Mechanical Branch Wounding Alters the BVOC Emission Patterns of Ficus Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
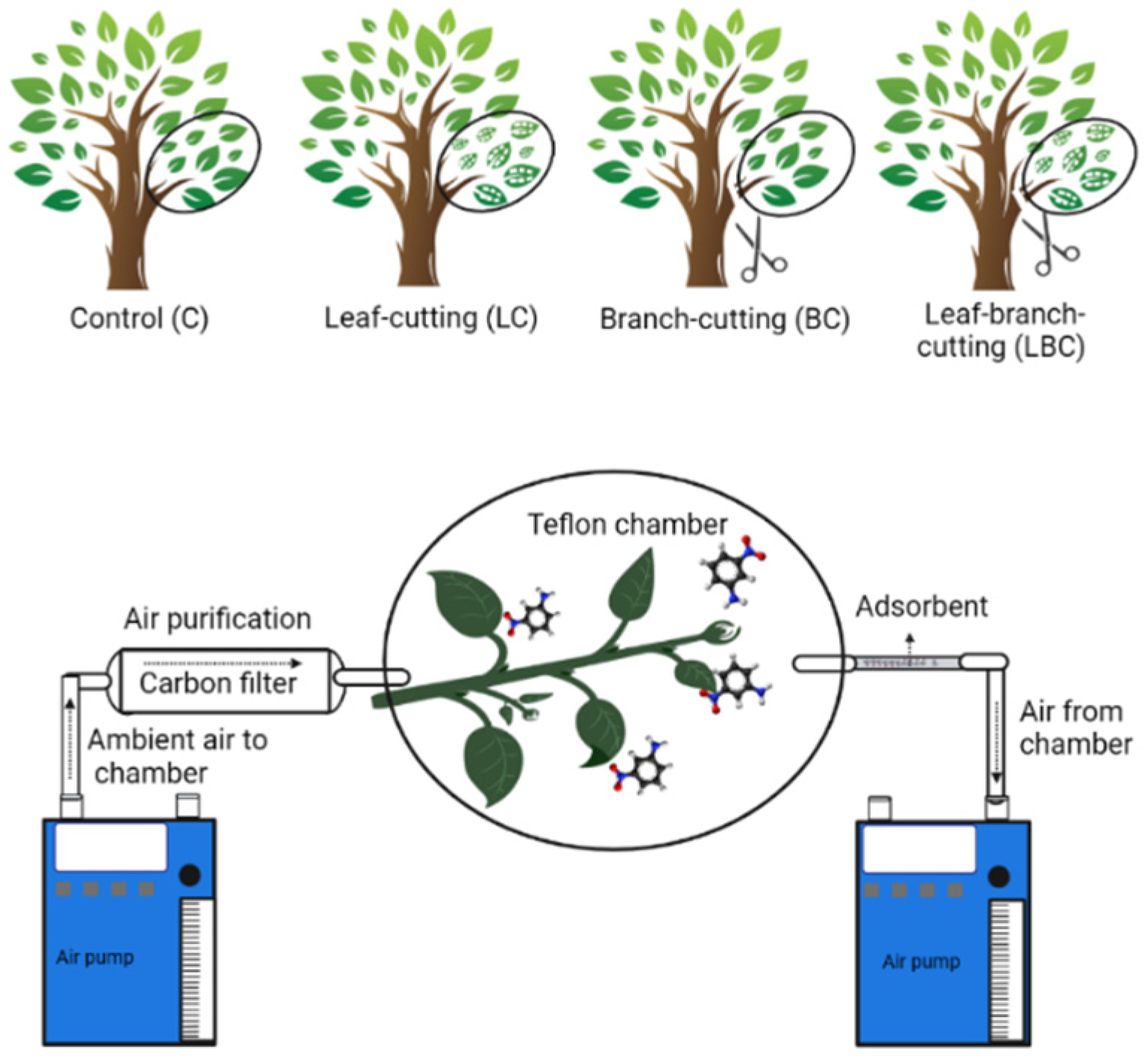
2.2. Experimental Manipulations
2.3. BVOC Sampling and Identification
2.4. Statistical Analysis
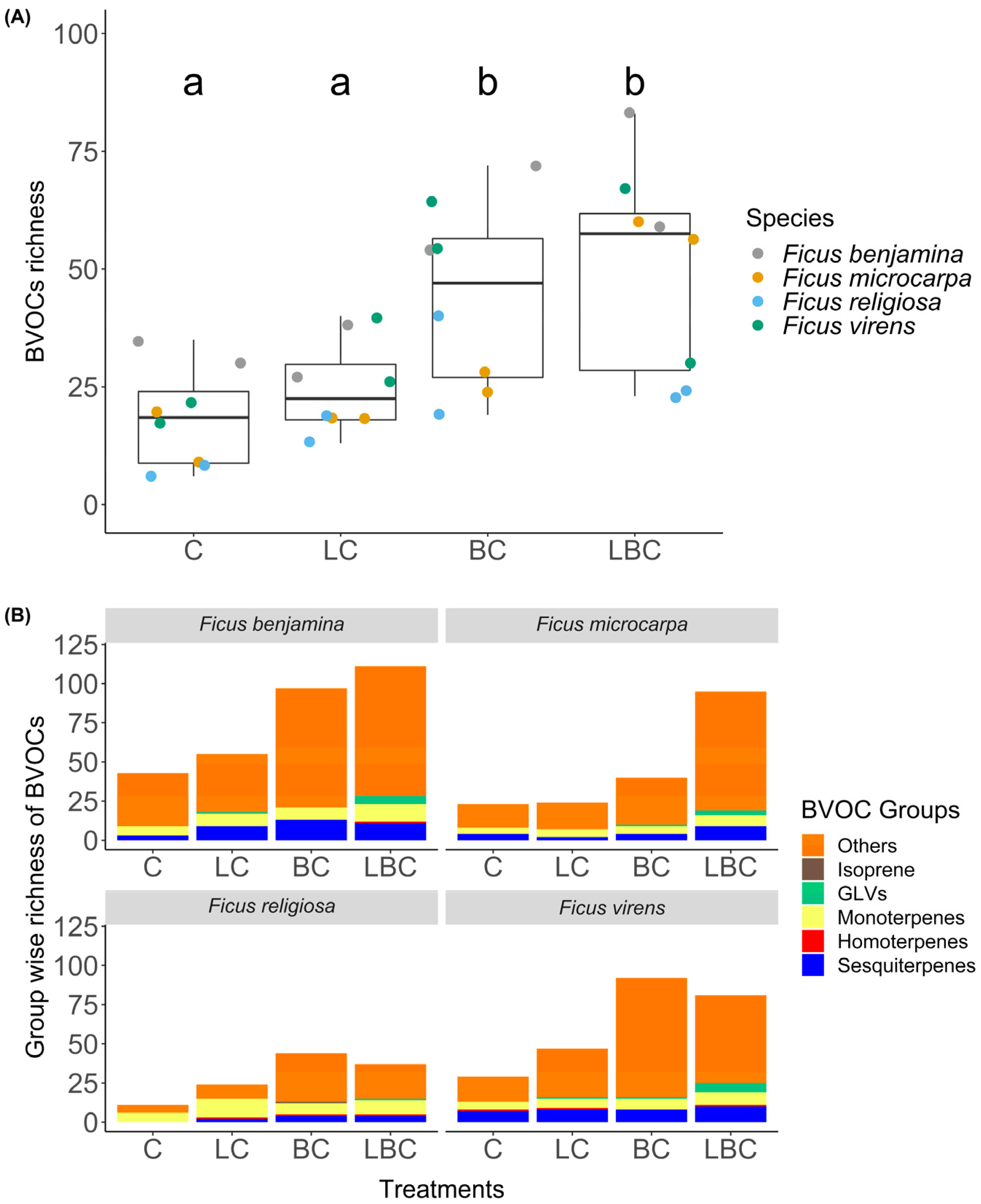
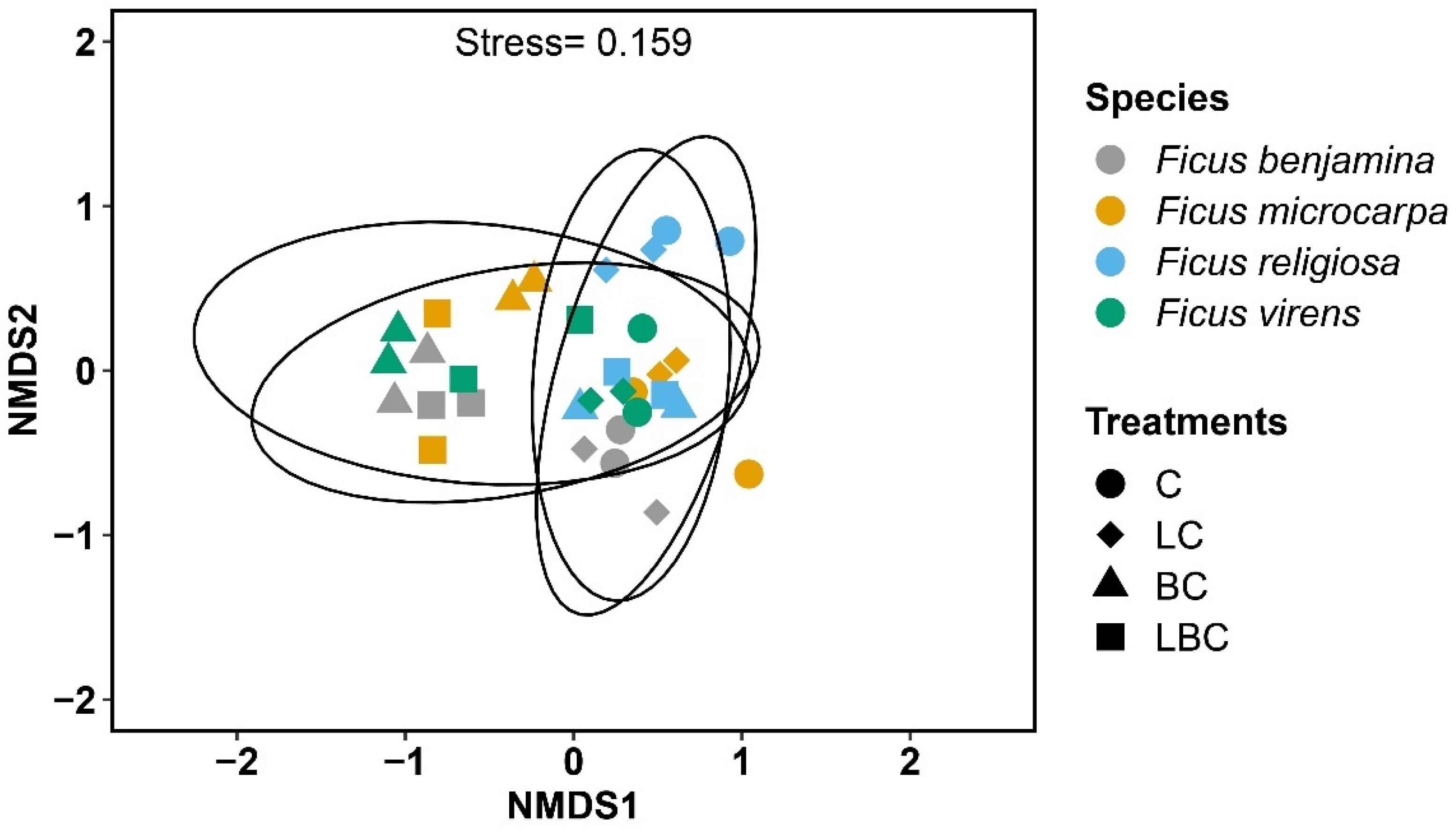
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, C.D.; Gadhavi, H.; Kumar Sahu, L.; Achuthan, J. Volatile Organic Compounds (VOCs) in the Air, their Importance and Measurements. Earth Sci. India 2017, 10, 1–15. [Google Scholar]
- Lin, Y.; Lun, X.; Tang, W.; Zhang, Z.; Jing, X.; Fan, C.; Wang, Q. Characteristics and Chemical Reactivity of Biogenic Volatile Organic Compounds from Dominant Forest Species in the Jing-Jin-Ji Area, China. For. Ecosyst. 2021, 8, 52. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Johnson, S.D. Pollinator-Mediated Evolution of Floral Signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Godfrey, A.J.R.; Potter, M.A.; Holopainen, J.K.; McCormick, A.C. Natural Variation in Volatile Emissions of the Invasive Weed Calluna vulgaris in New Zealand. Plants 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douma, J.C.; Ganzeveld, L.N.; Unsicker, S.B.; Boeckler, G.A.; Dicke, M. What Makes a Volatile Organic Compound a Reliable Indicator of Insect Herbivory? Plant Cell Environ. 2019, 42, 3308–3325. [Google Scholar] [CrossRef] [Green Version]
- Grote, R.; Sharma, M.; Ghirardo, A.; Schnitzler, J.P. A New Modeling Approach for Estimating Abiotic and Biotic Stress-Induced de Novo Emissions of Biogenic Volatile Organic Compounds from Plants. Front. For. Glob. Chang. 2019, 2, 26. [Google Scholar] [CrossRef]
- Schade, G.W.; Goldstein, A.H. Increase of Monoterpene Emissions from a Pine Plantation as a Result of Mechanical Dis-turbances. Geophys. Res. Lett. 2003, 30, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Räisänen, T.; Ryyppö, A.; Kellomäki, S. Impact of Timber Felling on the Ambient Monoterpene Concentration of a Scots Pine (Pinus sylvestris L.) Forest. Atmos. Environ. 2008, 42, 6759–6766. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Haase, K.B.; Jordan, C.; Mentis, E.; Cottrell, L.; Mayne, H.R.; Talbot, R.; Sive, B.C. Atmospheric Chemistry and Physics Changes in Monoterpene Mixing Ratios during Summer Storms in Rural New Hampshire (USA). Atmos. Chem. Phys. 2011, 11, 11465–11476. [Google Scholar] [CrossRef] [Green Version]
- Juráň, S.; Grace, J.; Urban, O. Temporal Changes in Ozone Concentrations and their Impact on Vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M. Indirect Plant Defense against Insect Herbivores: A Review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Heidy, R.; Bezerra, S.; Sousa-souto, L.; Euzébio, A.; Santana, G.; Ambrogi, B.G. Indirect Plant Defenses: Volatile Organic Compounds and Extrafloral Nectar. Arthropod-Plant Interact. 2021, 15, 467–489. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of Synthesis, Biochemistry and Functional Role of Plant Volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Nascetti, P.; Graverini, A.; Mannozzi, M. Emission and Content of Monoterpenes in Intact and Wounded Needles of the Mediterranean Pine, Pinus pinea. Funct. Ecol. 2000, 14, 589–595. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H. Systemic Release of Chemical Signals by Herbivore-Injured Corn. Proc. Natl. Acad. Sci. USA 1992, 89, 8399–8402. [Google Scholar] [CrossRef] [Green Version]
- Paré, P.W.; Tumlinson, J.H. De Novo Biosynthesis of Volatiles Induced by Insect Herbivory in Cotton Plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, J.K.; Gershenzon, J. Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Fontana, A.; Reichelt, M.; Hempel, S.; Gershenzon, J.; Unsicker, S.B. The Effects of Arbuscular Mycorrhizal Fungi on Direct and Indirect Defense Metabolites of Plantago lanceolata L. J. Chem. Ecol. 2009, 35, 833–843. [Google Scholar] [CrossRef] [Green Version]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The Specificity of Herbivore-Induced Plant Volatiles in Attracting Herbivore Enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, L.; Han, R. Analysis of Volatile Components in Different Ophiocordyceps sinensis and Insect Host Products. Molecules 2020, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-Induced Nocturnal Plant Volatiles Repel Conspecific Females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Vancanneyt, G.; Sanz, C.; Farmaki, T.; Paneque, M.; Ortego, F.; Castañera, P.; Sánchez-Serrano, J.J. Hydroperoxide Lyase Depletion in Transgenic Potato Plants Leads to an Increase in Aphid Performance. Proc. Natl. Acad. Sci. USA 2001, 98, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangione, A.M.; Dearing, M.D.; Karasov, W.H. Creosote Bush (Larrea tridentata) Resin Increases Water Demands and Reduces Energy Availability in Desert Woodrats (Neotoma lepida). J. Chem. Ecol. 2004, 30, 1409–1429. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Köllner, T.G.; Held, M.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. The Products of a Single Maize Sesquit-erpene Synthase Form a Volatile Defense Signal that Attracts Natural Enemies of Maize Herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Arimura, G.; Matsui, K.; Takabayashi, J. Chemical and Molecular Ecology of Herbivore-Induced Plant Volatiles: Proximate Factors and Their Ultimate Functions. Plant Cell Physiol. 2009, 50, 911–923. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; Von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [Green Version]
- Winer, A.M.; Arey, J.; Atkinson, R.; Aschmann, S.M. Emission Rates of Organics from Vegetation in California’s Central Valley. Atmos. Environ. Part A. Gen. Top. 1992, 26, 2647–2659. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative Patterns between Plant Volatile Emissions Induced by Biotic Stresses and the Degree of Damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Tunings, T.C.J.; Wäckers, F.L. Recruitment of Predators and Parasitoids by Herbivore-Injured Plants. Adv. Insect Chem. Ecol. 2004, 21–75. [Google Scholar] [CrossRef]
- Erb, M.; Foresti, N.; Turlings, T.C.J. A Tritrophic Signal That Attracts Parasitoids to Host-Damaged Plants Withstands Disruption by Non-Host Herbivores. BMC Plant Biol. 2010, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Ann. Rev. Èntomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, M.; So, S.; Compton, S.G.; Corlett, R. Fig-Eating by Vertebrate Frugivores: A Global Review. Biol. Rev. 2001, 76, 529–572. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.D. Figs and the Diversity of Tropical Rainforests. Bioscience 2005, 55, 1053–1064. [Google Scholar] [CrossRef]
- Diaz-Martin, Z.; Swamy, V.; Terborgh, J.; Alvarez-Loayza, P.; Cornejo, F. Identifying Keystone Plant Resources in an Ama-zonian Forest Using a Long-Term Fruit-Fall Record. J. Trop. Ecol. 2014, 30, 291–301. [Google Scholar] [CrossRef]
- Villard, C.; Larbat, R.; Munakata, R.; Hehn, A. Defense Mechanisms of Ficus: Pyramiding Strategies to Cope with Pests and Pathogens. Planta 2019, 249, 617–633. [Google Scholar] [CrossRef]
- Zhao, J.; Segar, S.T.; McKey, D.; Chen, J. Macroevolution of Defense Syndromes in Ficus (Moraceae). Ecol. Monogr. 2021, 91, e01428. [Google Scholar] [CrossRef]
- Basset, Y.; Novotny, V. Species Richness of Insect Herbivore Communities on Ficus in Papua New Guinea. Biol. J. Linn. Soc. 1999, 67, 477–499. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Kazantsev, T.; Talts, E.; Tosens, T.; Niinemets, Ü. Emission Timetable and Quantitative Patterns of Wound-Induced Volatiles Across Different Leaf Damage Treatments in Aspen (Populus tremula). J. Chem. Ecol. 2015, 41, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Portillo-Estrada, M.; Niinemets, Ü. Massive Release of Volatile Organic Compounds Due to Leaf Midrib Wounding in Populus tremula. Plant Ecol. 2018, 219, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Xiaoming, Z.; Matthew, W.; Hua, Z. Tropical Forests of Xishuangbanna, China. Biotropica 2006, 38, 306–309. [Google Scholar] [CrossRef]
- Hagiwara, T.; Shiojiri, K. Within-Plant Signaling via Volatiles in Beech (Fagus crenata Blume). J. Plant Interact. 2020, 15, 50–53. [Google Scholar] [CrossRef]
- Kim, L.; Galbally, I.E.; Porter, N.; Weeks, I.A.; Lawson, S.J. BVOC Emissions from Mechanical Wounding of Leaves and Branches of Eucalyptus sideroxylon (Red Ironbark). J. Atmos. Chem. 2011, 68, 265–279. [Google Scholar] [CrossRef]
- Brilli, F.; Hortnagl, L.; Bamberger, I.; Schnitzhofer, R.; Ruuskanen, T.M.; Hansel, A.; Loreto, F.; Wohlfahrt, G. Qualitative and Quantitative Characterization of Volatile Organic Compound Emissions from Cut Grass. Environ. Sci. Technol. 2012, 46, 3859–3865. [Google Scholar] [CrossRef]
- Geron, C.; Owen, S.; Guenther, A.; Greenberg, J.; Rasmussen, R.; Hui Bai, J.; Li, Q.J.; Baker, B. Volatile Organic Compounds from Vegetation in Southern Yunnan Province, China: Emission Rates and Some Potential Regional Implications. Atmos. Environ. 2006, 40, 1759–1773. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Guenther, A.; Turnipseed, A.; Duhl, T.; Greenberg, J. Seasonal and Interannual Variations in Whole-Ecosystem BVOC Emissions from a Subtropical Plantation in China. Atmos. Environ. 2017, 161, 176–190. [Google Scholar] [CrossRef]
- Kalske, A.; Shiojiri, K.; Uesugi, A.; Sakata, Y.; Morrell, K.; Kessler, A. Insect Herbivory Selects for Volatile-Mediated Plant-Plant Communication. Curr. Biol. 2019, 29, 3128–3133.e3. [Google Scholar] [CrossRef]
- Faiola, C.; Taipale, D. Impact of Insect Herbivory on Plant Stress Volatile Emissions from Trees: A Synthesis of Quantitative Measurements and Recommendations for Future Research. Atmos. Environ. X 2020, 5, 100060. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Pirożnikow, E.; Spirina, V.L.; Vasyanin, A.N. Emission of Volatile Organic Compounds by Plants on the Floor of Boreal and Mid-Latitude Forests. J. Atmos. Chem. 2022, 79, 153–166. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 7 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Staudt, M.; Lhoutellier, L. Monoterpene and Sesquiterpene Emissions from Quercus coccifera Exihibit Interacting Responses to Light and Temperature. Biogeosciences 2011, 8, 2757–2771. [Google Scholar] [CrossRef] [Green Version]
- Saunier, A.; Ormeño, E.; Boissard, C.; Wortham, H.; Temime-Roussel, B.; Lecareux, C.; Armengaud, A.; Fernandez, C. Effect of Mid-term Drought on Quercus pubescens BVOCs’ Emission Seasonality and their Dependency on Light and/or Temperature. Atmos. Chem. Phys. 2017, 17, 7555–7566. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Hartmann, H.; Hellén, H.; Wisthaler, A.; Perreca, E.; Weinhold, A.; Rücker, A.; van Dam, N.M.; Gershenzon, J.; Trumbore, S.E.; et al. New Perspectives on CO2, Temperature and Light Effects on BVOC Emissions Using Online Measurements by PTR-MS and Cavity Ring-down Spectroscopy. Environ. Sci. Technol. 2018, 52, 13811–13823. [Google Scholar] [CrossRef]
- Dicke, M.; Van Loon, J.J.A.; Soler, R. Chemical Complexity of Volatiles from Plants Induced by Multiple Attack. Nat. Chem. Biol 2009, 5, 317–324. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Unsicker, S.B.; Reichelt, M.; Kesselmeier, J.; Gershenzon, J.; Weisser, W.W. Emission of Volatile Organic Compounds after Herbivory from Trifolium pratense (L.) under Laboratory and Field Conditions. J. Chem. Ecol. 2009, 35, 1335–1348. [Google Scholar] [CrossRef] [Green Version]
- McCormick, A.C.; Boeckler, G.A.; Köllner, T.G.; Gershenzon, J.; Unsicker, S.B. The Timing of Herbivore-Induced Volatile Emission in Black Poplar (Populus nigra) and the Influence of Herbivore Age and Identity Affect the Value of Individual Volatiles as Cues for Herbivore Enemies. BMC Plant Biol. 2014, 14, 304. [Google Scholar] [CrossRef] [Green Version]
- Aboshi, T.; Toda, A.; Ashitani, T.; Murayama, T. A Herbivore-Induced Homoterpene Volatile is Emitted from Basella alba Leaves. Biosci. Biotechnol. Biochem. 2019, 83, 1989–1991. [Google Scholar] [CrossRef]
- Mithöfer, A.; Wanner, G.; Boland, W. Effects of Feeding Spodoptera littoralis on Lima Bean Leaves. II. Continuous Mechanical Wounding Resembling Insect Feeding is Sufficient to Elicit Herbivory-Related Volatile Emission. Plant Physiol. 2005, 137, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Sabina, S.; Jithesh, M.N. Mechanical Wounding of Leaf Midrib and Lamina Elicits Differential Biochemical Response and Mitigates Salinity Induced Damage in Tomato. J. Appl. Hortic. 2021, 23, 3–10. [Google Scholar] [CrossRef]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic Mechanical Wounding (MecWorm) versus Herbivore-Induced Responses: Early Signaling and Volatile Emission in Lima Bean (Phaseolus Lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Klinger, L.F.; Li, Q.J.; Guenther, A.B.; Greenberg, J.P.; Baker, B.; Bai, J.H. Assessment of Volatile Organic Compound Emissions from Ecosystems of China. J. Geophys. Res. Atmos. 2002, 107, ACH-16. [Google Scholar] [CrossRef]
- Zhi-Hui, W.; Yu-hua, B.; Zhao-rong, L.; Xue-song, W.; Qing-jun, L.; Klinger, L.F. Investigation of Natural VOC Emitted from Tropical Vegetations in China. J. Environ. 2005, 17, 8–13. [Google Scholar]
- Burkle, L.A.; Runyon, J.B. Drought and Leaf Herbivory Influence Floral Volatiles and Pollinator Attraction. Glob. Change Biol. 2016, 22, 1644–1654. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Matsui, K.; Kishimoto, K.; Kugimiya, S.; Takabayashi, J. Role of the Lipoxygenase/Lyase Pathway of Host-Food Plants in the Host Searching Behavior of Two Parasitoid Species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol. 2006, 32, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, R.; Shiojiri, K.; Matsui, K.; Takabayashi, J. Intermittent Exposure to Traces of Green Leaf Volatiles Triggers the Production of (Z)-3-Hexen-1-yl Acetate and (Z)-3-Hexen-1-ol in Exposed Plants. Plant Signal. Behav. 2013, 8, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Clavijo Mccormick, A.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-Induced Volatile Emission in Black Poplar: Regulation and Role in Attracting Herbivore Enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Köllner, T.G.; Zhang, W.; Wu, J.; Wu, J.; Guo, Y.; Zhang, Y. Identification and Characterization of (E)-β-Caryophyllene Synthase and α/β-Pinene Synthase Potentially Involved in Constitutive and Herbivore-Induced Terpene Formation in Cotton. Plant Physiol. Biochem. 2013, 73, 302–308. [Google Scholar] [CrossRef]
- Pinto-Zevallos, D.M.; Hellén, H.; Hakola, H.; Van Nouhuys, S.; Holopainen, J.K. Induced Defenses of Veronica spicata: Variability in Herbivore-Induced Volatile Organic Compounds. Phytochem. Lett. 2013, 6, 653–656. [Google Scholar] [CrossRef]
- Estell, R.E.; Fredrickson, E.L.; Anderson, D.M.; Havstad, K.M.; Remmenga, M.D. Effects of Four Mono- and Sesquiterpenes on the Consumption of Alfalfa Pellets by Sheep. J. Anim. Sci. 2002, 80, 3301–3306. [Google Scholar] [CrossRef]
- Rice, R.L.; Lincoln, D.E.; Langenheim, J.H. Palatability of Monoterpenoid Compositional Types of Satureja douglasii to a Generalist Molluscan Herbivore, Ariolimax dolichophallus. Biochem. Syst. Ecol. 1978, 6, 45–53. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Hosseini, B.; Estaji, A. Chemical Composition and Insecticidal Properties of the Essential Oil of Salvia leriifolia Benth (Lamiaceae) at Two Developmental Stages. J. Essent. Oil-Bear. Plants 2013, 16, 806–816. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Weidinmyer, C.; Palmer, P.I.; Geron, C. Estimates of Global Terrestrial Isoprene Emissions Using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.C.; McMahon, S.M.; Smith, M.N.; Boyle, B.; Violle, C.; van Haren, J.; Simova, I.; Meir, P.; Ferreira, L.V.; de Camargo, P.B.; et al. Isoprene Emission Structures Tropical Tree Biogeography and Community Assembly Responses to Climate. New Phytol. 2018, 220, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A.; et al. Estimations of Isoprenoid Emission Capacity from Enclosure Studies: Measurements, Data Processing, Quality and Standardized Measurement Protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Miyake, A.; Shinya, T.; Galis, I. Oral Secretions Affect HIPVs Induced by Generalist (Mythimna loreyi) and Specialist (Parnara guttata) Herbivores in Rice. J. Chem. Ecol. 2017, 43, 929–943. [Google Scholar] [CrossRef]
- Kollasch, A.M.; Abdul-Kafi, A.R.; Body, M.J.A.; Pinto, C.F.; Appel, H.M.; Cocroft, R.B. Leaf Vibrations Produced by Chewing Provide a Consistent Acoustic Target for Plant Recognition of Herbivores. Oecologia 2020, 194, 1–13. [Google Scholar] [CrossRef]
- Arimura, G. Making Sense of the Way Plants Sense Herbivores. Trends Plant Sci. 2021, 26, 288–298. [Google Scholar] [CrossRef]
- Uemura, T.; Arimura, G.I. Current Opinions about Herbivore-Associated Molecular Patterns and Plant Intracellular Signaling. Plant Signal. Behav. 2019, 14, 8–11. [Google Scholar] [CrossRef]
- Steinbrenner, A.D.; Munoz-Amatriain, M.; Chaparro, A.F.; Aguilar-Venegas, J.M.; Lo, S.; Okuda, S.; Glauser, G.; Dongiovanni, J.; Shi, D.; Hall, M.; et al. A Receptor-like Protein Mediates Plant Immune Responses to Herbivore-Associated Molecular Patterns. Proc. Natl. Acad. Sci. USA 2020, 117, 31510–31518. [Google Scholar] [CrossRef]
- Li, G.; Bartram, S.; Guo, H.; Mithöfer, A.; Kunert, M.; Boland, W. SpitWorm, a Herbivorous Robot: Mechanical Leaf Wounding with Simultaneous Application of Salivary Components. Plants 2019, 8, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, H.; Vecchi, G.A.; Underwood, S. Increasing Frequency of Extremely Severe Cyclonic Storms Over the Arabian Sea. Nat. Clim. Chang. 2017, 7, 885–889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panthee, S.; Ashton, L.A.; Tani, A.; Sharma, B.; Nakamura, A. Mechanical Branch Wounding Alters the BVOC Emission Patterns of Ficus Plants. Forests 2022, 13, 1931. https://doi.org/10.3390/f13111931
Panthee S, Ashton LA, Tani A, Sharma B, Nakamura A. Mechanical Branch Wounding Alters the BVOC Emission Patterns of Ficus Plants. Forests. 2022; 13(11):1931. https://doi.org/10.3390/f13111931
Chicago/Turabian StylePanthee, Shristee, Louise A. Ashton, Akira Tani, Bimal Sharma, and Akihiro Nakamura. 2022. "Mechanical Branch Wounding Alters the BVOC Emission Patterns of Ficus Plants" Forests 13, no. 11: 1931. https://doi.org/10.3390/f13111931
APA StylePanthee, S., Ashton, L. A., Tani, A., Sharma, B., & Nakamura, A. (2022). Mechanical Branch Wounding Alters the BVOC Emission Patterns of Ficus Plants. Forests, 13(11), 1931. https://doi.org/10.3390/f13111931





