Can We Predict Male Strobili Production in Araucaria angustifolia Trees with Dendrometric and Morphometric Attributes?
Abstract
:1. Introduction
2. Material and Methods
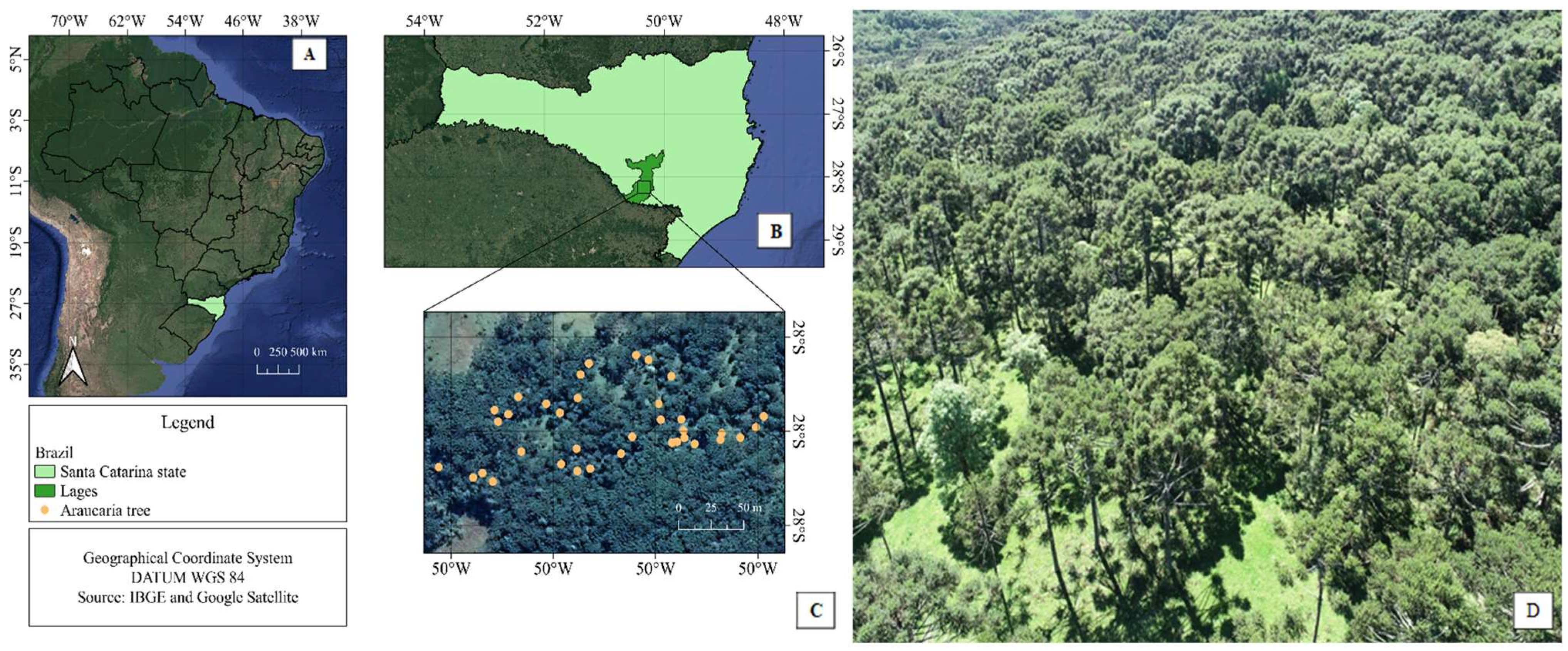
2.1. Study Site
2.2. Data Measurement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reitz, R.; Klein, R.M. Flora Ilustrada Catarinense: Araucariaceas; Herbário Barbosa Rodrigues: Itajaí, Brazil, 1966. [Google Scholar]
- Hess, A.F.; Atanazio, K.A.; Borsoi, G.A.; Schorr, L.P.B.; Souza, I.A.; Costa, E.A.; Klein, D.R.; Krefta, S.M.; Stepka, T.F.; Abatti, R.; et al. Crown efficiency and pine cones production for Brazilian pine (Araucaria angustifolia (Bertol.) Kuntze) in south Brazil. J. Agric. Sci. 2019, 11, 247–259. [Google Scholar] [CrossRef]
- Atanazio, K.A.; Hess, A.F.; Krefta, S.M.; Schorr, L.P.B.; Sousa, I.A.; Domiciano, C.A.R.; Cuchi, T.; Moraes, G.C. Modelagem das relações morfométricas com a produção de pinhas de Arraucaria angustifolia (Bertol.) Kuntze no sul do Brasil. Ciência Florest. 2022, 32, 1247–1267. [Google Scholar] [CrossRef]
- Wrege, M.S.; Fritzsons, E.; Soares, M.T.S.; Bognola, I.A.; Sousa, V.A.; Sousa, L.P.; Gomes, J.B.V.; Aguiar, A.V.; Gomes, G.C.; Matos, M.F.S.; et al. Distribuição natural e habitat da araucária frente às mudanças climáticas globais. Pesqui. Florest. Bras. 2017, 37, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Wrege, M.S.; Fritzsons, E.; Soares, M.T.S.; Souza, V.A. Variáveis climáticas relacionadas aos serviços ambientais: Estudo de caso da araucária. In Serviços Ambientais em Sistemas Agrícolas e Florestais do Bioma Atlântica; Parron, L.M., Garcia, J.G., Oliveira, E.B., Brown, G.G., Prado, R.B., Eds.; Embrapa: Brasília, Brazil, 2015; pp. 243–247. [Google Scholar]
- Wrege, M.S.; Steinmetz, S.; Reisser Júnior, C.; Almeida, I.R. Atlas Climático da Região Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul, 2nd ed.; Embrapa: Brasília, Brazil, 2012; 333p. [Google Scholar]
- Puchalski, A.; Mantovani, M.; Reis, M.S. Variações em populações naturais de Araucaria angustifolia (Bert.) Kuntze associada a condições edafo-climáticas. Sci. Florest. 2006, 70, 137–148. [Google Scholar]
- Schliemann, S.A.; Bockheim, J.G. Methods for studying treefall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Stan, A.; Daniel, L.D. Growth releases across a natural canopy gap-forest gradient in old-growth forests. For. Ecol. Manag. 2014, 313, 98–103. [Google Scholar] [CrossRef]
- Whitmore, T.C. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Zanon, M.L.B.; Finger, C.A.G.; Schneider, P.R. Proporção da dióicia e distribuição diamétrica de árvores masculinas e femininas de Araucaria angustifolia (Bertol.) Kuntze, em povoamentos implantandos. Ciência Florest. 2009, 19, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Mattos, J.R. O Pinheiro Brasileiro; Grêmio Politécnico: São Paulo, Brazil, 1972; 620p. [Google Scholar]
- Ferri, M.G. Botânica: Morfologia Externa das Plantas, 15th ed.; Nobel: São Paulo, Brazil, 1983; 148p. [Google Scholar]
- Mantovani, A.; Morellato, P.C.; Reis, M.S. Fenologia reprodutiva e produção de sementes em Araucaria angustifolia (Bert.) O. Kuntze. Rev. Bras. De Botânica 2004, 27, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Anselmini, J.I.; Zanette, F. Polinização controlada em Araucaria angustifolia. Cerne 2012, 18, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.O.; Finger, C.A.G.; Costa, E.A.; Campoe, O.C.; Schons, C.T. Using crown characterisation variables as indicator of the vigor, competition and growth of Brazilian pine. Southern Forests: A J. For. Sci. 2021, 83, 240–253. [Google Scholar] [CrossRef]
- Costa, E.A.; Finger, C.A.G.; Fleig, F.D.; Hess, A.F.; Marangon, G.P. Density management diagram of araucaria uneven aged forest. Floresta 2016, 46, 173–184. [Google Scholar] [CrossRef]
- Hess, A.F.; da Silveira, A.C.; Krefta, S.M.; dos Santos, D.V.; Filho, M.D.H.V.; Atanazio, K.A.; Schorr, L.P.B.; Santos, I.A.; Borsoi, G.A.; Stepka, T.F.; et al. Crown dynamics of Brazilian pine (Araucaria angustifolia) in Santa Catarina region of Brazil. Aust. J. Crop Sci. 2018, 12, 449–457. [Google Scholar] [CrossRef]
- Klein, D.R.; Hess, A.F.; Krefta, S.M.; Filho, M.D.H.V.; Ciarnoscki, L.D.; Costa, E.A. Morphometric relations for Araucaria angustifolia (Bertol.) Kuntze in Santa Catarina, Brazil. Floresta 2017, 47, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Ricken, P.; Hess, A.F.; de Mattos, P.P.; Braz, E.M.; Nakajima, N.Y.; Hosokawa, R.T. Mophometry of Araucaria angustifolia at different altitudes in Southern Brazil. Pesqui. Florest. Bras. 2020, 40, 1–11. [Google Scholar] [CrossRef]
- da Silveira, A.C.; Hess, A.F.; Schorr, L.P.B.; Stepka, T.F.; Krefta, S.M.; Atanazio, K.A. Variáveis de copa na determinação da densidade máxima de florestas de Araucaria angustifolia (Bertol.) Kuntze. Sci. For. 2021, 49, 1–13. [Google Scholar] [CrossRef]
- Bauhus, J.; Forrester, D.I.; Gardiner, B.; Jactel, H.; Vallejo, R.; Pretzsch, H. Ecological stability of mixed-species forests. In Mixed-Species Forests; Ecology and Managament, Pretzsch, H., Forrester, D.I., Bauhus, J., Eds.; Springer: Berlin, Germany, 2017; pp. 337–382. [Google Scholar]
- Del Río, M.; Pretzsc, H.; Ruiz-Peinado, R.; Ampoorter, E.; Annighöfer, p.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Drössler, L.; et al. Species interactions increase the temporal stability community productivity in Pinus sylvestris-Fagus sylvatica mixtures across Europe. J. Ecol. 2017, 105, 1032–1043. [Google Scholar]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.D.; Ammer, C. Site adapted admixed tree species reduce drought susceptibility of mature European beach. Glob. Change Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.P.; Konle, U.; Nagel, J.; Spellmann, H.; Zassada, M.; et al. Productivity of mixed versus pure stands of oak (Quercus pretraea (Matt.) Liebl and Quercus robur L.) and European beach (Fagus sykvatica L.) along an ecological gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistence of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, A.C.; Hess, A.F.; Schorr, L.P.B.; Krefta, S.M.; dos Santos, D.V.; Filho, M.D.H.V.; Atanazio, K.A.; Costa, E.A.; Stepka, T.F.; Borsoi, G.A. Management of Brazilian pine (Araucaria angustifolia (Bertol) Kuntze) based on the Liocourt model in a mixed ombrophilous forest in Southern Brazil. Aust. J. Crop Sci. 2018, 12, 311–317. [Google Scholar] [CrossRef]
- Solórzano-Filho, J.A. Demografia e ecologia da dispersão de sementes de Araucaria angustifolia (Bert.) Kutze (Araucariaceae), numa população relictual em Campos do Jordão, SP. Master Thesis, University of São Paulo, Sao Paulo, SP, Brazil, 2001. Available online: https://repositorio.usp.br/item/001181122 (accessed on 1 October 2022).
- Wendling, I.; Zanette, F. Araucária: Propagação e Manejo de Plantios; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Statsoft Inc. Statistica 2014; Version 10.20; Windows. Dell.; Statsoft Inc.: Tulsa, OK, USA, 2014. [Google Scholar]
- CONAB—Companhia Nacional de Abastecimento. Bol. Da Socio-Biodivers. 2017, 1, 1–67.
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras; Embrapa: Brasília, Brazil, 2003. [Google Scholar]
- Bauhus, J. Rooting patterns of old-growth forests: Is aboveground structural and functional diversity mirrored belowground? In Old-Growth Forests; Ecological Studies, Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 207, pp. 211–229. [Google Scholar]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Biber, P. Size-symmetric versus size-asymmetric competition and growth of coarse roots and stem of Picea marina. J. For. Res. 2010, 40, 370–384. [Google Scholar]
- Triviño, M.; Pohjanmies, T.; Mazziotta, A.; Juutinen, A.; Podkopaev, D.; Tortorec, E.L.; Mönkkönen, M. Optimizing management to enhance multifunctionality in a boreal forest landscape. J. Appl. Ecol. 2017, 54, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Binkley, D.; Campoe, O.C.; Gspaltl, M.; Forrester, D. Light absorption and use efficiency in forest: Why differ for trees and stands. For. Ecol. Manag. 2013, 288, 5–13. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For.Ecol. Manag. 2013, 312, 282–292. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Enno, U.; Dahlhausen, J.; Rötzer, T.; Caldentey, J.; Koike, T.; Con, T.V.; Chavanne, A.; Seifert, T.; et al. Crow size and growing space requirement of common tree species in urban centers, parks, and forests. Urban For. Urban Green. 2015, 14, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Rickli-Horst, H.C.; Bona, C.; Sant’ Anna-Santos, B.F.; Koehler, H.S.; Wendling, I.; Zuffellato-Ribas, K.C. Visual and anatomical analysis of welding quality x scion survival in Araucaria angustifolia. Acta Sci. 2021, 43, e45509. [Google Scholar] [CrossRef]
- da Costa, N.C.F.; Stedile, L.I.B.; Lauterjung, M.B.; Montagna, T.; Ribeiro, R.C.; Bernardi, A.P.; Mantovani, A.; dos Reis, M.S.; Nodari, R.O. Spatiotemporal variation in mating system and genetic diversity of Araucaria angustifolia: Implications for conservation and seed collection. For. Ecol. Manag. 2021, 481, 118716. [Google Scholar] [CrossRef]
- Lo Monaco, A.; Macinnis-Ng, C.; Rajora, O.P. Forests for a Better Future: Sustainability, Innovation and Interdisciplinarity. Forests 2022, 13, 941. [Google Scholar] [CrossRef]
- Wendling, I. Tecnologia de Enxertia de Araucaria Angustifolia Para Produção Precoce de Pinhões; (Comunicado Técnico, 351); Embrapa Florestas: Colombo, Brazil, 2015. [Google Scholar]
- Dornelles, L.; “Vem de berço”: A Importância da Safra Para Quem Vive de Renda do Pinhão em SC. Florianópolis, Santa Catarina. 2021. Available online: https://ndmais.com.br/economia-sc/vem-de-berco-a-importancia-da-safra-para-quem-vive-da-renda-do-pinhao-em-sc/ (accessed on 1 October 2022).
- de Souza, I.A.; Hess, A.F.; Costa, E.A.; da Silveira, A.C.; Schorr, L.P.B.; Atanazio, K.A. Development of models to a id decision making in the management of Araucaria angustifolia (Bertol.) Kuntze. Floresta 2020, 50, 1854–1863. [Google Scholar] [CrossRef]
- Orman, O.; Wrzesinski, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and Silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2021, 482, 118866. [Google Scholar] [CrossRef]
- Krůček, M.; Trochta, J.; Cibrulka, M.; Král, K. Beyond the cones: How crown shape plasticity alters aboveground competition for space and light—Evidence for terrestrial laser scanning. Agric. For. Meteorol. 2019, 264, 188–199. [Google Scholar] [CrossRef]
- Costa, E.A.; Hess, A.F.; Finger, C.A.G.; Schons, C.T.; Klein, D.R.; Barbosa, L.O.; Borsoi, G.A.; Liesenberg, V.; Bispo, P.d.C. Enhancing Height Predictions of Brazilian Pine for Mixed, Uneven-Aged Forests Using Artificial Neural Networks. Forests 2022, 13, 1284. [Google Scholar] [CrossRef]
- Costa, E.A.; Liesenberg, V.; Hess, A.F.; Finger, C.A.G.; Schneider, P.R.; Longhi, R.V.; Schons, C.T.; Borsoi, G.A. Simulating Araucaria angustifolia (Bertol.) Kuntze Timber Stocks with Liocourt’s Law in a Natural Forest in Southern Brazil. Forests 2020, 11, 339. [Google Scholar] [CrossRef] [Green Version]
- de Mattos, P.P.; Curto, R.A.; Braz, E.M.; Netto, S.P. How do Araucaria angustifolia trees grow in overstocked stands? Dendrochronologia 2022, 74, 125976. [Google Scholar] [CrossRef]
- Beckert, S.M.; Rosot, M.A.D.; Rosot, N.C. Crescimento e dinâmica de Araucaria angustifolia (Bert.) O. Ktze. em fragmento de Floresta Ombrófila Mista. Sci. For. 2014, 42, 209–218. [Google Scholar]
- Li, Y.; Kröber, W.; Bruelheide, H.; Härdtle, W.; von Oheimb, G. Crown and leaf traits as predictors of subtropical tree sapling growth rates. J. Plant Ecol. 2017, 10, 136–145. [Google Scholar] [CrossRef]
- Hadad, M.A.; Roig, F.A.; Molina, J.G.A.; Hacket-Pain, A. Growth of male and female Araucaria araucana trees respond differently to regional mast events, creating sex-specific patterns in their tree-ring chronologies. Ecol. Indic. 2021, 122, 107245. [Google Scholar] [CrossRef]
- Biffi, L.J.; Mitishita, E.; Liesenberg, L.; Santos, A.A.; Gonçalves, D.D.; Estrabis, N.V.; Silva, J.A.; Osco, L.P.; Ramos, A.P.M.; Centeno, J.A.S.; et al. ATSS Deep Learning-Based Approach to Detect Apple Fruits. Remote Sens. 2021, 13, 54. [Google Scholar] [CrossRef]
- Martins, D.A.P.; Erdmann, J.M.; Lemos, A.M.; Walter, F.F.; Lanzarini, A.C.; Kanieski, M.R. Espécies potenciais para recuperação de áreas degradadas a partir de levantamento florístico realizado no Planalto Catarinense. Rev. De Ciências Agroveterinárias 2019, 18, 38–46. [Google Scholar] [CrossRef]




| Variable | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| an | 317 | 80 | 885 | 180.2 |
| d | 40.1 | 16.4 | 69.7 | 9.0 |
| h | 14.2 | 8.5 | 18.7 | 2.2 |
| API10 | 0.56 | 0.24 | 0.98 | 0.20 |
| cd | 9.57 | 4.7 | 19.6 | 3.28 |
| csa | 80.21 | 17.35 | 301.72 | 59.71 |
| ci | 0.68 | 0.36 | 1.17 | 0.21 |
| hd | 39 | 21 | 84 | 14 |
| cl | 6.9 | 2.7 | 12.2 | 2.23 |
| CE1 | 0.01042 | 0.00110 | 0.03558 | 0.00771 |
| CE2 | 5.03287 | 1.92449 | 11.39336 | 2.13079 |
| Equation | b0 | SE | b1 | D | AIC | BIC | LF |
|---|---|---|---|---|---|---|---|
| 9 | 4.7573 * | 0.24 | 0.0237 * | 7.5 | 456.7 | 461.4 | G–ln(µ) |
| 10 | 163.8949 * | 36.12 | 1.899 * | 7.4 | 456.2 | 460.9 | G(µ) |
| 11 | 6.2944 * | 0.21 | −0.0143 * | 9.4 | 464.7 | 469.4 | G–ln(µ) |
| Component | Eigenvalue | % Total Variance | Cumulative Eigenvalue | Cumulative % |
|---|---|---|---|---|
| 1 | 6.67 | 66.67 | 6.67 | 66.67 |
| 2 | 2.4 | 23.99 | 9.07 | 90.66 |
| 3 | 0.55 | 5.54 | 9.62 | 96.20 |
| 4 | 0.31 | 3.01 | 9.93 | 99.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demétrio, L.; Hess, A.F.; de Sousa, A.N.; Costa, E.A.; Liesenberg, V.; Freisleben, M.J.; Schimalski, M.B.; Finger, C.A.G.; Hofiço, N.d.S.A.; Bispo, P.d.C. Can We Predict Male Strobili Production in Araucaria angustifolia Trees with Dendrometric and Morphometric Attributes? Forests 2022, 13, 2074. https://doi.org/10.3390/f13122074
Demétrio L, Hess AF, de Sousa AN, Costa EA, Liesenberg V, Freisleben MJ, Schimalski MB, Finger CAG, Hofiço NdSA, Bispo PdC. Can We Predict Male Strobili Production in Araucaria angustifolia Trees with Dendrometric and Morphometric Attributes? Forests. 2022; 13(12):2074. https://doi.org/10.3390/f13122074
Chicago/Turabian StyleDemétrio, Laryssa, André Felipe Hess, Alex Nascimento de Sousa, Emanuel Arnoni Costa, Veraldo Liesenberg, Maurício Jean Freisleben, Marcos Benedito Schimalski, César Augusto Guimarães Finger, Noé dos Santos Ananias Hofiço, and Polyanna da Conceição Bispo. 2022. "Can We Predict Male Strobili Production in Araucaria angustifolia Trees with Dendrometric and Morphometric Attributes?" Forests 13, no. 12: 2074. https://doi.org/10.3390/f13122074






