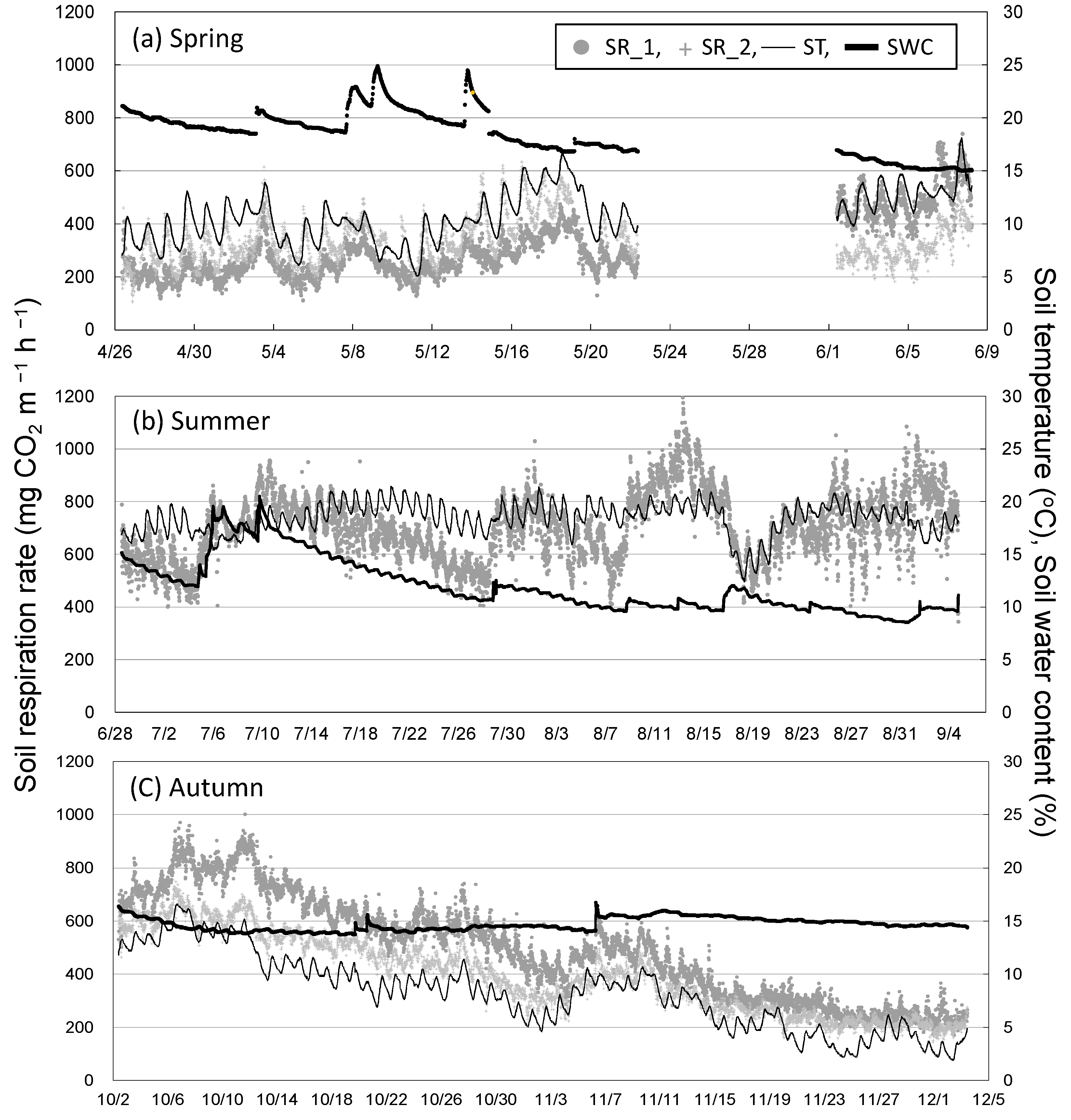
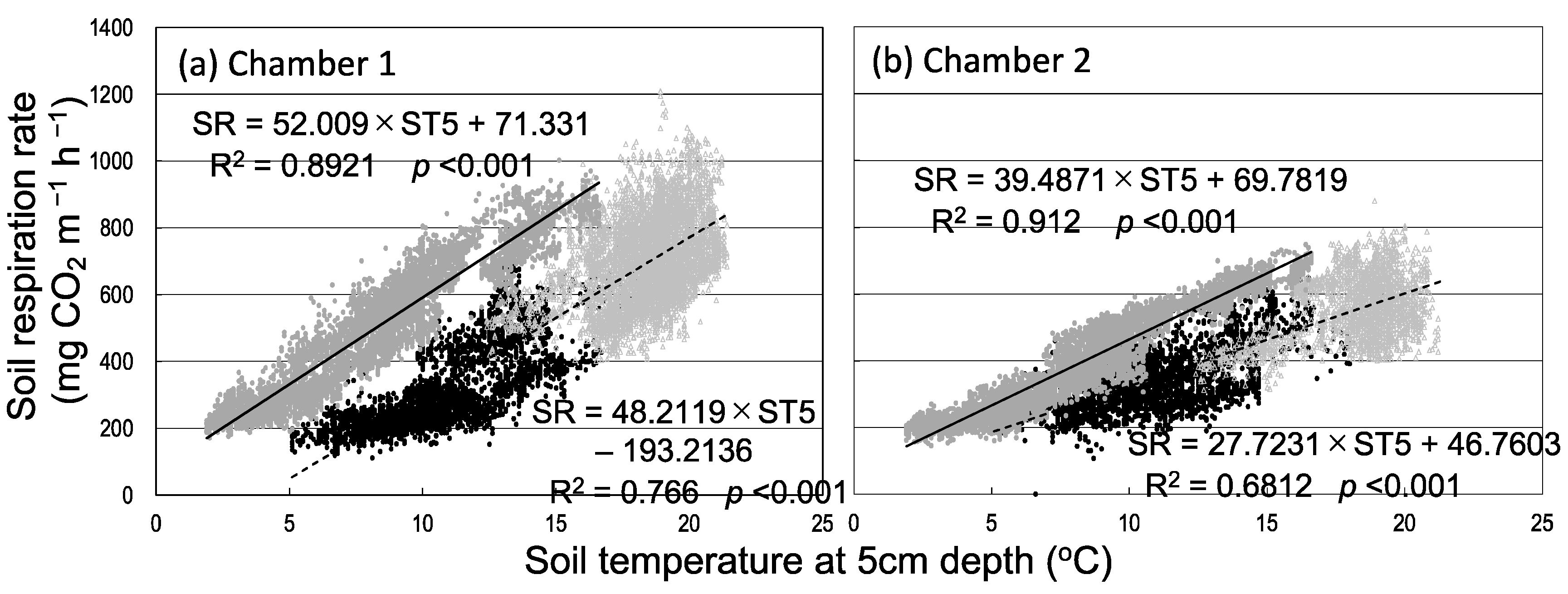
3.1. Seasonal and Diurnal Variation in Soil Respiration and Environmental Factors
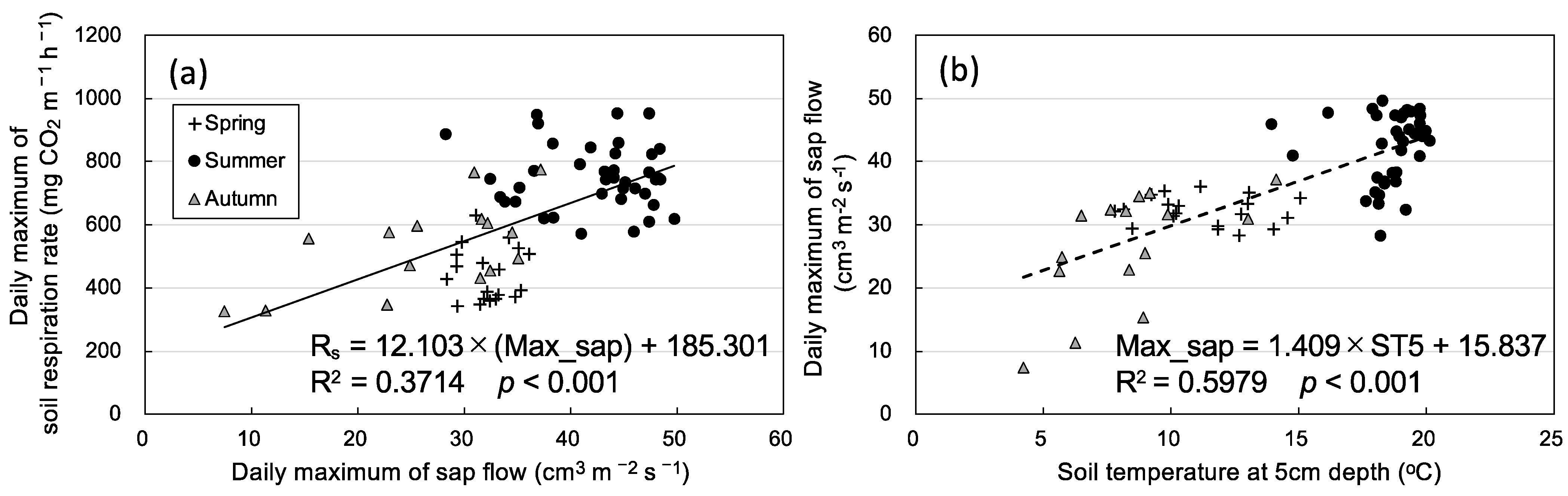
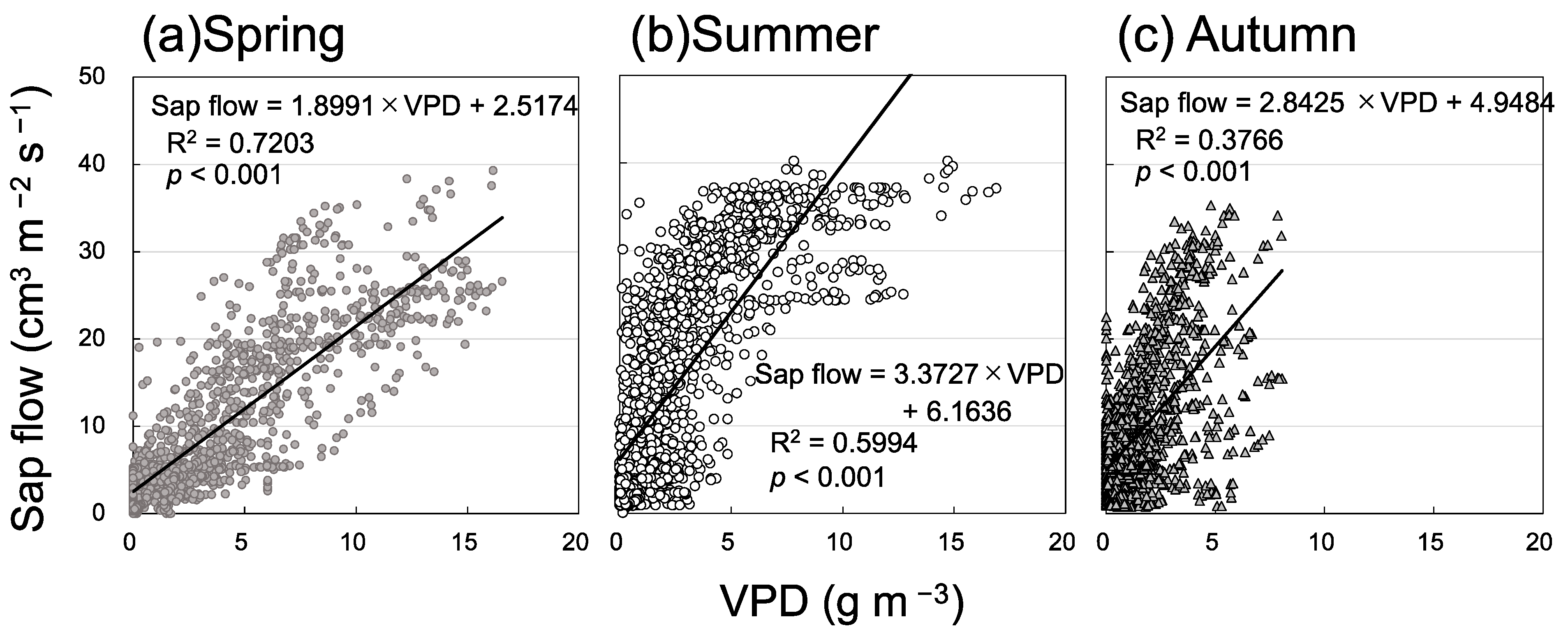
3.2. Seasonal Variation in Soil Respiration and Sap Flow
4. Discussion
| Direction of Rs Hysteresis | Dominant Species | Forest Type | Depth of Soil Temperature Measurement (cm) | Forest Age (Years) | Annual Air Temperature (°C) | Annual Precipitation (mm yr−1) | References No. |
|---|---|---|---|---|---|---|---|
| Clockwise | Populus tremuloides | MF | 2 * | 81 | 0.3 | 456 | [7] |
| Clockwise | Quercus kelloggii | MF | 8 * | No data | 10.3 | 507 | [14] |
| Clockwise | Quercus serrata | MF | 5 * | 30 | 15.5 | 1388 | [38] |
| Clockwise | Pinus densiflora | MF | 0 *, 5 *, 10 * | 40 | 6.7 † | 1132 † | [29] |
| Clockwise | Chamaecyparis obtusa | PT | 2 | 50 | 13.4 | 1595 | [16] |
| Counterclockwise | Pinus tabuliformis | PT | 10 | 4 | 10.8 | 454 | [39] |
| Counterclockwise | Picea abies, Pinus sylvestris | MF | 5 | 70 | 5.5 | 527 | [40] |
| Counterclockwise | Quercus robur | PT | 2 | 65 | 9.8 | 750 | [41] |
| Counterclockwise | Cryptomeria japonica | PT | 5 | 2–3 | 10.6 | 1734 | [42] |
| Counterclockwise | Pinus densiflora | MF | 5 | 55 | 6.8 | 1086 | This study |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Mo, W.; Lee, M.; Uchida, M.; Inatomi, M.; Saigusa, N.; Mariko, S.; Koizumi, H. Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agric. For. Meteorol. 2005, 134, 81–94. [Google Scholar] [CrossRef]
- Teramoto, M.; Liang, N.; Takahashi, Y.; Zeng, J.; Saigusa, N.; Ide, R.; Zhao, X. Enhanced understory carbon flux components and robustness of net CO2 exchange after thinning in a larch forest in central Japan. Agric. For. Meteorol. 2019, 274, 106–117. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Moyano, F.E.; Vasilyeva, N.; Bouckaert, L.; Cook, F.; Craine, J.; Yuste, J.C.; Don, A.; Epron, D.; Formanek, P.; Franzluebbers, A.; et al. The moisture response of soil heterotrophic respiration interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Jassal, R.S.; Nesic, Z. Interpreting the dependence of soil respiration on soil temperature and water content in a boreal aspen stand. Agric. For. Meteorol. 2006, 140, 220–235. [Google Scholar] [CrossRef]
- Cai, Y.; Nishimura, T.; Ida, H.; Hirota, M. Spatial variation in soil respiration is determined by forest canopy structure through soil water content in a mature beech forest. For. Ecol. Manag. 2021, 501, 119673. [Google Scholar] [CrossRef]
- Subke, J.-A.; Inglima, I.; Cotrufo, M.F. Trends and methodological impacts in soil CO2 efflux partitioning: A metanalytical review. Glob. Chang. Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between heterotrophic and autotrophic components of soil respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Raich, J.; Lambers, H.; Oliver, D. Respiration in terrestrial ecosystems. Treatise Geochem. 2014, 10, 613–649. [Google Scholar] [CrossRef]
- Makita, N.; Fujimoto, R.; Tamura, A. The contribution of roots, mycorrhizal hyphae, and soil free-living Microbes to soil respiration and its temperature sensitivity in larch forest. Forests 2021, 12, 1410. [Google Scholar] [CrossRef]
- Zhang, Q.; Phillips, R.P.; Manzoni, S.; Scott, R.L.; Oishi, A.C.; Finzi, A.; Daly, E.; Vargas, R.; Novick, K.A. Changes in photosynthesis and soil moisture drive the seasonal soil respiration-temperature hysteresis relationship. Agric. For. Meteorol. 2018, 259, 184–195. [Google Scholar] [CrossRef]
- Vargas, R.; Allen, M.F. Environmental controls and the influence of vegetation type, fine roots and rhizomorphs on diel and seasonal variation in soil respiration. New Phytol. 2008, 179, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Chen, N.; Qiao, L.; Zhao, C. Photosynthesis regulates the diel hysteresis pattern between soil respiration and soil temperature in a steppe grassland. Geoderma 2022, 408, 115561. [Google Scholar] [CrossRef]
- Makita, N.; Kosugi, Y.; Sakabe, A.; Kanazawa, A.; Ohkubo, S.; Tani, M. Seasonal and diurnal patterns of soil respiration in an evergreen coniferous forest: Evidence from six years of observation with automatic chambers. PLoS ONE 2018, 13, e0192622. [Google Scholar] [CrossRef] [PubMed]
- Heinemeyer, A.; Wilkinson, M.; Vargas, R.; Subke, J.-A.; Casella, E.; Morison, J.I.L.; Ineson, P. Exploring the “overflow tap” theory: Linking forest soil CO2 fluxes and individual mycorrhizosphere components to photosynthesis. Biogeosciences 2012, 9, 79–95. [Google Scholar] [CrossRef]
- Klein, T.; Rotenberg, E.; Tatarinov, F.; Yakir, D. Association between sap flow-derived and eddy covariance-derived measurements of forest canopy CO2 uptake. New Phytol. 2016, 209, 436–446. [Google Scholar] [CrossRef]
- Kume, T.; Onozawa, Y.; Kamatsu, H.; Tsuruta, K.; Shinohara, Y.; Umebayashi, T.; Otsuki, K. Stand-scale transpiration estimates in a Moso bamboo forest: (I) Applicability of sap flux measurements. For. Ecol. Manag. 2010, 260, 1287–1294. [Google Scholar] [CrossRef]
- Schäfer, K.V.; Oren, R.; Lai, C.T.; Katul, G.G. Hydrologic balance in an intact temperature forest ecosystem under ambient and elevated atmospheric CO2 concentration. Glob. Chang. Biol. 2002, 8, 895–911. [Google Scholar] [CrossRef]
- Moore, D.J.P.; Gonzalez-Meler, M.A.; Taneva, L.; Pippen, J.S.; Kim, H.S.; DeLucia, E.H. The effect of carbon dioxide enrichment on apparent stem respiration from Pinus taeda L. is confounded by high levels of soil carbon dioxide. Oecologia 2008, 158, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Aoki, S.; Nagasawa, H.; Mabuchi, T.; Kubota, K.; Inoue, S.; Utsumi, Y.; Otsuki, K. Effect of tree-to-tree and radial variations on sap flow estimates of transpiration in Japanese cedar. Agric. For. Meteorol. 2005, 135, 110–116. [Google Scholar] [CrossRef]
- Kato, J.; Hayasi, I. Quantitative analysis of a stand of Pinus densiflora undergoing succession to Quercus mongolica ssp. crispula: 1. A 31-year record of growth and population dynamics of the canopy trees. Ecol. Res. 2006, 21, 503–509. [Google Scholar] [CrossRef]
- Iimura, Y.; Fujimoto, M.; Hirota, M.; Tamura, K.; Higashi, T.; Yonebayashi, K.; Fujitake, N. Effects of ecological succession on surface mineral horizons in Japanese volcanic ash soil. Geoderma 2010, 159, 122–130. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujitake, N.; Ueda, Y.; Oji, Y. Vertical distribution of main soil hydroxyanthraquinones in soil profiles. Soil Sci. Plant Nutr. 1999, 45, 551–561. [Google Scholar] [CrossRef]
- Suchewaboripont, V.; Ando, M.; Yoshitake, S.; Iimura, Y.; Hirota, M.; Ohtsuka, T. Spatial upscaling of soil respiration under a complex canopy structure in an old-growth deciduous forest, Central Japan. Forests 2017, 8, 36. [Google Scholar] [CrossRef]
- Sakabe, A.; Kosugi, Y.; Takahashi, K.; Itoh, M.; Kanazawa, A.; Makita, N.; Ataka, M. One year of continuous measurements of soil CH4 and CO2 fluxes in a Japanese cypress forest: Temporal and spatial variations associated with Asian monsoon rainfall. J. Geophys. Res. Biogeosci. 2015, 120, 585–599. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-Fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Oe, Y.; Yamamoto, A.; Mariko, S. Characteristics of soil respiration temperature sensitivity in a Pinus/Betula mixed forest during periods of rising and falling temperature under the Japanese monsoon climate. J. Ecol. Field Biol. 2011, 34, 193–202. [Google Scholar] [CrossRef]
- Bekku, S.Y.; Sakata, T.; Nakano, T.; Koizumi, H. Middy depression in root respiration of Quercus crispula and Chamaecyparis obtusa: Its implication for estimating carbon cycling in forest ecosystems. Ecol. Res. 2009, 24, 865–871. [Google Scholar] [CrossRef]
- Tomotsune, M.; Yoshitake, S.; Watanabe, S.; Koizumi, H. Separation of root and heterotrophic respiration within soil respiration by trenching, root biomass regression, and root excising methods in a cool-temperate deciduous forest in Japan. Ecol. Res. 2013, 28, 259–269. [Google Scholar] [CrossRef]
- Hermans, R.; McKenzie, R.; Andersen, R.; Teh, Y.A.; Cowie, N.; Subke, J.A. Net soil carbon balance in afforested peatlands and separating autotrophic and heterotrophic soil CO2 effluxes. Biogeosciences 2022, 19, 313–327. [Google Scholar] [CrossRef]
- Teramoto, M.; Liang, N.; Ishida, S.; Zeng, J. Long-term stimulatory warming effect on soil heterotrophic respiration in a coo-temperate boad-leaved deciduous forest in Northern Japan. J. Geophys. Res. Biogeosci. 2018, 123, 1161–1177. [Google Scholar] [CrossRef]
- Baraba, J.; Yuste, J.C.; Poyatos, R.; Janssens, I.A.; Lloret, F. Strong resilience of soil respiration components to drought-induced die-off resulting in forest secondary succession. Oecologia 2016, 182, 27–41. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Negishi, M.; Sugita, K.; Iimura, Y.; Hirota, M. Carbon cycling and sequestration in a Japanese red pine (Pinus densiflora) forest on lava flow of Mt. Fuji. Ecol. Res. 2013, 28, 855–867. [Google Scholar] [CrossRef]
- Han, T.; Huang, W.; Liu, J.; Zhou, G.; Xiao, Y. Different soil respiration responses to litter manipulation in three subtropical successional forests. Sci. Rep. 2015, 5, 18166. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.M.; Wang, G.G.; Xu, Z.J.; Zong, Y.Y.; Zhang, X.L.; Li, J.J.; Wang, H.; Chen, F.S. Litter addition and understory removal influenced soil organic carbon quality and mineral nitrogen supply in a subtropical plantation forest. Plant Soil 2021, 460, 527–540. [Google Scholar] [CrossRef]
- Kominami, Y.; Joumura, M.; Ataka, M.; Tamai, K.; Miyama, T.; Dannoura, M.; Makita, N.; Yoshimura, K. Heterotrophic respiration causes seasonal hysteresis in soil respiration in a warm-temperate forest. J. For. Res. 2012, 17, 296–304. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.; Wu, B.; Zhang, Y.; Chen, W.; Wang, X.; Yu, H.; He, G. Temperature response of soil respiration in a Chinese pine plantation: Hysteresis and seasonal vs. Diel Q10. PLoS ONE 2013, 8, e57858. [Google Scholar] [CrossRef]
- Morén, A.-S.; Lindroth, A. CO2 exchange at the floor of a boreal forest. Agric. For. Meteorol. 2000, 101, 1–14. [Google Scholar] [CrossRef]
- Yuste, J.C.; Janssens, I.A.; Ceulemans, R. Calibration and validation of an empirical approach to model soil CO2 efflux in a decisuous forest. Biogeochemistry 2005, 73, 209–230. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lee, J.-S.; Koizumi, H. Temporal variation in CO2 efflux from soil and snow surfaces in a Japanese cedar (Cryptomeria japonica) plantation, central Japan. Ecol. Res. 2008, 23, 777–785. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, M.; Hobara, Y.; Saitoh, T.M.; Hirota, M. Temporal Variation and Hysteresis of Soil Respiration and Sap Flow of Pinus densiflora in a Cool Temperate Forest, Japan. Forests 2022, 13, 1833. https://doi.org/10.3390/f13111833
Adachi M, Hobara Y, Saitoh TM, Hirota M. Temporal Variation and Hysteresis of Soil Respiration and Sap Flow of Pinus densiflora in a Cool Temperate Forest, Japan. Forests. 2022; 13(11):1833. https://doi.org/10.3390/f13111833
Chicago/Turabian StyleAdachi, Minaco, Yudai Hobara, Taku M. Saitoh, and Mitsuru Hirota. 2022. "Temporal Variation and Hysteresis of Soil Respiration and Sap Flow of Pinus densiflora in a Cool Temperate Forest, Japan" Forests 13, no. 11: 1833. https://doi.org/10.3390/f13111833
APA StyleAdachi, M., Hobara, Y., Saitoh, T. M., & Hirota, M. (2022). Temporal Variation and Hysteresis of Soil Respiration and Sap Flow of Pinus densiflora in a Cool Temperate Forest, Japan. Forests, 13(11), 1833. https://doi.org/10.3390/f13111833







