Dynamic Changes of Rhizosphere Soil Microbiome and Functional Genes Involved in Carbon and Nitrogen Cycling in Chinese Fir Monoculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Samples Collection and Chemical Property Analysis
2.3. Metagenomic Sequencing of Rhizosphere Microbiota
2.4. Data Analysis
3. Results
3.1. Analysis of Soil Nutrient Properties
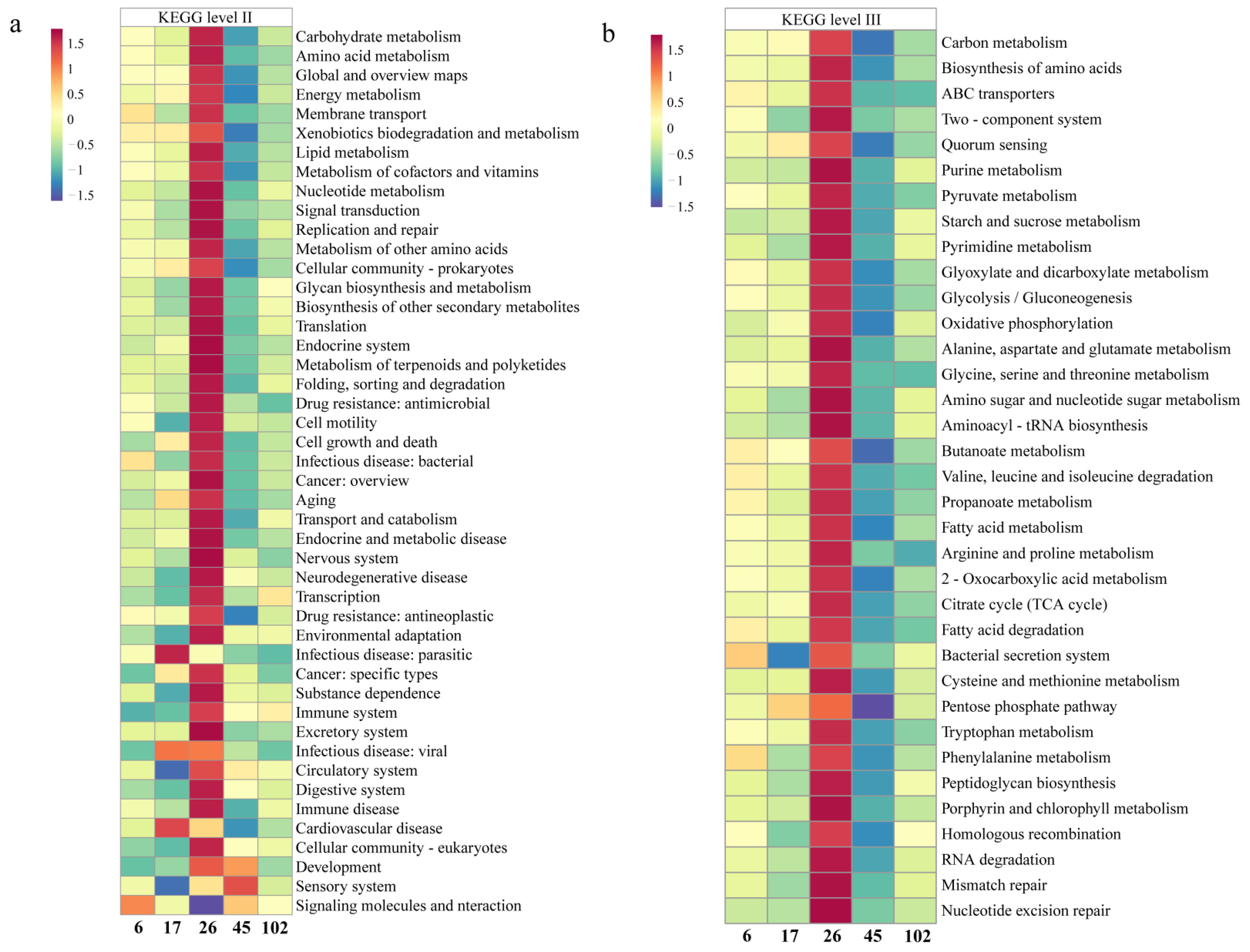
3.2. The Abundant Functional Groups Based on The KEGG Database
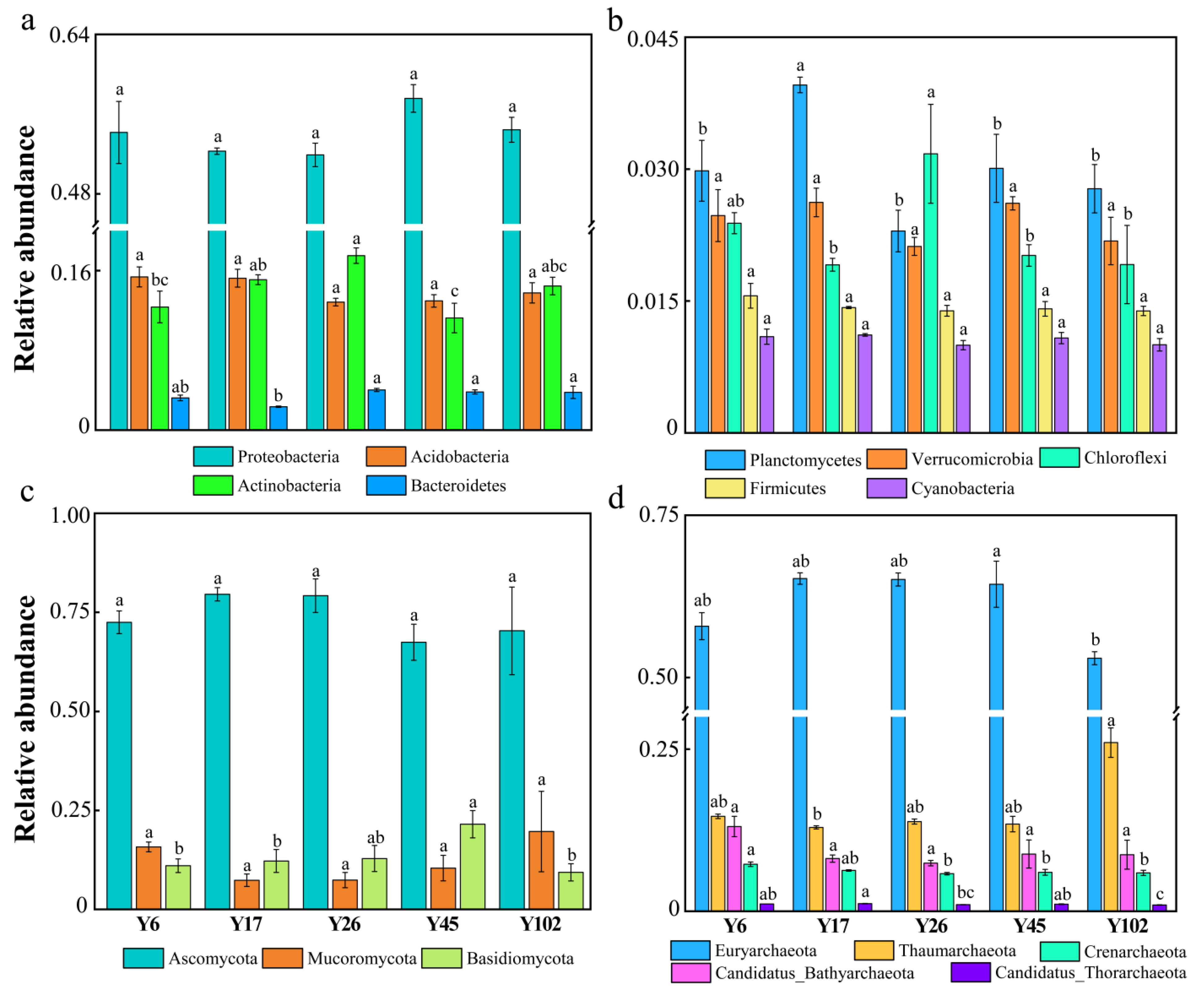
3.3. Assemblage Characteristics of Rhizosphere Microbial Community
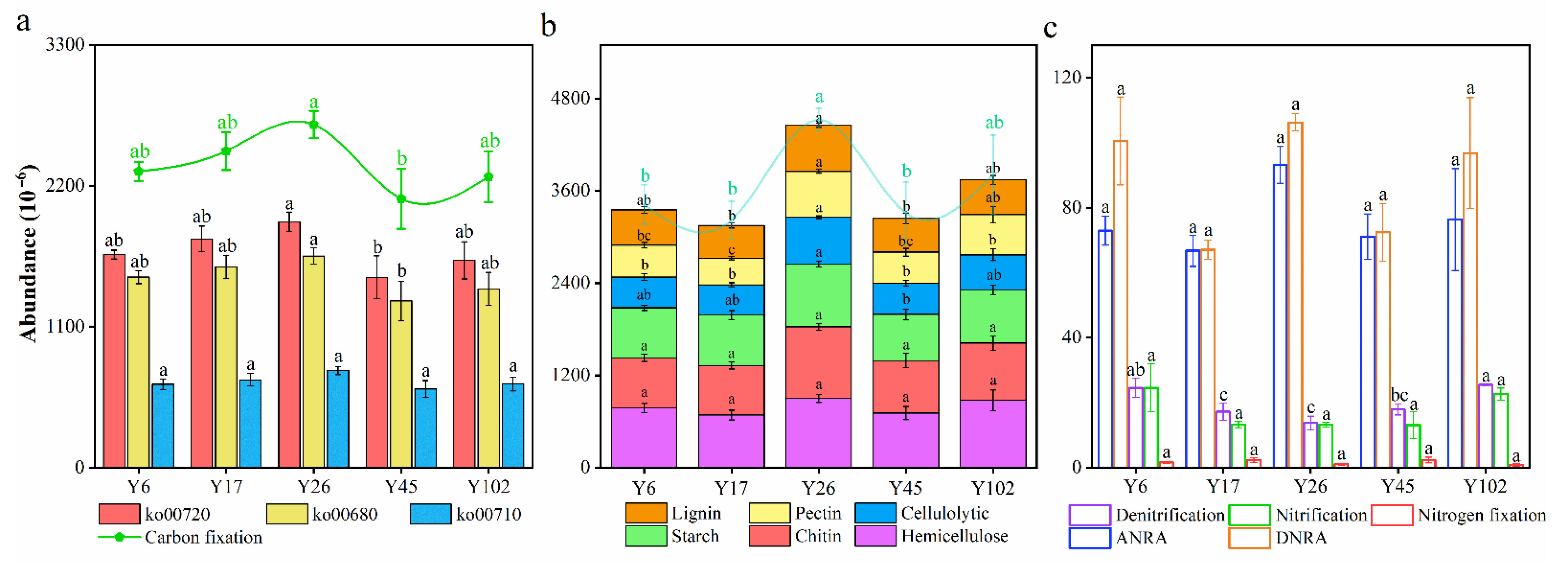
3.4. Function Pathways Involved in C Cycling
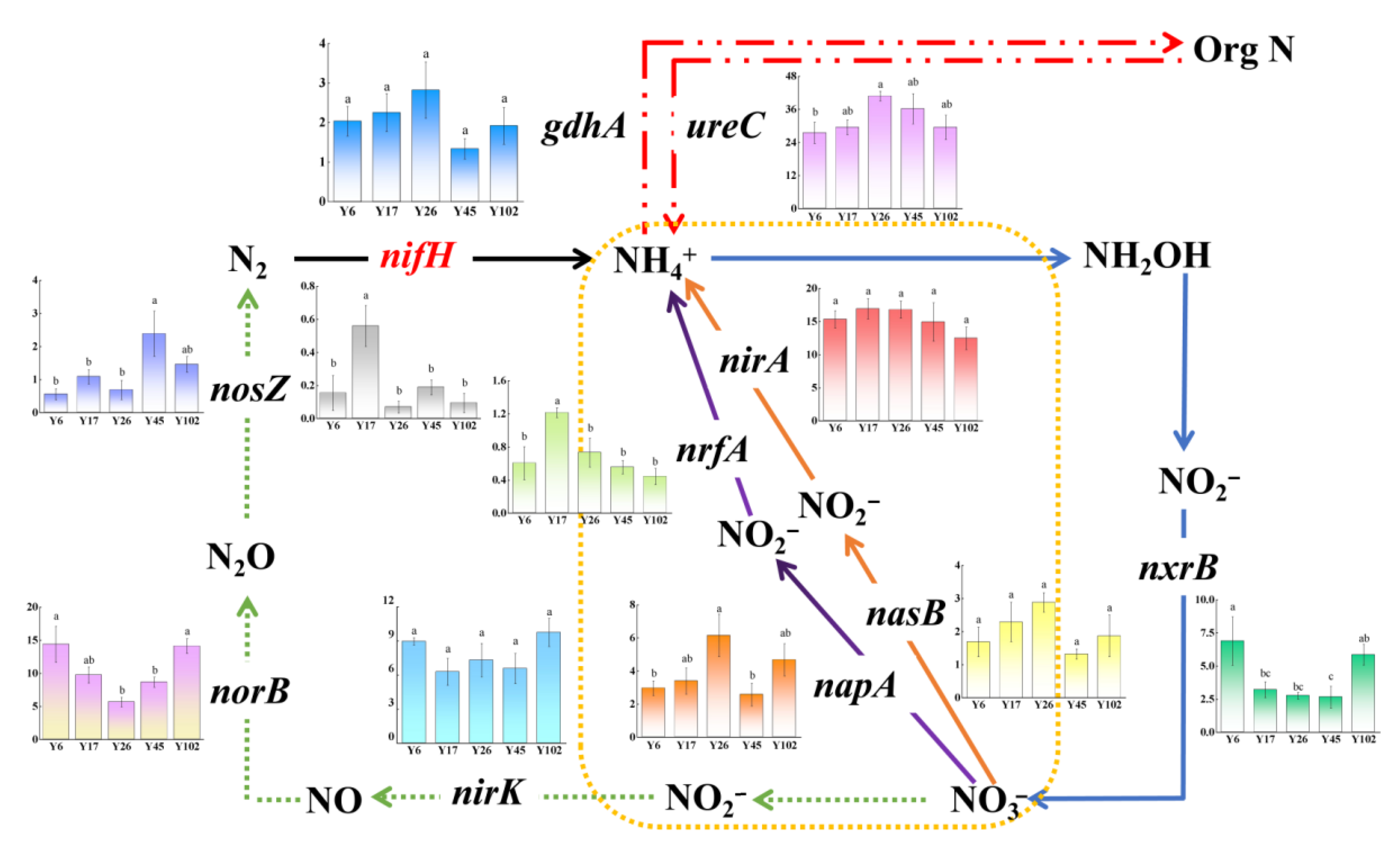
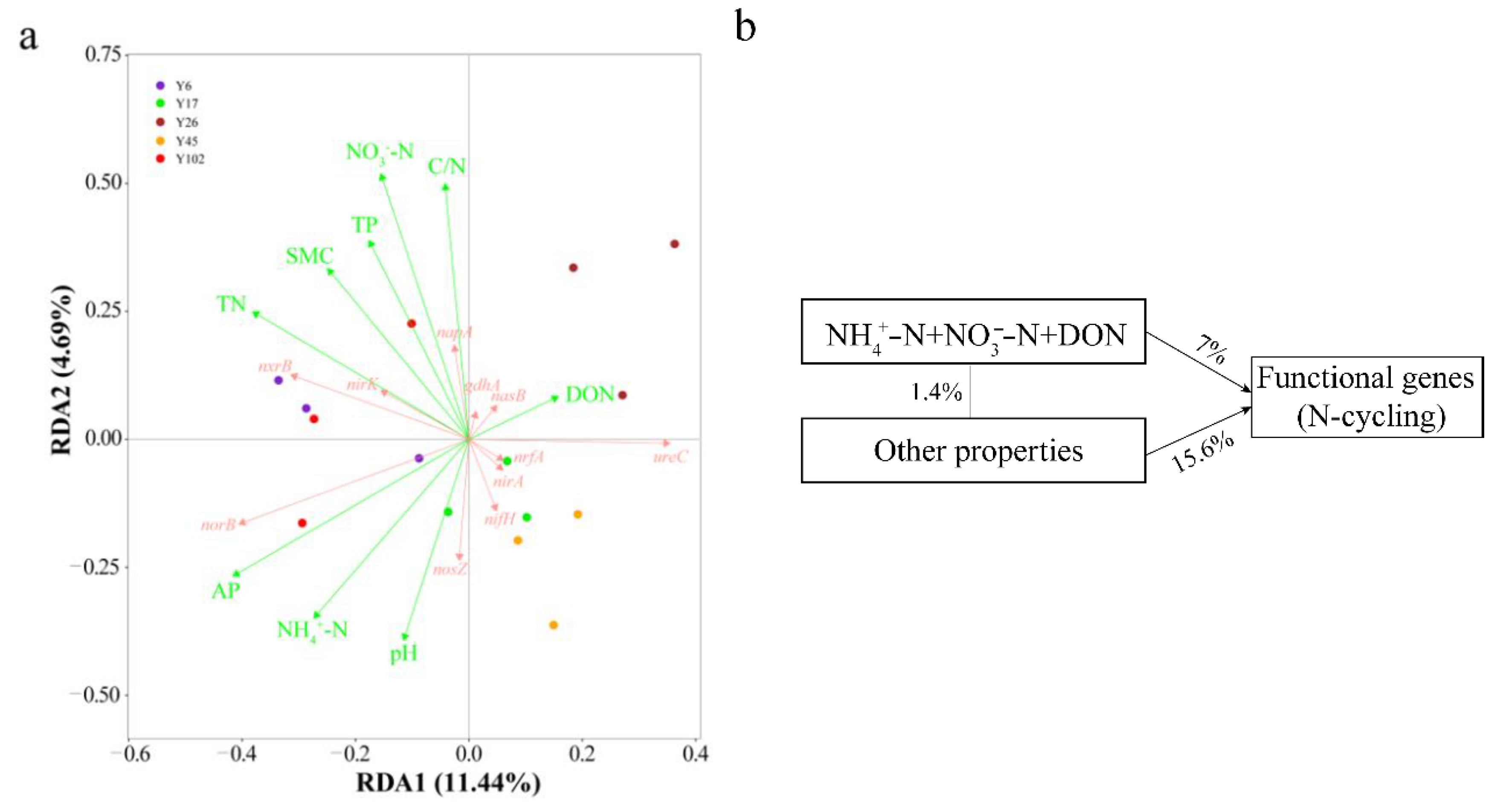
3.5. Functional Pathways Involved in N Cycling
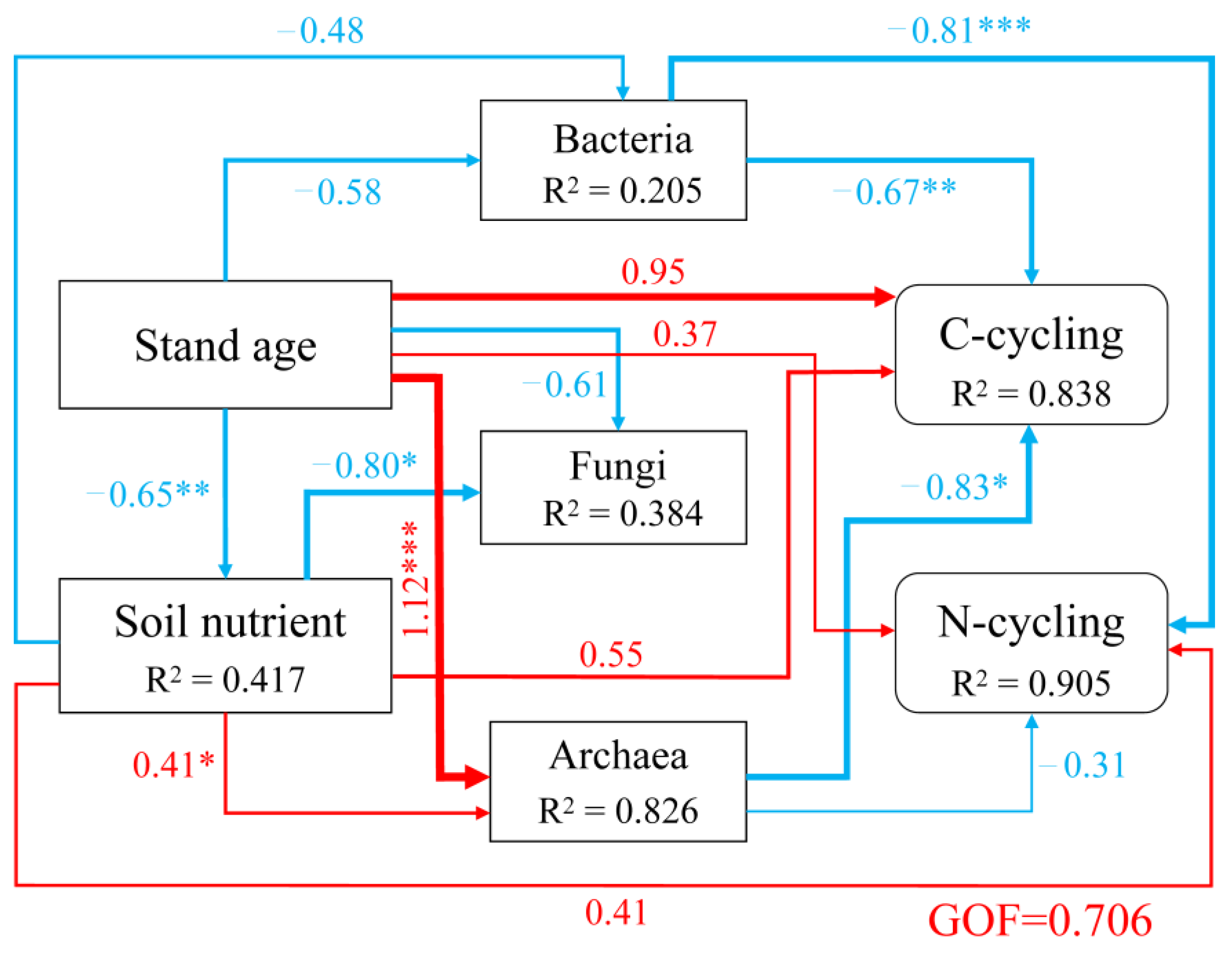
3.6. The Linkage between Stand Age, Soil Nutrient Contents and the Abundance of C and N Functional Pathways
4. Discussion
4.1. Functional Pathways Characteristics of Rhizosphere Microbial Community in Chinese Fir Plantations at Different Developmental Stages
4.2. Microbial Composition and Assemblage in the Chinese Fir Rhizosphere
4.3. Nitrogen-Cycling Processes in Rhizosphere of Chinese Fir Plantation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NFGA. Forest Resources in China—The 9th National Forest Inventory; National Forestry and Grassland Administration: Beijing, China, 2019. [Google Scholar]
- FAO. Global Forest Resource Assessment 2020; Food and Agricultural Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Huang, Z.J.; Selvalakshmi, S.; Vasu, D.; Liu, Q.Q.; Hao, C.; Guo, F.T.; Ma, X.Q. Identification of indicators for evaluating and monitoring the effects of Chinese fir monoculture plantations on soil quality. Ecol. Indic. 2018, 93, 547–554. [Google Scholar]
- Ma, X.Q.; Heal, K.V.; Liu, A.Q.; Jarvis, P.G. Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. For. Ecol. Manag. 2007, 243, 61–74. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Sun, J.; Yao, X.D.; Wang, X.H.; Huang, J.X.; Xiong, D.C.; Chen, G.S. Fine root nutrient foraging ability in relation to carbon availability along a chronosequence of Chinese fir plantations. For. Ecol. Manag. 2022, 507, 120003. [Google Scholar] [CrossRef]
- Islam, W.; Saqib, H.S.A.; Tayyab, M.; Wang, Z.Y.; Ding, X.X.; Su, X.P.; Huang, Z.Q.; Chen, H.Y.H. Natural forest chronosequence maintains better soil fertility indicators and assemblage of total belowground soil biota than Chinese fir monoculture in subtropical ecosystem. J. Clean. Prod. 2022, 334, 130228. [Google Scholar] [CrossRef]
- Revillini, D.; Gehring, C.A.; Johnson, N.C. The role of locally adapted mycorrhizas and rhizobacteria in plant-soil feedback systems. Funct. Ecol. 2016, 30, 1086–1098. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.D.; Qiu, X.Y.; Zhang, J.; Zhang, J.Y.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.M.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Harbort, C.J.; Hashimoto, M.; Inoue, H.; Niu, Y.L.; Guan, R.; Rombola, A.D.; Kopriva, S.; Voges, M.J.; Sattely, E.S.; Garrido-Oter, R.; et al. Root-Secreted Coumarins and the Microbiota Interact to Improve Iron Nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Ren, Y.; Xun, W.B.; Yan, H.; Ma, A.Y.; Xiong, W.; Shen, Q.R.; Zhang, R.F. Functional compensation dominates the assembly of plant rhizospheric bacterial community. Soil Biol. Biochem. 2020, 150, 107968. [Google Scholar] [CrossRef]
- Farooq, T.H.; Kumar, U.; Yan, Y.; Arif, M.S.; Shakoor, A.; Tayyab, M.; Rathod, P.H.; Altaf, M.M.; Wu, P.F. Receptiveness of soil bacterial diversity in relation to soil nutrient transformation and canopy growth in Chinese fir monoculture influenced by varying stand density. Trees-Struct. Funct. 2022, 36, 1149–1160. [Google Scholar] [CrossRef]
- Farooq, T.H.; Kumar, U.; Shakoor, A.; Albasher, G.; Alkahtani, S.; Rizwana, H.; Tayyab, M.; Dobaria, J.; Hussain, M.I.; Wu, P.F. Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture. Sustainability 2021, 13, 10688. [Google Scholar] [CrossRef]
- Islam, W.; Saqib, H.S.A.; Adnan, M.; Wang, Z.Y.; Tayyab, M.; Huang, Z.Q.; Chen, H.Y.H. Differential response of soil microbial and animal communities along the chronosequence of Cunninghamia lanceolata at different soil depth levels in subtropical forest ecosystem. J. Adv. Res. 2022, 38, 41–54. [Google Scholar] [CrossRef]
- Sun, S.; Badgley, B.D. Changes in microbial functional genes within the soil metagenome during forest ecosystem restoration. Soil Biol. Biochem. 2019, 135, 163–172. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef]
- Chen, J.; Sinsabaugh, R.L. Linking microbial functional gene abundance and soil extracellular enzyme activity: Implications for soil carbon dynamics. Glob. Change Biol. 2021, 27, 1322–1325. [Google Scholar] [CrossRef]
- Yang, C.; Lv, D.T.; Jiang, S.Y.; Lin, H.; Sun, J.Q.; Li, K.J.; Sun, J. Soil salinity regulation of soil microbial carbon metabolic function in the Yellow River Delta, China. Sci. Total Environ. 2021, 790, 148258. [Google Scholar] [CrossRef]
- Chen, G.S.; Yang, Z.J.; Gao, R.; Xie, J.S.; Guo, J.F.; Huang, Z.Q.; Yang, Y.S. Carbon storage in a chronosequence of Chinese fir plantations in southern China. For. Ecol. Manag. 2013, 300, 68–76. [Google Scholar] [CrossRef]
- Li, Y.; Heal, K.; Wang, S.Z.; Cao, S.; Zhou, C.F. Chemodiversity of Soil Dissolved Organic Matter and Its Association with Soil Microbial Communities Along a Chronosequence of Chinese Fir Monoculture Plantations. Front. Microbiol. 2021, 12, 729344. [Google Scholar] [CrossRef]
- Kumar, S.; Garkoti, S.C. Rhizosphere influence on soil microbial biomass and enzyme activity in banj oak, chir pine and banj oak regeneration forests in the central Himalaya. Geoderma 2022, 409, 115626. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Jiao, P.Y.; Guo, W.; Du, D.J.; Hu, Y.L.; Tan, X.; Liu, X. Changes in Bulk and Rhizosphere Soil Microbial Diversity and Composition Along an Age Gradient of Chinese Fir (Cunninghamia lanceolate) Plantations in Subtropical China. Front. Microbiol. 2022, 12, 777862. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Liu, C.M.; Luo, R.B.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; LoCascio, P.F.; Hauser, L.J.; Uberbacher, E.C. Gene and translation initiation site prediction in metagenomic sequences. Bioinformatics 2012, 28, 2223–2230. [Google Scholar] [CrossRef]
- Li, W.; Jaroszewski, L.; Godzik, A. Clustering of highly homologous sequences to reduce the size of large protein databases. Bioinformatics 2001, 17, 282–283. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Huson, D.H.; Beier, S.; Flade, I.; Gorska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.J.; Tappu, R. MEGAN Community Edition-Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef]
- Yin, Y.B.; Mao, X.Z.; Yang, J.C.; Chen, X.; Mao, F.L.; Xu, Y. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012, 40, W445–W451. [Google Scholar] [CrossRef]
- Zhu, F.X.; Doyle, E.; Zhu, C.Y.; Zhou, D.M.; Gu, C.; Gao, J. Metagenomic analysis exploring microbial assemblages and functional genes potentially involved in di (2-ethylhexyl) phthalate degradation in soil. Sci. Total Environ. 2020, 715, 137037. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Yu, K. Application of microbial gene databases in the annotation of nitrogen cycle functional genes. Microbiol. China 2020, 47, 3021–3038. (In Chinese) [Google Scholar]
- Dai, Z.M.; Zang, H.D.; Chen, J.; Fu, Y.Y.; Wang, X.H.; Liu, H.T.; Shen, C.C.; Wang, J.J.; Kuzyakov, Y.; Becker, J.N.; et al. Metagenomic insights into soil microbial communities involved in carbon cycling along an elevation climosequences. Environ. Microbiol. 2016, 23, 4631–4645. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéja, R.; Zuhlke, D.; Vetrovsky, T.; Becher, D.; Riedel, K.; Baldrian, P. Decoding the complete arsenal for cellulose and hemicellulose deconstruction in the highly efficient cellulose decomposer Paenibacillus O199. Biotechnol. Biofuels 2016, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.Z.; Li, Y.; Zhang, X.D.; Yan, Z.Q.; Wu, H.D.; Li, M.; Yan, L.; Zhang, K.R.; Wang, J.Z.; Kang, X.M. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Zhang, X.P.; Li, Q.L.; Zhong, Z.K.; Huang, Z.Y.; Bian, F.Y.; Yang, C.B.; Wen, X. Changes in Soil Organic Carbon Fractions and Fungal Communities, Subsequent to Different Management Practices in Moso Bamboo Plantations. J. Fungi 2022, 8, 640. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Yun, Y.; Gui, Z.; Su, T.; Tian, X.; Wang, S.; Chen, Y.; Su, Z.; Fan, H.; Xie, J.; Li, G.; et al. Deep mining decreases the microbial taxonomic and functional diversity of subsurface oil reservoirs. Sci. Total Environ. 2022, 821, 153564. [Google Scholar] [CrossRef]
- Huuskonen, S.; Domisch, T.; Finer, L.; Hantula, J.; Hynynen, J.; Matala, J.; Miina, J.; Neuvonen, S.; Nevalainen, S.; Niemisto, P.; et al. What is the potential for replacing monocultures with mixed-species stands to enhance ecosystem services in boreal forests in Fennoscandia? For. Ecol. Manag. 2021, 479, 118558. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zou, G.Y.; Zuo, Q.; Li, C.Z.; Gu, J.L.; Kang, L.Y.; Ma, M.T.; Liang, K.Y.; Liu, D.S.; Du, L.F. Soil bacterial community and metabolism showed a more sensitive response to PBAT biodegradable mulch residues than that of LDPE mulch residues. J. Hazard. Mater. 2022, 438, 129507. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Yao, L. Soil microbial community and physicochemical properties together drive soil organic carbon in Cunninghamia lanceolata plantations of different stand ages. Peer J. 2022, 10, e13873. [Google Scholar] [CrossRef]
- Fan, H.B.; Su, B.Q. Biogeochemical cycle within ecosystem of Chinese fir plantations I: Dynamics of nutrients returning to ecosystem. Chin. J. Appl. Environ. Biol. 2000, 2, 127–132. (In Chinese) [Google Scholar]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Zhong, Y.Q.W.; Yan, W.M.; Wang, R.W.; Wang, W.; Shangguan, Z.P. Decreased occurrence of carbon cycle functions in microbial communities along with long-term secondary succession. Soil Biol. Biochem. 2018, 123, 207–217. [Google Scholar] [CrossRef]
- Tayyab, M.; Yang, Z.Q.; Zhang, C.F.; Islam, W.; Lin, W.X.; Zhang, H. Sugarcane monoculture drives microbial community composition, activity and abundance of agricultural-related microorganisms. Environ. Sci. Pollut. Res. 2021, 28, 48080–48096. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Yan, B.F.; Liu, N.; Liu, M.H.; Du, X.Y.; Shang, F.; Huang, Y. Soil actinobacteria tend to have neutral interactions with other co-occurring microorganisms, especially under oligotrophic conditions. Environ. Microbiol. 2021, 23, 4126–4140. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, L.M.; Shen, J.P.; Zheng, Y.M.; Di, H.J.; He, J.Z. Distribution and diversity of archaeal communities in selected Chinese soils. FEMS Microbiol. Ecol. 2011, 80, 146–158. [Google Scholar] [CrossRef]
- Angel, R.; Soares, M.I.M.; Ungar, E.D.; Gillor, O. Biogeography of soil archaea and bacteria along a steep precipitation gradient. ISME J. 2010, 4, 553–563. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea are interactive components of complex microbiomes. Trends Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef]
- Zhalnina, K.; de Quadros, P.D.; Camargo, F.A.O.; Triplett, E.W. Drivers of archaeal ammonia-oxidizing communities in soil. Front. Microbiol. 2012, 3, 210. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Pearson Education: Upper Saddle River, NJ, USA, 2006; Chapter 13. [Google Scholar]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Llado, S.; Zifcakova, L.; Vetrovsky, T.; Eichlerova, I.; Baldrian, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; DeAngelis, K.M.; Pold, G. Draft Genome Sequence of Acidobacteria Group 1 Acidipila sp. Strain EB88, Isolated from Forest Soil. Microbiol. Resour. Announc. 2019, 8, e01464-18. [Google Scholar] [CrossRef]
- Pang, Z.Q.; Dong, F.; Liu, Q.; Lin, W.X.; Hu, C.H.; Yuan, Z.N.A. Soil metagenomics reveals effects of continuous sugarcane cropping on the structure and functional pathway of rhizospheric microbial community. Front. Microbiol. 2021, 12, 627569. [Google Scholar] [CrossRef]
- Kuypers, M.; Marchant, H.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Zhou, S.T.; Xue, K.; Zhang, B.; Tang, L.; Pang, Z.; Wang, F.; Che, R.X.; Ran, Q.W.; Xia, A.Q.; Wang, K.; et al. Spatial patterns of microbial nitrogen-cycling gene abundances along a precipitation gradient in various temperate grasslands at a regional scale. Geoderma 2021, 404, 115236. [Google Scholar] [CrossRef]
- Petersen, D.G.; Blazewicz, S.J.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [CrossRef]
- Minick, K.J.; Pandey, C.B.; Fox, T.R.; Subedi, S. Dissimilatory nitrate reduction to ammonium and N2O flux: Effect of soil redox potential and N fertilization in loblolly pine forests. Biol. Fertil. Soils 2016, 52, 601–614. [Google Scholar] [CrossRef]
- Zhao, B.J.; Li, X.S.; Wang, Y.; Tan, X.; Qi, W.H.; Li, H.R.; Wei, J.W.; You, Y.; Shi, W.J.; Zhang, Q.F. Dissimilatory nitrate reduction and functional genes in two subtropical rivers, China. Environ. Sci. Pollut. Res. 2021, 28, 68155–68173. [Google Scholar] [CrossRef]
- Braker, G.; Tiedje, J.M. Nitric Oxide Reductase (norB) Genes from Pure Cultures and Environmental Samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef]
- Ni, B.; Zhao, W.; Zuo, X.H.; You, J.; Li, Y.L.; Li, J.N.; Du, Y.D.; Chen, X. Deyeuxia angustifolia Kom. encroachment changes soil physicochemical properties and microbial community in the alpine tundra under climate change. Sci. Total Environ. 2022, 813, 152615. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; He, L.Y.; Xu, X.F.; Ren, C.J.; Wang, J.; Guo, Y.X.; Zhao, F.Z. Linkage between microbial functional genes and net N mineralisation in forest soils along an elevational gradient. Eur. J. Soil Sci. 2022, 73, e13276. [Google Scholar] [CrossRef]
- Helen, D.; Kim, H.; Tytgat, B.; Anne, W. Highly diverse nirK genes comprise two major clades that harbour ammonium-producing denitrifiers. BMC Genom. 2016, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.Y.; Peng, Z.H.; Tian, J.; Song, F.Q.; Wei, G.H.; Wang, H.L. Distinct abundance patterns of nitrogen functional microbes in desert soil profiles regulatesoil nitrogen storage potential along a desertification development gradient. CATENA 2020, 194, 104716. [Google Scholar] [CrossRef]
- Hayden, H.L.; Drake, J.; Imhof, M.; Oxley, A.P.A.; Norng, S.; Mele, P.M. The abundance of nitrogen cycle genes amoA and nifH depends on land-uses and soil types in South-Eastern Australia. Soil Biol. Biochem. 2010, 42, 1774–1783. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Lin, Q.M.; Xu, J.M. Soil Biochemistry; Higher Education Press: Beijing, China, 2015; pp. 198–202. (In Chinese) [Google Scholar]

| Stand Ages (Years) | 6 | 17 | 26 | 45 | 102 |
|---|---|---|---|---|---|
| pH | 5.09 ± 0.07 a | 4.77 ± 0.09 b | 4.70 ± 0.01 b | 5.15 ± 0.07 a | 4.71 ± 0.07 b |
| DOC (mg kg−1) | 242.65 ± 13.17 ab | 288.95 ± 30.27 a | 303.14 ± 29.28 a | 186.80 ± 4.52 b | 218.68 ± 10.52 b |
| DON (mg kg−1) | 51.55 ± 4.76 a | 66.91 ± 6.82 a | 58.19 ± 5.06 a | 49.53 ± 3.95 a | 48.79 ± 5.46 a |
| NH4+-N (mg kg−1) | 10.29 ± 1.12 a | 10.35 ± 1.13 a | 7.94 ± 0.95 a | 8.58 ± 1.07 a | 9.56 ± 2.82 a |
| NO3--N (mg kg−1) | 5.29 ± 0.26 bc | 4.85 ± 0.25 c | 6.89 ± 0.74 ab | 4.56 ± 0.28 c | 7.96 ± 1.11 a |
| TN (g kg−1) | 1.20 ± 0.10 a | 1.03 ± 0.15 a | 1.13 ± 0.07 a | 0.90 ± 0.10 a | 1.27 ± 0.12 a |
| TC (g kg−1) | 18.37 ± 2.02 a | 15.40 ± 2.12 ab | 18.73 ± 1.62 a | 10.83 ± 1.11 b | 17.83 ± 2.09 a |
| C/N | 15.54 ± 0.09 a | 14.80 ± 0.38 ab | 16.04 ± 0.60 a | 12.19 ± 0.17 c | 14.07 ± 0.62 b |
| SMC (Soil moisture content) | 0.31 ± 0.02 a | 0.26 ± 0.02 ab | 0.26 ± 0.01 bc | 0.16 ± 0.01 d | 0.22 ± 0.00 cd |
| AP (mg kg−1) | 3.21 ± 0.5 ab | 2.46 ± 0.24 ab | 1.91 ± 0.51 b | 3.89 ± 1.11 ab | 3.98 ± 0.50 a |
| Soil Property | Mantel r | ||
|---|---|---|---|
| Bacterial Community | Fungal Community | Archaeal Community | |
| pH | −0.04 | 0.09 | 0.24 * |
| TN | −0.04 | 0.01 | 0.19 |
| TC | −0.06 | −0.03 | 0.04 |
| C/N | 0.05 | −0.05 | 0.02 |
| SMC | 0.09 | −0.12 | 0.30 * |
| DOC | −0.05 | −0.16 | −0.13 |
| NH4+-N | 0.01 | 0.10 | 0.09 |
| NO3−-N | 0.05 | 0.14 | 0.12 |
| TP | 0.27 * | −0.11 | 0.14 |
| AP | −0.04 | 0.36* | 0.30 * |
| DON | −0.10 | 0.00 | −0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, W.; Gao, Q.; Zhou, C. Dynamic Changes of Rhizosphere Soil Microbiome and Functional Genes Involved in Carbon and Nitrogen Cycling in Chinese Fir Monoculture. Forests 2022, 13, 1906. https://doi.org/10.3390/f13111906
Wang S, Chen W, Gao Q, Zhou C. Dynamic Changes of Rhizosphere Soil Microbiome and Functional Genes Involved in Carbon and Nitrogen Cycling in Chinese Fir Monoculture. Forests. 2022; 13(11):1906. https://doi.org/10.3390/f13111906
Chicago/Turabian StyleWang, Shuzhen, Wenwen Chen, Qianqian Gao, and Chuifan Zhou. 2022. "Dynamic Changes of Rhizosphere Soil Microbiome and Functional Genes Involved in Carbon and Nitrogen Cycling in Chinese Fir Monoculture" Forests 13, no. 11: 1906. https://doi.org/10.3390/f13111906
APA StyleWang, S., Chen, W., Gao, Q., & Zhou, C. (2022). Dynamic Changes of Rhizosphere Soil Microbiome and Functional Genes Involved in Carbon and Nitrogen Cycling in Chinese Fir Monoculture. Forests, 13(11), 1906. https://doi.org/10.3390/f13111906





