Response of Soil Respiration to Simulated Acid Rain with Different Ratios of
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experiment Design
2.3. Measurements of Rs, Rh, Soil Temperature and Soil Moisture
2.4. Soil Properties and Root Biomass
2.5. Statistical Analysis
3. Results
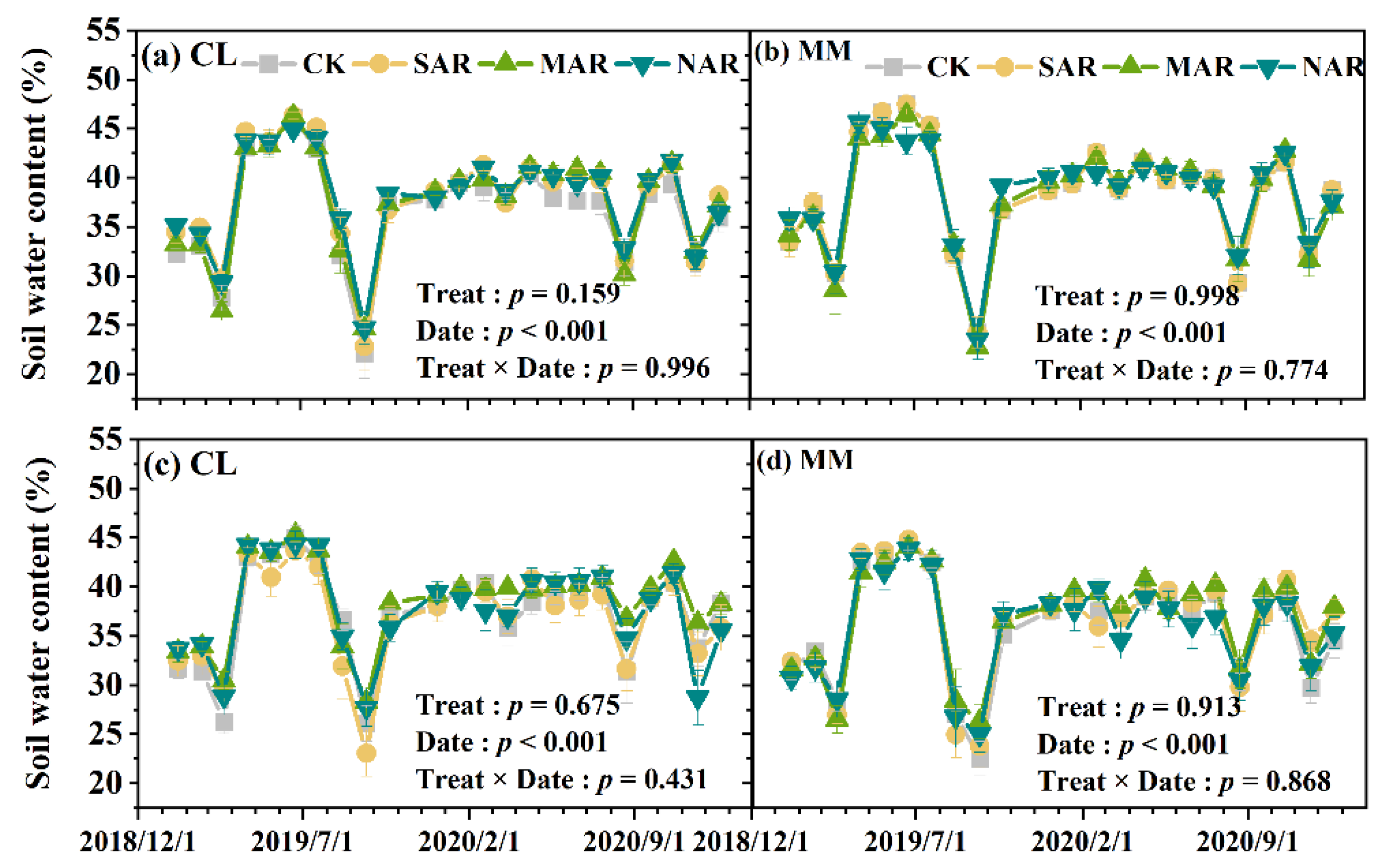
3.1. Soil Temperature and Moisture
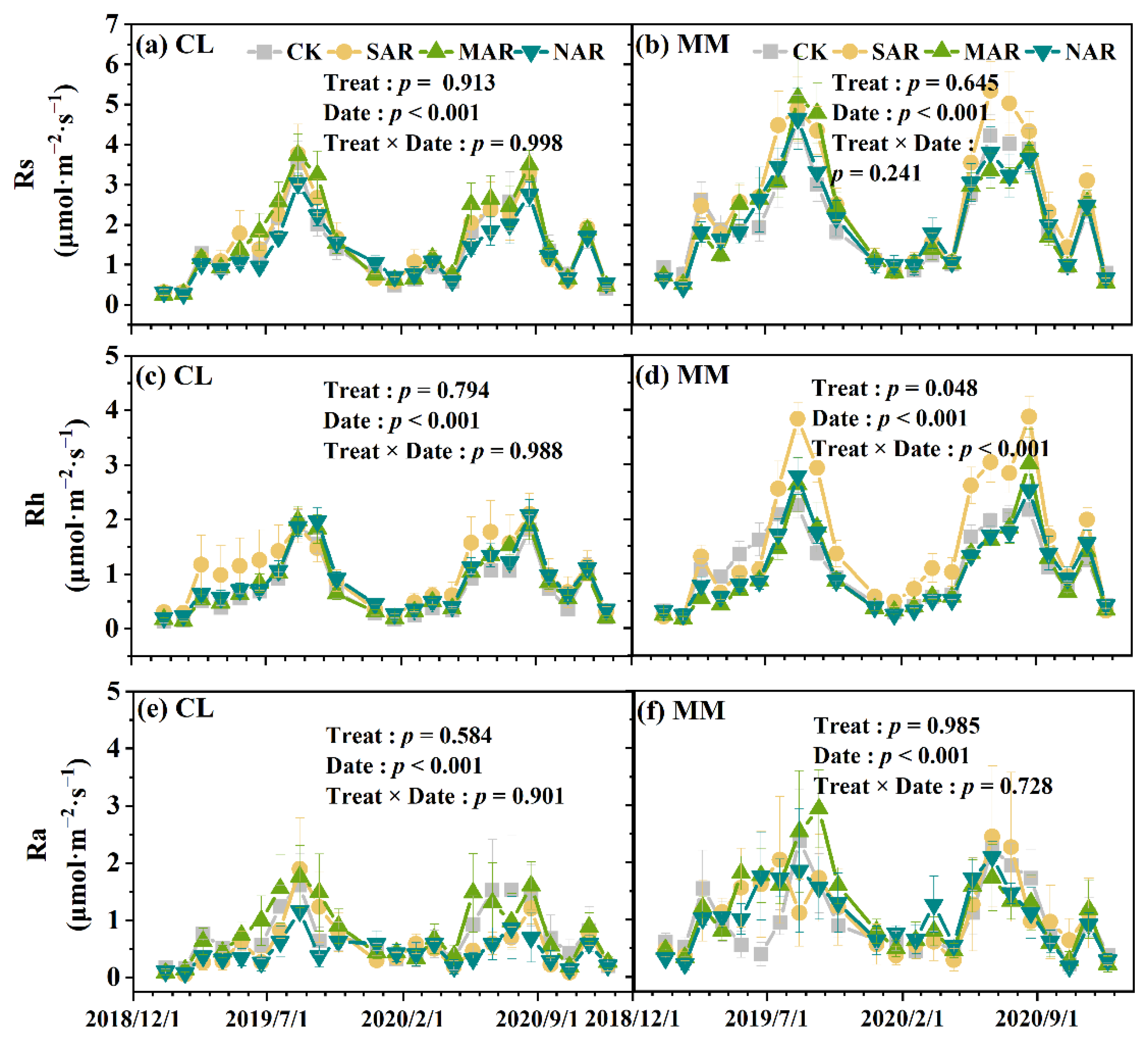
3.2. Rs and Its Components
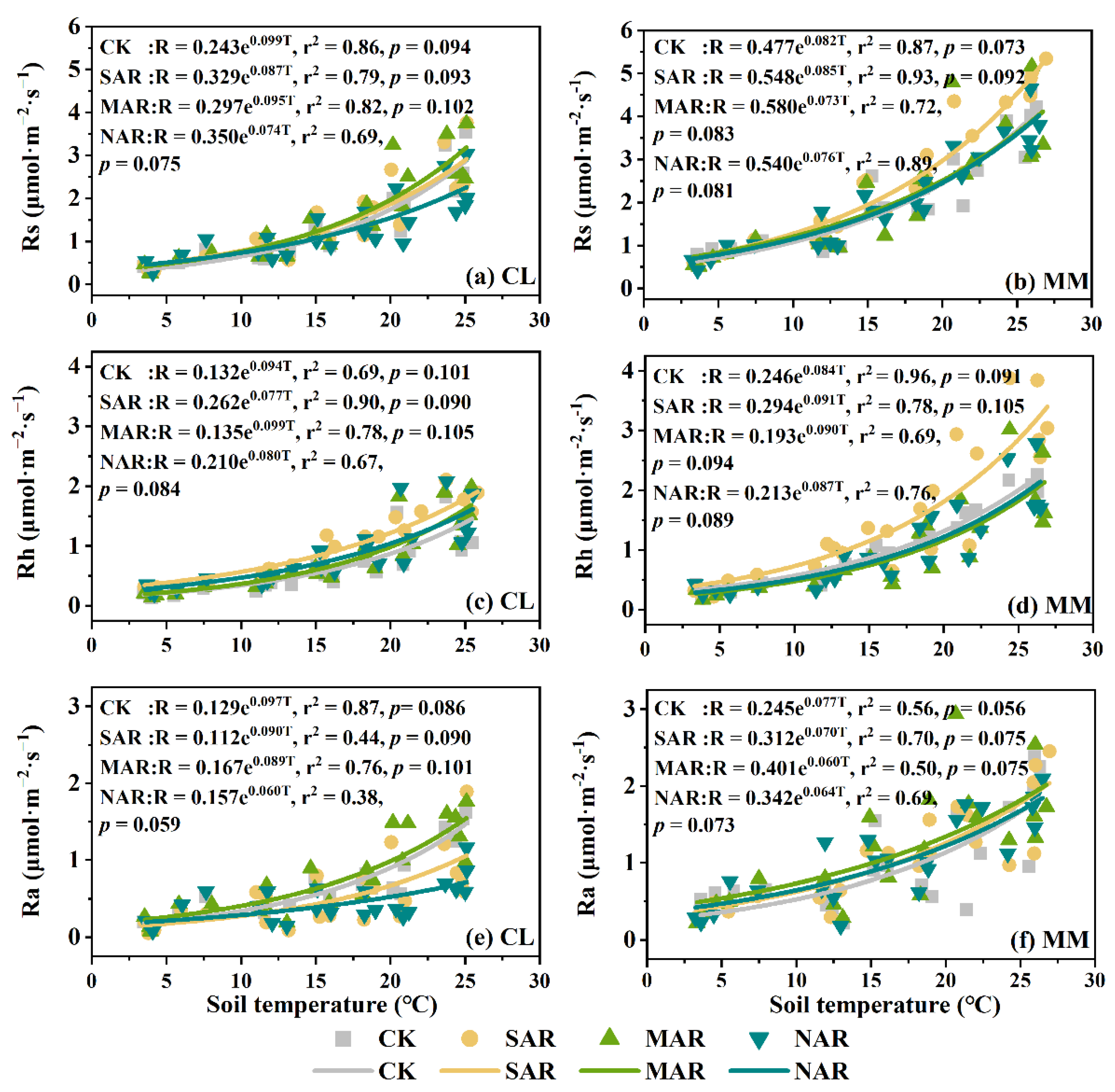
3.3. Temperature Sensitivity of Rs and Its Components
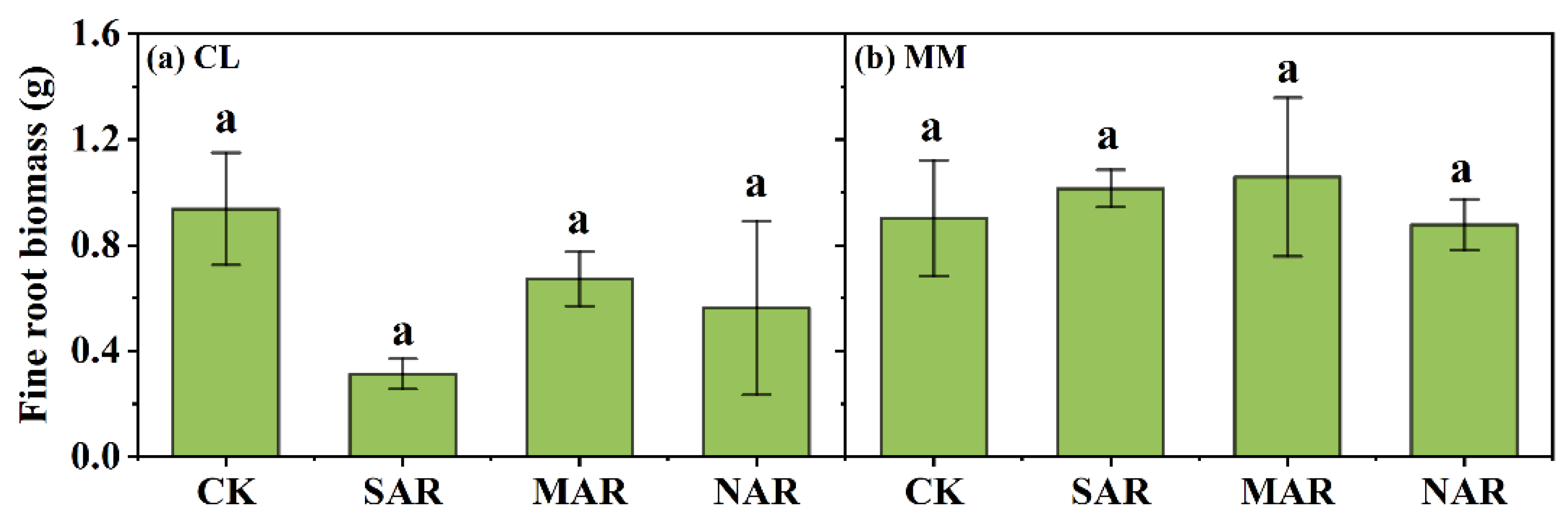
3.4. Soil Properties, Root Biomass, Microbial Community and Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kita, I.; Sato, T.; Kase, Y.; Mitropoulos, P. Neutral rains at Athens, Greece: A natural safeguard against acidification of rains. Sci. Total Environ. 2004, 327, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.E.; Ouyang, Y.; Ling, D.J. Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 2007, 67, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wang, Y.Q.; Zhang, W.Q. Impact of simulated acid rain on the composition of soil microbial communities and soil respiration in typical subtropical forests in Southwest China. Ecotoxicol. Environ. Saf. 2021, 215, 112152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wang, Y.Q.; Wang, Y.J.; Wang, B. Effects of simulated acid rain on soil respiration and its component in a mixed coniferous-broadleaved forest of the three gorges reservoir area in Southwest China. For. Ecosyst. 2019, 6, 32. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.R.; Meng, M.J.; Fu, Z.Y.; Xu, L.H.; Zha, Y.; Yue, J.M.; Zhang, S.F.; Zhang, J.C. Comparative effects of simulated acid rain of different ratios of to on fine root in subtropical plantation of China. Sci. Total Environ. 2018, 618, 336–346. [Google Scholar]
- Carslaw, D.C.; Beevers, S.D.; Tate, J.E.; Westmoreland, E.J.; Williams, M.L. Recent evidence concerning higher NOx emissions from passenger cars and light duty vehicles. Atmos. Environ. 2011, 45, 7053–7063. [Google Scholar] [CrossRef]
- Yu, H.L.; He, N.P.; Wang, Q.F.; Zhu, J.X.; Gao, Y.; Zhang, Y.H.; Jia, Y.L.; Yu, G.R. Development of atmospheric acid deposition in China from the 1990s to the 2010s. Environ. Pollut. 2017, 231, 182–190. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yi, X.Q.; Gao, X.Z.; Wang, M.H.; Shao, C.Y.; Lv, Z.D.; Chen, J.J.; Liu, Z.H.; Shen, C.W. Physiological and biochemical responses of tea seedlings (Camellia sinensis) to simulated acid rain conditions. Ecotoxicol. Environ. Saf. 2020, 192, 110315. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Luo, Q.; Chen, Y.J.; Hu, J.Q.; He, M.; Gao, J.; Zhou, L.Y.; Liu, H.Y.; Zhou, X.H. Interactive effects of grazing and global change factors on soil and ecosystem respiration in grassland ecosystems: A global synthesis. J. Appl. Ecol. 2019, 56, 2007–2019. [Google Scholar] [CrossRef]
- Liang, G.H.; Liu, X.Z.; Chen, X.M.; Qiu, Q.Y.; Zhang, D.Q.; Chu, G.W.; Liu, J.X.; Liu, S.Z.; Zhou, G.Y. Response of soil respiration to acid rain in forests of different maturity in southern China. PLoS ONE 2013, 8, e62207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Shi, Z.J.; Wei, H.; Zhang, J.E. Acid rain reduces soil CO2 emission and promotes soil organic carbon accumulation in association with decreasing the biomass and biological activity of ecosystems: A meta-analysis. Catena 2022, 208, 105714. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Lee, X.Q.; Cao, F. Chemical characteristics and sources of organic acids in precipitation at a semi-urban site in Southwest China. Atmos. Environ. 2011, 45, 413–419. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Chen, S.T.; Sun, L.; Zhang, X.; Shen, X.S.; Liu, Y.F.; Ren, J.Q. Contrasting effects of long-term acid rain simulation on temperature sensitivity of soil respiration and enzymatic activities in a subtropical forest. J. Soils Sediments 2019, 20, 412–424. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, D.F.; Zhang, J.E.; Saleem, M.; Zhang, Y.; Ma, R.; He, Y.N.; Yang, J.Y.; Xiang, H.M.; Wei, H. Effect of simulated acid rain on soil CO2, CH4 and N2O emissions and microbial communities in an agricultural soil. Geoderma 2020, 366, 114222. [Google Scholar] [CrossRef]
- Zheng, S.Z.; Fan, J.; Hu, H.Q. The effect of different rates and forms of sulphur applied on soil microbial biomass and activity. J. Food. Agric. Environ. 2011, 9, 898–906. [Google Scholar]
- Lv, Y.N.; Wang, C.Y.; Jia, Y.Y.; Wang, W.W.; Ma, X.; Du, J.J.; Pu, G.Z.; Tian, X.J. Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl. Soil Ecol. 2014, 79, 1–9. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.S.; Zeng, H.; Li, Z.L.; Yi, C.X.; Niu, S.L. Global soil acidification impacts on belowground processes. Environ. Res. Lett. 2019, 14, 074003. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Jandl, R. Carbon losses due to soil warming: Do autotrophic and heterotrophic soil respiration respond equally? Glob. Chang. Biol. 2009, 15, 901–913. [Google Scholar] [CrossRef]
- Chen, S.T.; Shen, X.S.; Hu, Z.H.; Chen, H.S.; Shi, Y.S.; Liu, Y. Effects of simulated acid rain on soil CO2 emission in a secondary forest in subtropical China. Geoderma 2012, 189–190, 65–71. [Google Scholar] [CrossRef]
- Zeng, W.J.; Zhang, J.Y.; Wang, W. Strong root respiration response to nitrogen and phosphorus addition in nitrogen-limited temperate forests. Sci. Total Environ. 2018, 642, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.B.; Shi, Z.H.; Li, Z.W.; Wang, L.; Chen, J.; Wang, J. Responses of soil respiration and its temperature sensitivity to nitrogen addition: A meta-analysis in China. Appl. Soil Ecol. 2020, 150, 103484. [Google Scholar] [CrossRef]
- Yang, Q.P.; Liu, L.L.; Zhang, W.D.; Xu, M.; Wang, S.L. Different responses of stem and soil CO2 efflux to pruning in a Chinese fir (Cunninghamia lanceolata) plantation. Trees-Struct. Funct. 2015, 29, 1207–1218. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y.Q. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Zheng, W.H.; Li, R.S.; Yang, Q.P.; Zhang, W.D.; Huang, K.; Guan, X.; Wang, S.L. Short-term response of soil respiration to simulated acid rain in Cunninghamia lanceolata and Michelia macclurei plantations. J. Soils Sediments 2018, 19, 1239–1249. [Google Scholar] [CrossRef]
- Chen, S.T.; Zhang, X.; Liu, Y.F.; Hu, Z.H.; Shen, X.S.; Ren, J.Q. Simulated acid rain changed the proportion of heterotrophic respiration in soil respiration in a subtropical secondary forest. Appl. Soil Ecol. 2015, 86, 148–157. [Google Scholar] [CrossRef]
- Janssens, I.A.; Pilegaard, K. Large seasonal changes in Q10 of soil respiration in a beech forest. Glob. Chang. Biol. 2003, 9, 911–918. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.U.; Tigabu, M.; Gilani, M.M.; Zou, X.H.; Wu, P.F. Chinese fir (Cunninghamia lanceolata) a green gold of China with continues decline in its productivity over the successive rotations: A review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Hougton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Tang, L.; Lin, Y.H.; He, X.B.; Han, G.M. Acid rain decelerates the decomposition of Cunninghamia lanceolata needle and Cinnamomum camphora leaf litters in a karst region in China. Ecol. Res. 2019, 34, 193–200. [Google Scholar] [CrossRef]
- Chen, B.H.; Wang, J.; Duan, X.; Zhao, F.X.; Zhang, W.D.; Guan, X.; Chen, L.C.; Wang, Q.K.; Wang, S.L.; Yang, Q.P. Nitrogen Addition Decreases Rhizodeposition by Chinese Fir (Cunninghamia lanceolata (Lamb.) Hook) Seedlings and Its Distribution in Soil Aggregates. Forests 2022, 13, 1166. [Google Scholar] [CrossRef]
- Noh, N.J.; Kuribayashi, M.; Saitoh, T.M.; Nakaji, T.; Nakamura, M.; Hiura, T.; Muraoka, H. Responses of soil, heterotrophic, and autotrophic respiration to experimental open-field soil warming in a cool-temperate deciduous forest. Ecosystems 2015, 19, 504–520. [Google Scholar] [CrossRef]
- De Boer, G.J. Simultaneous determination of ammonium and nitrate in soils. Commun. Soil Sci. Plant Anal. 1996, 27, 803–808. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Lavkulich, L.M. A comparison of methods to determine total, organic, and available phosphorus in forest soils. Commun. Soil Sci. Plant Anal. 1997, 28, 651–663. [Google Scholar] [CrossRef]
- Tan, Z.; Mclaren, R.G.; Cameron, K.C. Forms of sulfur extracted from soils after different methods of sample preparation. Aust. J. Soil Res. 1994, 32, 823–834. [Google Scholar] [CrossRef]
- White, D.C.; Davis, W.M.; Nickels, J.S.; King, J.D.; Bobbie, R.J. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegård, Å. Changes in soil fungal: Bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Ushio, M.; Balser, T.C.; Kitayama, K. Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 2013, 365, 157–170. [Google Scholar] [CrossRef]
- Denef, K.; Roobroeck, D.; Wadu, M.C.M.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects oflong term nitrogen deposition on extracellular enzyme activity in an Acer saccharum, forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Guadalix, M.E.; Pardo, M.T. Sulfate sorption by variable charge soils. J. Soil Sci. 1991, 42, 607–614. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- De Vries, W.; Posch, M.; Kämäri, J. Simulation of the long-term soil response to acid deposition in various buffer ranges. Water Air Soil Pollut. 1989, 48, 349–390. [Google Scholar] [CrossRef]
- Liang, G.H.; Hui, D.F.; Wu, X.Y.; Wu, J.P.; Liu, J.X.; Zhou, G.Y. Effects of simulated acid rain on soil respiration and its components in a subtropical mixed conifer and broadleaf forest in southern China. Environ. Sci. Process. Impacts 2016, 18, 246–255. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, J.; Li, W.J.; Xu, D.H.; Knops, J.M.H.; Du, G.Z. Plant functional groups, grasses versus forbs, differ in their impact on soil carbon dynamics with nitrogen fertilization. Eur. J. Soil Biol. 2016, 75, 79–87. [Google Scholar] [CrossRef]
- Brookshire, E.N.J.; Gerber, S.; Menge, D.N.L.; Hedin, L.O. Large losses of inorganic nitrogen from tropical rainforests suggest a lack of nitrogen limitation. Ecol. Lett. 2012, 15, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Oulehle, F.; Tahovská, K.; Chuman, T.; Evans, C.D.; Hruška, J.; Růžek, M.; Bárta, J. Comparison of the impacts of acid and nitrogen additions on carbon fluxes in European conifer and broadleaf forests. Environ. Pollut. 2018, 238, 884–893. [Google Scholar] [CrossRef]
- Tanikawa, T.; Sobue, A.; Hirano, Y. Acidification processes in soils with different acid buffering capacity in Cryptomeria japonica and Chamaecyparis obtusa forests over two decades. For. Ecol. Manag. 2014, 334, 284–292. [Google Scholar] [CrossRef]
- Tang, X.L.; Du, J.; Shi, Y.H.; Lei, N.F.; Chen, G.; Cao, L.X.; Pei, X.J. Global patterns of soil heterotrophic respiration—A meta-analysis of available dataset. Catena 2020, 191, 104574. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Bradford, M.A.; Keiser, A.D.; Davies, C.A.; Mersmann, C.A.; Strickland, M.S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 2013, 113, 271–281. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef]
- Peng, S.S.; Piao, S.L.; Wang, T.; Sun, J.Y.; Shen, Z.H. Temperature sensitivity of soil respiration in different ecosystems in China. Soil Biol. Biochem. 2009, 41, 1008–1014. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-Bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Chang. Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Craine, J.M.; Gelderman, T.M. Soil moisture controls on temperature sensitivity of soil organic carbon decomposition for a mesic grassland. Soil Biol. Biochem. 2011, 43, 455–457. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Spatial and seasonal variations of Q10 determined by soil respiration measurements at a Sierra Nevadan Forest. Glob. Biogeochem. Cycles 2001, 15, 687–696. [Google Scholar] [CrossRef]
- Larssen, T.; Lydersen, E.; Tang, D.G.; He, Y.; Gao, J.X.; Liu, H.Y.; Duan, L.; Seip, H.M.; Vogt, R.D.; Mulder, J.; et al. Acid rain in China. Environ. Sci. Technol. 2006, 40, 418–425. [Google Scholar] [CrossRef]


| Plantation | Treatment | |||
|---|---|---|---|---|
| CK | SAR | MAR | NAR | |
| CL | 0.57 ± 0.15 a | 0.63 ± 0.10 a | 0.55 ± 0.10 a | 0.67 ± 0.02 a |
| MM | 0.55 ± 0.08 a | 0.72 ± 0.19 a | 0.49 ± 0.08 a | 0.51 ± 0.05 a |
| Plantation | Treatment | pH | Inorganic N (mg·g−1) | Availability P (mg·kg−1) | Availability S (mg·kg−1) | |
|---|---|---|---|---|---|---|
| CL | Untrenched | CK | 4.16 ± 0.04 | 13.68 ± 0.82 | 1.09 ± 0.12 | 25.44 ± 1.21 |
| SAR | 4.10 ± 0.05 | 14.63 ± 0.76 | 1.26 ± 0.08 | 24.80 ± 1.83 | ||
| MAR | 4.03 ± 0.08 | 14.57 ± 0.62 | 1.30 ± 0.14 | 26.76 ± 1.32 | ||
| NAR | 4.14 ± 0.15 | 16.24 ± 0.51 | 1.34 ± 0.11 | 25.32 ± 1.61 | ||
| Trenched | CK | 4.11 ± 0.04 | 11.51 ± 0.90 b | 1.05 ± 0.30 | 25.49 ± 1.49 | |
| SAR | 4.06 ± 0.06 | 14.09 ± 0.64 a | 1.07 ± 0.06 | 24.28 ± 2.72 | ||
| MAR | 4.00 ± 0.05 | 13.36 ± 0.41 ab | 0.93 ± 0.04 | 26.95 ± 0.75 | ||
| NAR | 4.11 ± 0.06 | 12.00 ± 0.30 b | 1.05 ± 0.07 | 26.21 ± 0.55 | ||
| MM | Untrenched | CK | 4.20 ± 0.02 | 10.69 ± 1.06 | 1.30 ± 0.09 | 24.90 ± 2.06 |
| SAR | 4.19 ± 0.03 | 9.50 ± 0.97 | 1.05 ± 0.05 | 26.10 ± 1.12 | ||
| MAR | 4.31 ± 0.10 | 12.56 ± 1.60 | 1.20 ± 0.13 | 23.74 ± 2.36 | ||
| NAR | 4.18 ± 0.07 | 12.58 ± 1.36 | 1.24 ± 0.16 | 27.66 ± 0.27 | ||
| Trenched | CK | 4.28 ± 0.05 | 8.22 ± 0.97 | 0.95 ± 0.09 | 25.35 ± 1.28 | |
| SAR | 4.20 ± 0.02 | 7.55 ± 0.78 | 0.97 ± 0.06 | 27.62 ± 0.76 | ||
| MAR | 4.32 ± 0.08 | 10.15 ± 0.74 | 0.99 ± 0.06 | 27.08 ± 1.17 | ||
| NAR | 4.19 ± 0.04 | 9.47 ± 0.44 | 1.06 ± 0.11 | 28.16 ± 0.58 |
| Plantation | Treatment | Total Biomass (nmol·g−1) | Fungi (nmol·g−1) | G+ (nmol·g−1) | G− (nmol·g−1) | G+/G− | Fungi/Bacterial | |
|---|---|---|---|---|---|---|---|---|
| CL | Untrenched | CK | 30.83 ± 6.01 | 0.50 ± 0.08 | 8.54 ± 1.66 | 8.95 ± 1.70 | 0.98 ± 0.11 | 0.03 ± 0.00 |
| SAR | 21.08 ± 6.61 | 0.39 ± 0.08 | 5.47 ± 1.79 | 6.61 ± 2.32 | 0.87 ± 0.06 | 0.04 ± 0.01 | ||
| MAR | 28.89 ± 0.88 | 0.97 ± 0.39 | 7.20 ± 0.40 | 9.06 ± 0.68 | 0.80 ± 0.05 | 0.06 ± 0.02 | ||
| NAR | 34.15 ± 14.00 | 0.42 ± 0.06 | 5.80 ± 1.55 | 7.61 ± 2.52 | 0.86 ± 0.13 | 0.04 ± 0.01 | ||
| Trenched | CK | 21.86 ± 3.11 | 0.30 ± 0.05 | 6.29 ± 1.16 | 6.24 ± 1.13 | 1.07 ± 0.24 | 0.02 ± 0.01 | |
| SAR | 24.79 ± 7.08 | 0.39 ± 0.11 | 6.87 ± 1.61 | 7.83 ± 2.81 | 1.05 ± 0.18 | 0.03 ± 0.01 | ||
| MAR | 24.70 ± 3.05 | 0.35 ± 0.06 | 7.59 ± 0.51 | 7.43 ± 1.23 | 1.07 ± 0.11 | 0.02 ± 0.00 | ||
| NAR | 52.84 ± 29.63 | 0.31 ± 0.05 | 6.63 ± 0.49 | 6.02 ± 0.37 | 1.10 ± 0.07 | 0.02 ± 0.00 | ||
| MM | Untrenched | CK | 35.16 ± 6.50 | 0.79 ± 0.14 | 9.38 ± 1.37 | 11.87 ± 2.82 | 0.84 ± 0.08 | 0.04 ± 0.00 |
| SAR | 35.06 ± 5.76 | 0.97 ± 0.17 | 9.36 ± 1.29 | 11.15 ± 2.17 | 0.87 ± 0.05 | 0.05 ± 0.00 | ||
| MAR | 47.14 ± 7.64 | 1.01 ± 0.24 | 12.53 ± 2.47 | 16.17 ± 5.59 | 0.78 ± 0.08 | 0.04 ± 0.01 | ||
| NAR | 51.79 ± 5.61 | 1.00 ± 0.14 | 14.56 ± 1.56 | 16.56 ± 1.94 | 0.89 ± 0.05 | 0.03 ± 0.00 | ||
| Trenched | CK | 33.03 + 1.24 | 0.81 ± 0.06 | 8.82 ± 0.88 | 11.02 + 0.56 | 0.81 ± 0.10 | 0.04 ± 0.00 | |
| SAR | 33.66 ± 5.34 | 0.84 ± 0.09 | 8.87 ± 1.05 | 10.80 + 2.01 | 0.86 ± 0.07 | 0.04 ± 0.00 | ||
| MAR | 42.35 ± 8.77 | 1.06 ± 0.21 | 11.23 ± 2.45 | 13.91 + 3.07 | 0.82 ± 0.08 | 0.04 ± 0.01 | ||
| NAR | 38.20 ± 6.17 | 0.90 ± 0.16 | 10.13 ± 1.79 | 11.52 + 2.15 | 0.90 ± 0.07 | 0.04 ± 0.00 |
| Plantation | Treatment | BG (μmol·h−1·g−1) | NAG (μmol·h−1·g−1) | LAP (μmol·h−1·g−1) | AP (μmol·h−1·g−1) | PHO (μmol·h−1·g−1) | URE (μg·g−1) | |
|---|---|---|---|---|---|---|---|---|
| CL | Untrenched | CK | 811.84 ± 166.00 | 369.18 ± 177.75 | 455.36 ± 128.62 | 2313.62 ± 500.23 | 3261.57 ± 549.25 b | 156.70 ± 20.75 |
| SAR | 848.12 ± 59.14 | 353.30 ± 105.68 | 384.58 ± 103.11 | 1686.90 ± 307.12 | 4256.55 ± 567.32 ab | 220.55 ± 54.31 | ||
| MAR | 791.95 ± 93.12 | 333.88 ± 163.71 | 530.20 ± 275.20 | 2651.00 ± 741.47 | 4746.67 ± 760.78 ab | 214.35 ± 58.01 | ||
| NAR | 901.98 ± 114.44 | 412.97 ± 102.22 | 254.00 ± 21.13 | 2121.30 ± 202.19 | 5646.23 ± 779.41 a | 265.37 ± 50.72 | ||
| Trenched | CK | 874.62 ± 96.21 | 354.27 ± 149.39 | 156.26 ± 37.97 b | 1944.38 ± 183.65 b | 2822.85 ± 264.60 b | 136.75 ± 47.89 | |
| SAR | 871.60 ± 42.27 | 357.26 ± 109.92 | 659.87 ± 144.11 a | 3227.34 ± 376.87 a | 4937.89 ± 941.18 a | 261.50 ± 71.84 | ||
| MAR | 820.48 ± 76.42 | 332.52 ± 144.43 | 341.46 ± 93.88 b | 2004.47 ± 216.54 b | 3024.80 ± 124.09 b | 193.71 ± 25.67 | ||
| NAR | 808.04 ± 75.18 | 335.48 ± 150.61 | 412.28 ± 92.50 ab | 2707.51 ± 321.10 ab | 4069.72 ± 643.20 ab | 215.11 ± 45.96 | ||
| MM | Untrenched | CK | 1119.50 ± 85.49 | 859.97 ± 43.32 a | 612.56 ± 290.74 | 3899.86 ± 715.74 | 5708.89 ± 438.11 | 379.14 ± 66.70 |
| SAR | 951.59 ± 37.41 | 758.34 ± 40.40 ab | 387.46 ± 68.06 | 3081.71 ± 418.84 | 4564.59 ± 491.18 | 335.07 ± 76.02 | ||
| MAR | 918.49 ± 61.27 | 753.55 ± 57.73 ab | 425.14 ± 52.94 | 4215.41 ± 825.04 | 6180.58 ± 1246.20 | 349.31 ± 97.69 | ||
| NAR | 947.19 ± 137.39 | 688.74 ± 44.45 b | 234.21 ± 50.91 | 2846.32 ± 470.58 | 4991.83 ± 972.13 | 221.48 ± 72.89 | ||
| Trenched | CK | 1255.61 ± 148.54 | 949.82 ± 71.29 | 1013.56 ± 131.14 a | 3622.70 ± 381.73 | 3482.39 ± 570.14 | 319.77 ± 67.85 | |
| SAR | 1064.37 ± 286.63 | 826.51 ± 141.20 | 708.32 ± 313.91 ab | 3948.73 ± 760.23 | 3697.56 ± 804.21 | 354.35 ± 77.61 | ||
| MAR | 1072.72 ± 121.11 | 781.20 ± 104.45 | 318.44 ± 153.13 b | 3907.05 ± 770.03 | 3020.00 ± 323.02 | 286.89 ± 100.31 | ||
| NAR | 980.64 ± 137.42 | 748.93 ± 130.62 | 403.78 ± 88.67 b | 3612.26 ± 694.84 | 3876.82 ± 623.44 | 255.92 ± 65.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, Q.; Zhang, W.; Chen, L.; Guan, X.; Huang, K.; Li, R.; Zheng, W.; Wang, Q.; Wang, S.
Response of Soil Respiration to Simulated Acid Rain with Different Ratios of
Wang J, Yang Q, Zhang W, Chen L, Guan X, Huang K, Li R, Zheng W, Wang Q, Wang S.
Response of Soil Respiration to Simulated Acid Rain with Different Ratios of
Wang, Jiao, Qingpeng Yang, Weidong Zhang, Longchi Chen, Xin Guan, Ke Huang, Renshan Li, Wenhui Zheng, Qingkui Wang, and Silong Wang.
2022. "Response of Soil Respiration to Simulated Acid Rain with Different Ratios of
Wang, J., Yang, Q., Zhang, W., Chen, L., Guan, X., Huang, K., Li, R., Zheng, W., Wang, Q., & Wang, S.
(2022). Response of Soil Respiration to Simulated Acid Rain with Different Ratios of






