Effects of Drying and Rewetting Cycles on Carbon Dioxide Emissions and Soil Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Site Description
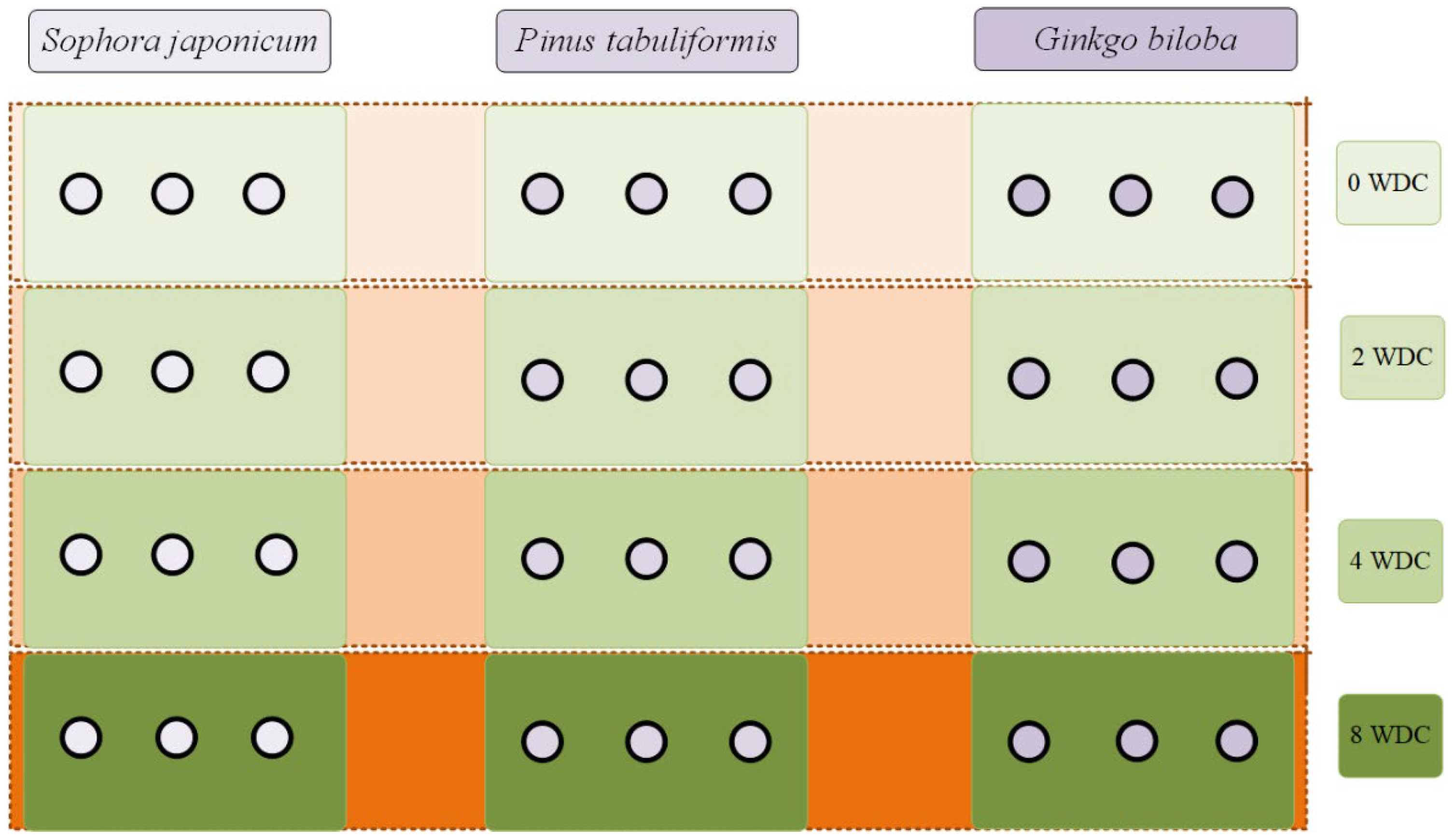
2.2. Experimental Design
2.3. Determination of Soil Physicochemical Properties and Microbial Biomass Carbon and Nitrogen
2.4. Determination of Soil CO2 Fluxes
2.5. DNA Extraction and High-Throughput Sequencing of Soil Microorganisms
2.6. Statistical Analyses
3. Results
3.1. Soil Physicochemical Properties and Microbial Biomass Carbon and Nitrogen
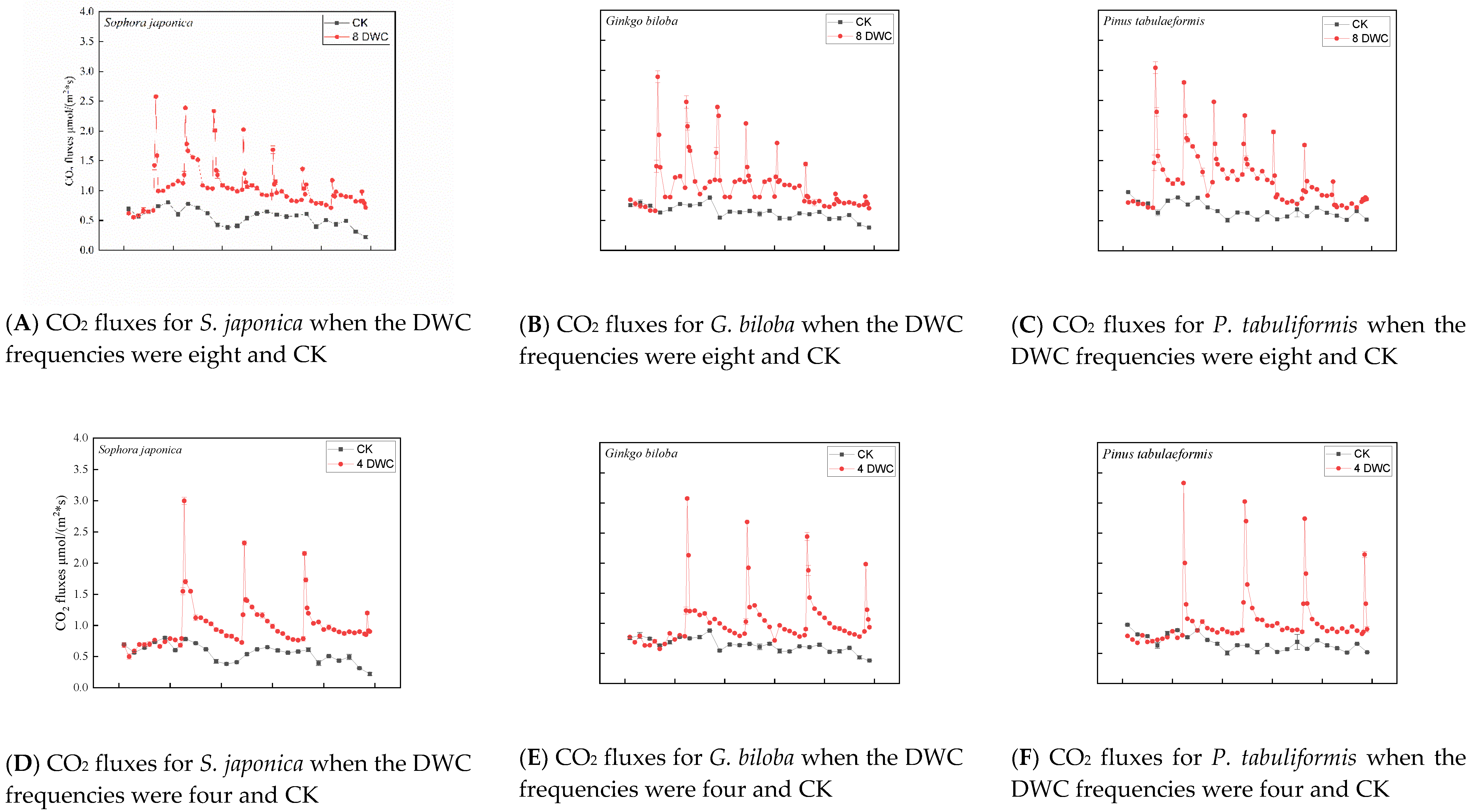
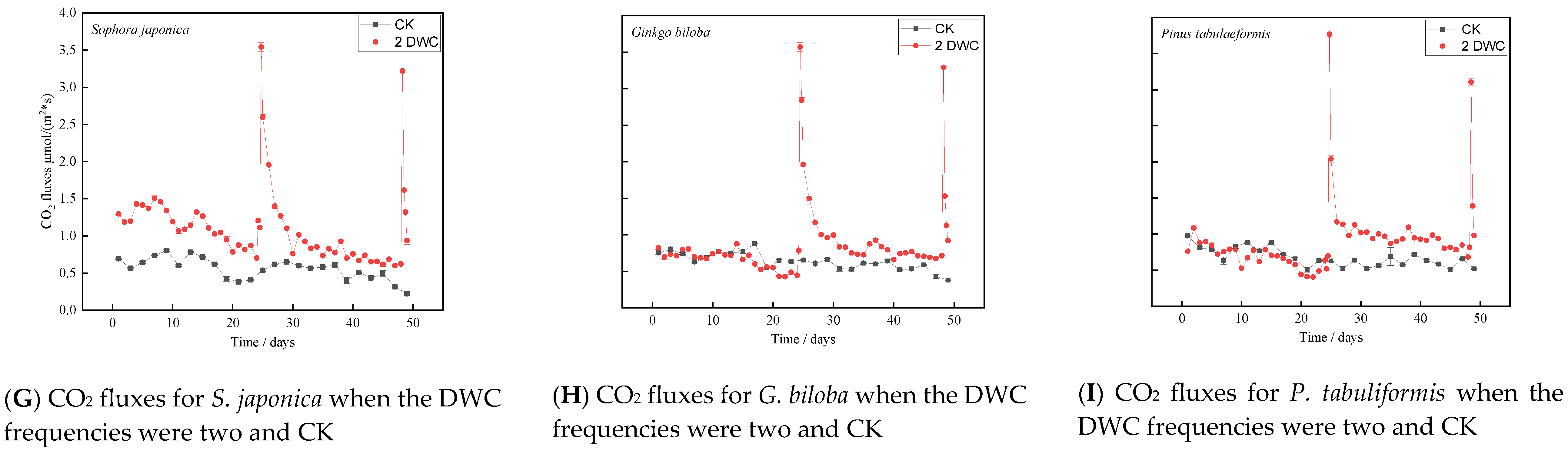
3.2. Effects of Drying and Rewetting Cycle Frequencies on CO2 Fluxes
3.3. Effects of Drying and Rewetting Cycle Frequencies on Cumulative CO2 Fluxes
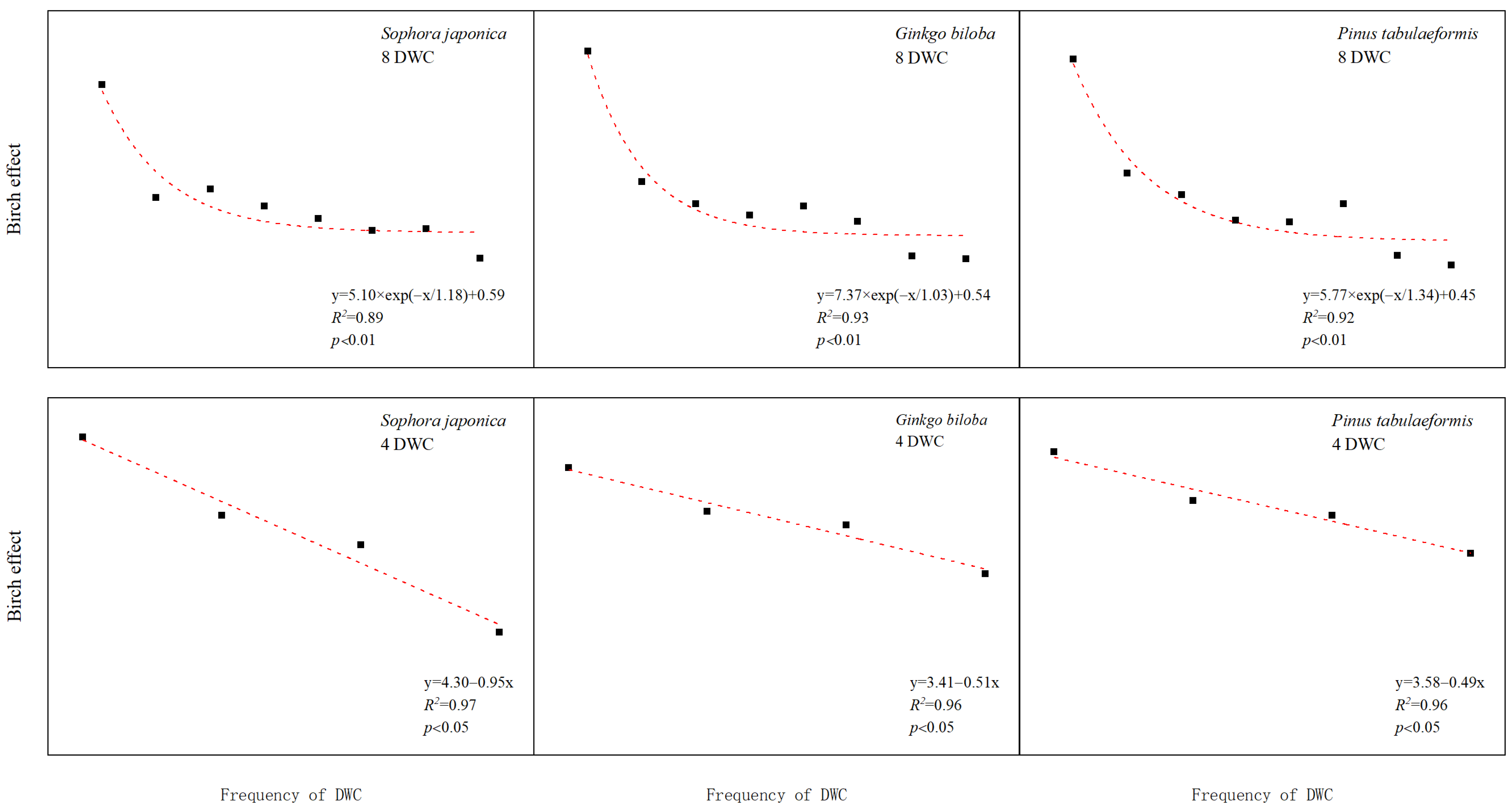
3.4. Effects of Drying and Rewetting Cycle Frequencies on the Birch Effect
3.5. Effects of Drying and Rewetting Cycles on Soil Bacterial Community
3.5.1. Soil Bacterial Community Diversity
3.5.2. Abundance of Bacterial Communities at the Phylum and Genus Levels
3.5.3. Correlation Analysis between Soil Physicochemical Properties and Bacterial Communities
4. Discussion
4.1. Effects of Drying and Rewetting Cycles on CO2 Emissions
4.2. Effects of Drying and Rewetting Cycles on Microbial Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marengo, J.A.; Jones, R.; Alves, L.M.; Valverde, M.C. Future change of temperature and precipitation extremes in South America as derived from the PRECIS regional climate modeling system. Int. J. Climatol. 2010, 29, 2241–2255. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shao, Q.; Tao, Y.; Peng, S.; Sun, F. Changes in daily temperature and precipitation extremes in the Yellow River Basin, China. Stoch. Environ. Res. Risk Assess. 2012, 27, 401–421. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Deluca, T.H.; Boisvenue, C. Boreal forest soil carbon: Distribution, function and modelling. Forestry 2012, 34, 4678–4689. [Google Scholar]
- Ouyang, Y.; Li, X. Recent research progress on soil microbial responses to drying–rewetting cycles. Acta Ecol. Sin. 2013, 33, 1–6. [Google Scholar] [CrossRef]
- Piao, S.; Zhang, X.; Chen, A.; Liu, Q.; Lian, X.; Wang, X.; Peng, S.; Wu, X. The impacts of climate extremes on the terrestrial carbon cycle: A review. Sci. China 2019, 62, 1551–1563. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef]
- Dorothea, F.; Markus, R.; Michael, B.; Kirsten, T.; David, F.; Miguel, D.M.; Pete, S. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Change Biol. 2015, 21, 2861–2880. [Google Scholar]
- Skansi, M.; Brunet, M.; Sigró, J.; Aguilar, E.; Jones, P.D. Warming and wetting signals emerging from analysis of changes in climate extreme indices over South America. Glob. Planet. Change 2013, 100, 295–307. [Google Scholar] [CrossRef]
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global warming and 21st century drying. Clim. Dyn. 2014, 43, 2607–2627. [Google Scholar] [CrossRef] [Green Version]
- Jan, M.; Janine, F.; Werner, B. Drying–rewetting events reduce C and N losses from a Norway spruce forest floor. Soil Biol. Biochem. 2010, 42, 1303–1312. [Google Scholar]
- Chen, H.; Lai, L.; Zhao, X.; Li, G.; Lin, Q. Soil microbial biomass carbon and phosphorus as affected by frequent drying–rewetting. Soil Res. 2016, 54, 321–327. [Google Scholar] [CrossRef]
- Michelle, M.S.L.; Derrick, Y.F.L. Impacts of wetting-drying cycles on short-term carbon and nitrogen dynamics in Amynthas earthworm casts. Pedosphere 2021, 31, 423–432. [Google Scholar]
- Zhang, C.G.; Gao, Y.; Wang, G.S.; Shuang, L.I. Effects of drying-wetting and additional nitrogen on CO_2 and N_2O emissions from farmland soils. J. Agro-Environ. Sci. 2018, 37, 2079–2090. [Google Scholar]
- Chowdhury, N.; Nakatani, A.S.; Setia, R.; Marschner, P. Microbial activity and community composition in saline and non-saline soils exposed to multiple drying and rewetting events. Plant Soil 2011, 348, 103–113. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X. Impacts of drying-wetting cycles on CO2 and N2O emissions from soils in different ecosystems. Acta Ecol. Sin. 2013, 33, 1251–1259. [Google Scholar] [CrossRef]
- Peralta, A.L.; Ludmer, S.; Kent, A.D. Hydrologic history influences microbial community composition and nitrogen cycling under experimental drying/wetting treatments. Soil Biol. Biochem. 2013, 66, 29–37. [Google Scholar] [CrossRef]
- Fan, Z.P.; Hu, Y.L.; Li, J.T.; Zeng, D.H. Effects of drying-rewetting on soil C and N mineralization rates in Mongolian pine plantation in a semi-arid region. Chin. J. Ecol. 2015, 34, 3360–3367. [Google Scholar]
- Yemadje, P.L.; Chevallier, T.; Deleporte, P.; Bernoux, M. Effect of biomass management regimes and wetting-drying cycles on soil carbon mineralization in a Sudano-Sahelian region. J. Arid Environ. 2016, 127, 1–6. [Google Scholar] [CrossRef]
- Min, K.; Berhe, A.A.; Khoi, C.M.; Asperen, H.V.; Six, J. Differential effects of wetting and drying on soil CO2 concentration and flux in near-surface vs. deep soil layers. Biogeochemistry 2020, 148, 255–269. [Google Scholar] [CrossRef]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Y.Q.; Qin, S.G. Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosci. Discuss. 2013, 10, 9213–9242. [Google Scholar] [CrossRef] [Green Version]
- Li, J.T.; Wang, J.J.; Zeng, D.H.; Zhao, S.Y.; Huang, W.L.; Sun, X.K.; Hu, Y.L. The influence of drought intensity on soil respiration during and after multiple drying-rewetting cycles. Soil Biol. Biochem. 2018, 127, 82–89. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, W. Impacts of drying–wetting cycles on rhizosphere respiration and soil organic matter decomposition. Soil Biol. Biochem. 2013, 63, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.E.; Schimel, J.P.; Meixner, T.; Sickman, J.O.; Melack, J.M. Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol. Biochem. 2005, 37, 2195–2204. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Waring, B.G.; Powers, J.S. Unraveling the mechanisms underlying pulse dynamics of soil respiration in tropical dry forests. Environ. Res. Lett. 2016, 11, 105005. [Google Scholar] [CrossRef]
- Todman, L.C.; Fraser, F.C.; Pawlett, M.; Ritz, K.; Deeks, L.K. On the origin of carbon dioxide released from rewetted soils. Soil Biol. Biochem. 2016, 101, 1–5. [Google Scholar]
- Ouyang, Y.; Li, X. Effect of repeated drying-rewetting cycles on soil extracellular enzyme activities and microbial community composition in arid and semi-arid ecosystems. Eur. J. Soil Biol. 2020, 98, 103187. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Change Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, S.; Wang, R. Diverse soil respiration responses to extreme precipitation patterns in arid and semiarid ecosystems. Appl. Soil Ecol. 2021, 163, 103928. [Google Scholar] [CrossRef]
- Huygens, D.; Schouppe, J.; Roobroeck, D.; Alvarez, M.; Balocchi, O.; Valenzuela, E.; Pinochet, D.; Boeckx, P. Drying–rewetting effects on N cycling in grassland soils of varying microbial community composition and management intensity in south central Chile. Appl. Soil Ecol. 2011, 48, 270–279. [Google Scholar] [CrossRef]
- Yang, P.; Wang, N.a.; Zhao, L.; Su, B.; Niu, Z.; Zhao, H. Responses of grassland ecosystem carbon fluxes to precipitation and their environmental factors in the Badain Jaran Desert. Environ. Sci. Pollut. Res. 2022, 29, 75805–75821. [Google Scholar] [CrossRef] [PubMed]
- Dasheng, S.; Qingfang, B.; Kejie, L.; Jun, Z.; Qichun, Z.; Chongwei, J.; Lingli, L.; Xianyong, L. Effect of Soil Drying Intensity During an Experimental Drying-Rewetting Event on Nutrient Transformation and Microbial Community Composition. Pedosphere 2018, 28, 644–655. [Google Scholar]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Neergaard, A.D.; Jensen, L.S.; Luxhi, J.; Jensen, C.R. Alternate partial root-zone irrigation induced dry/wet cycles of soils stimulate N mineralization and improve N nutrition in tomatoes. Plant Soil 2010, 337, 167–177. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Andersen, M.N.; Jensen, C.R. Carbon retention in the soil-plant system under different irrigation regimes. Agric. Water Manag. 2010, 98, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Veach, A.M.; Zeglin, L.H. Historical Drought Affects Microbial Population Dynamics and Activity During Soil Drying and Re-Wet. Microb. Ecol. 2020, 79, 662–674. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Bottomley, P.J.; Jumpponen, A.; Rice, C.W.; Arango, M.; Lindsley, A.; McGowan, A.; Mfombep, P.; Myrold, D. Altered precipitation regime affects the function and composition of soil microbial communities on multiple time scales. Ecology 2013, 94, 2334–2345. [Google Scholar] [CrossRef]
- Nijs, E.D.; Hicks, L.C.; Leizeaga, A.; Tietema, A.; Rousk, J. Soil microbial moisture dependences and responses to drying-rewetting: The legacy of 18 years drought. Glob. Change Biol. 2018, 25, 1005–1015. [Google Scholar] [CrossRef]
- Xue, Z.; Dario, F.; Makoto, I.; Isao, A.; Zhang, R.; Jia, Z. The Resilience of Microbial Community under Drying and Rewetting Cycles of Three Forest Soils. Front. Microbiol. 2016, 7, 1101. [Google Scholar]
- Zhao, J.; Ouyang, Z.; Zheng, H.; Zhou, W.; Wang, X.; Xu, W.; Ni, Y. Plant species composition in green spaces within the built-up areas of Beijing, China. Plant Ecol. 2010, 209, 179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, G.; Zhang, T. Responses of foliar δ13C in Sabina przewalskii and Picea crassifolia to altitude and its mechanism in the Qilian Mountains, China. Chin. J. Plant Ecol. 2010, 34, 125–133. [Google Scholar]
- Wang, Y.; Liu, X.; Chen, F.; Huang, R.; Deng, X.; Jiang, Y. Seasonal dynamics of soil microbial biomass C and N of Keteleeria fortunei var.cyclolepis forests with different ages. J. For. Res. 2020, 31, 2377–2384. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Zhu, B.; Cheng, W. Decadally cycling soil carbon is more sensitive to warming than faster-cycling soil carbon. Glob. Change Biol. 2015, 21, 4602–4612. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [Green Version]
- Mendes, L.W.; Tsai, S.M.; Navarrete, A.A.; Hollander, M.; Veen, J.; Kuramae, E.E. Soil-Borne Microbiome: Linking Diversity to Function. Microb. Ecol. 2015, 70, 255–265. [Google Scholar] [CrossRef]
- Leon, E.; Vargas, R.; Bullock, S.; Lopez, E.; Panosso, A.R.; Scala, N.L. Hot spots, hot moments, and spatio-temporal controls on soil CO2 efflux in a water-limited ecosystem. Soil Biol. Biochem. 2014, 77, 12–21. [Google Scholar] [CrossRef]
- Smith, A.P.; Bond-Lamberty, B.; Benscoter, B.W.; Tfaily, M.M.; Hinkle, C.R.; Liu, C.; Bailey, V.L. Shifts in pore connectivity from precipitation versus groundwater rewetting increases soil carbon loss after drought. Nat. Commun. 2017, 8, 1335. [Google Scholar] [CrossRef]
- Sánchez-García, C.; Oliveira, B.; Keizer, J.J.; Doerr, S.H.; Urbanek, E. Water repellency reduces soil CO2 efflux upon rewetting. Sci. Total Environ. 2019, 708, 135014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lado-Monserrat, L.; Lull, C.; Bautista, I.; Lidón, A.; Herrera, R. Soil moisture increment as a controlling variable of the “Birch effect”. Interactions with the pre-wetting soil moisture and litter addition. Plant Soil 2014, 379, 21–34. [Google Scholar] [CrossRef]
- Gebremichael, A.; Orr, P.J.; Osborne, B. The impact of wetting intensity on soil CO2 emissions from a coastal grassland ecosystem. Geoderma 2019, 343, 86–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Li, M. Soil respiration, aggregate stability and nutrient availability affected by drying duration and drying-rewetting frequency. Geoderma 2022, 413, 115743. [Google Scholar] [CrossRef]
- Jla, C.; Ld, B.; Yla, C.; Jw, B.; Jie, W.B.; Zsab, C.; Lei, D. Soil organic carbon variation determined by biogeographic patterns of microbial carbon and nutrient limitations across a 3000-km humidity gradient in China. Catena 2022, 209, 105849. [Google Scholar]
- Chowdhury, N.; Burns, R.G.; Marschner, P. Recovery of soil respiration after drying. Plant Soil 2011, 348, 269–279. [Google Scholar] [CrossRef]
- Schmitt, A.; Glaser, B. Organic matter dynamics in a temperate forest soil following enhanced drying. Soil Biol. Biochem. 2011, 43, 478–489. [Google Scholar] [CrossRef]
- Cruz-Martinez, K.; Rosling, A.; Zhang, Y.; Song, M.; Andersen, G.L.; Banfield, J.F. Effect of Rainfall-Induced Soil Geochemistry Dynamics on Grassland Soil Microbial Communities. Appl. Environ. Microbiol. 2012, 78, 7587. [Google Scholar] [CrossRef]


| Number of Drying and Rewetting Cycles | Amount of Precipitation for Each Cycle (mm) | Precipitation Period (d) | Drought Period (d) | Total Amount of Precipitation (mm) | Experimental Duration (d) |
|---|---|---|---|---|---|
| 0 (0 DWCs/CK) | 0 | 0 | 48 | 0 | 48 |
| 2 (2 DWCs/R80) | 80 | 1 | 23 | 160 | 48 |
| 4 (4 DWCs/R40) | 40 | 1 | 11 | 160 | 48 |
| 8 (8 DWCs/R20) | 20 | 1 | 5 | 160 | 48 |
| Soil Properties | Soil Layer | Sophora japonica | Pinus tabulaeformis | Ginkgo biloba |
|---|---|---|---|---|
| pH | 0–10 cm | 7.55 ± 0.09 Aa | 7.71 ± 0.05 Aa | 7.64 ± 0.08 Aa |
| 10–20 cm | 7.62 ± 0.04 Aa | 7.63 ± 0.03 Aab | 7.67 ± 0.01 Aa | |
| 20–30 cm | 7.62 ± 0.01 Aa | 7.50 ± 0.12 Ab | 7.62 ± 0.07 Aa | |
| BD (g/cm3) | 0–10 cm | 1.43 ± 0.01 Aa | 1.38 ± 0.01 Ac | 1.30 ± 0.03 Bb |
| 10–20 cm | 1.45 ± 0.06 Aa | 1.48 ± 0.01 Ab | 1.53 ± 0.00 Aa | |
| 20–30 cm | 1.49 ± 0.06 Aa | 1.52 ± 0.01 Aa | 1.55 ± 0.01 Aa | |
| TC/TN | 0–10 cm | 12.55 ± 1.87 Aa | 13.95 ± 0.49 Aa | 12.47 ± 1.19 Aa |
| 10–20 cm | 13.30 ± 1.54 Aa | 11.41 ± 1.17 Ab | 12.39 ± 0.43 Aa | |
| 20–30 cm | 11.39 ± 0.31 Aa | 11.76 ± 0.37 Ab | 11.44 ± 0.24 Aa | |
| The proportion of clay (%) | 0–10 cm | 21.02 ± 0.17 Aab | 17.77 ± 1.79 Ba | 20.49 ± 0.33 Aa |
| 10–20 cm | 20.79 ± 0.66 Ab | 19.07 ± 0.93 Ba | 20.49 ± 0.33 ABa | |
| 20–30 cm | 23.18 ± 1.59 Aa | 20.35 ± 0.19 Aa | 21.40 ± 2.47 Aa | |
| MBC (mg/kg) | 0–10 cm | 188.60 ± 38.37 Ab | 75.26 ± 46.99 Bb | 150.23 ± 36.77 ABb |
| 10–20 cm | 215.14 ± 28.24 Aab | 132.21 ± 17.53 Bb | 194.54 ± 39.67 ABb | |
| 20–30 cm | 298.11 ± 51.94 Aa | 252.40 ± 16.14 Aa | 354.96 ± 82.53 Aa | |
| MBN (mg/kg) | 0–10 cm | 28.75 ± 17.25 Aa | 14.01 ± 10.08 Ab | 19.89 ± 3.84 Ab |
| 10–20 cm | 29.59 ± 5.49 Aa | 26.93 ± 5.72 Aab | 28.09 ± 1.11 Ab | |
| 20–30 cm | 45.87 ± 18.14 Aa | 31.36 ± 5.74 Aa | 68.98 ± 27.63 Aa | |
| MBN/TN | 0–10 cm | 370.41 ± 147.57 Aa | 221.59 ± 101.93 Aa | 238.94 ± 54.74 Ab |
| 10–20 cm | 394.37 ± 34.18 Aa | 296.34 ± 75.60 Aa | 321.58 ± 7.20 Aab | |
| 20–30 cm | 425.11 ± 158.09 Aa | 288.07 ± 70.30 Aa | 545.54 ± 172.97 Aa |
| Group | Shannon Index | Simpson Index | Chao1 Index | Observed Species |
|---|---|---|---|---|
| R20 | 10.05 ± 0.09 b | 0.9976 ± 0.0001 ab | 4128.46 ± 232.23 ab | 3084 ± 134.30 b |
| R40 | 10.13 ± 0.14 ab | 0.9977 ± 0.0006 ab | 4242.28 ± 82.09 ab | 3164 ± 88.86 ab |
| R80 | 10.04 ± 0.04 b | 0.9970 ± 0.0005 ab | 4107.14 ± 65.34 b | 3081 ± 25.98 b |
| CK | 9.98 ± 0.08 b | 0.9969 ± 0.0004 b | 4176.49 ± 108.01 ab | 3122 ± 62.13 ab |
| CS | 10.18 ± 0.05 a | 0.9978 ± 0.0001 a | 4351.69 ± 143.93 a | 3282 ± 121.38 a |
| Group | Actinobacteria (%) | Proteobacteria (%) | Acidobacteria (%) |
|---|---|---|---|
| R20 | 22.30 ± 4.97 b | 26.18 ± 2.47 a | 22.45 ± 2.96 a |
| R40 | 19.65 ± 2.62 b | 28.59 ± 2.76 a | 20.81 ± 5.37 ab |
| R80 | 26.06 ± 2.19 ab | 28.11 ± 3.83 a | 17.19 ± 3.36 b |
| CK | 35.29 ± 4.86 a | 20.90 ± 2.55 b | 17.42 ± 2.85 b |
| CS | 26.10 ± 5.99 ab | 24.03 ± 2.88 ab | 21.99 ± 3.36 ab |
| Group | Gp6 | Gaiella | Gemmatimonas | Gp4 |
|---|---|---|---|---|
| R20 | 10.70 ± 2.17 a | 4.68 ± 1.57 a | 4.31 ± 1.18 a | 3.70 ± 1.15 a |
| R40 | 10.37 ± 4.55 ab | 3.22 ± 0.55 a | 3.37 ± 0.53 a | 2.97 ± 0.78 ab |
| R80 | 7.67 ± 1.43 b | 3.76 ± 0.90 a | 3.56 ± 0.90 a | 2.44 ± 1.36 ab |
| CK | 8.49 ± 0.97 ab | 4.39 ± 1.27 a | 1.75 ± 0.47 b | 1.82 ± 0.92 b |
| CS | 10.86 ± 2.73 a | 4.56 ± 1.17 a | 2.86 ± 0.70 ab | 2.82 ± 0.96 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, X.; Liu, X.; Cui, Y.; Zhang, Y.; Zheng, X.; Zhang, W.; Fan, Y.; Zou, J. Effects of Drying and Rewetting Cycles on Carbon Dioxide Emissions and Soil Microbial Communities. Forests 2022, 13, 1916. https://doi.org/10.3390/f13111916
Zhang Y, Li X, Liu X, Cui Y, Zhang Y, Zheng X, Zhang W, Fan Y, Zou J. Effects of Drying and Rewetting Cycles on Carbon Dioxide Emissions and Soil Microbial Communities. Forests. 2022; 13(11):1916. https://doi.org/10.3390/f13111916
Chicago/Turabian StyleZhang, Yun, Xiaohan Li, Xinmei Liu, Yufei Cui, Ye Zhang, Xiaoying Zheng, Weiwei Zhang, Yue Fan, and Junliang Zou. 2022. "Effects of Drying and Rewetting Cycles on Carbon Dioxide Emissions and Soil Microbial Communities" Forests 13, no. 11: 1916. https://doi.org/10.3390/f13111916






