Soil Fungal Diversity and Functionality Changes Associated with Multispecies Restoration of Pinus massoniana Plantation in Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. DNA Extraction and Bioinformatics
2.3. Soil Properties
2.4. Statistical Analysis
3. Results
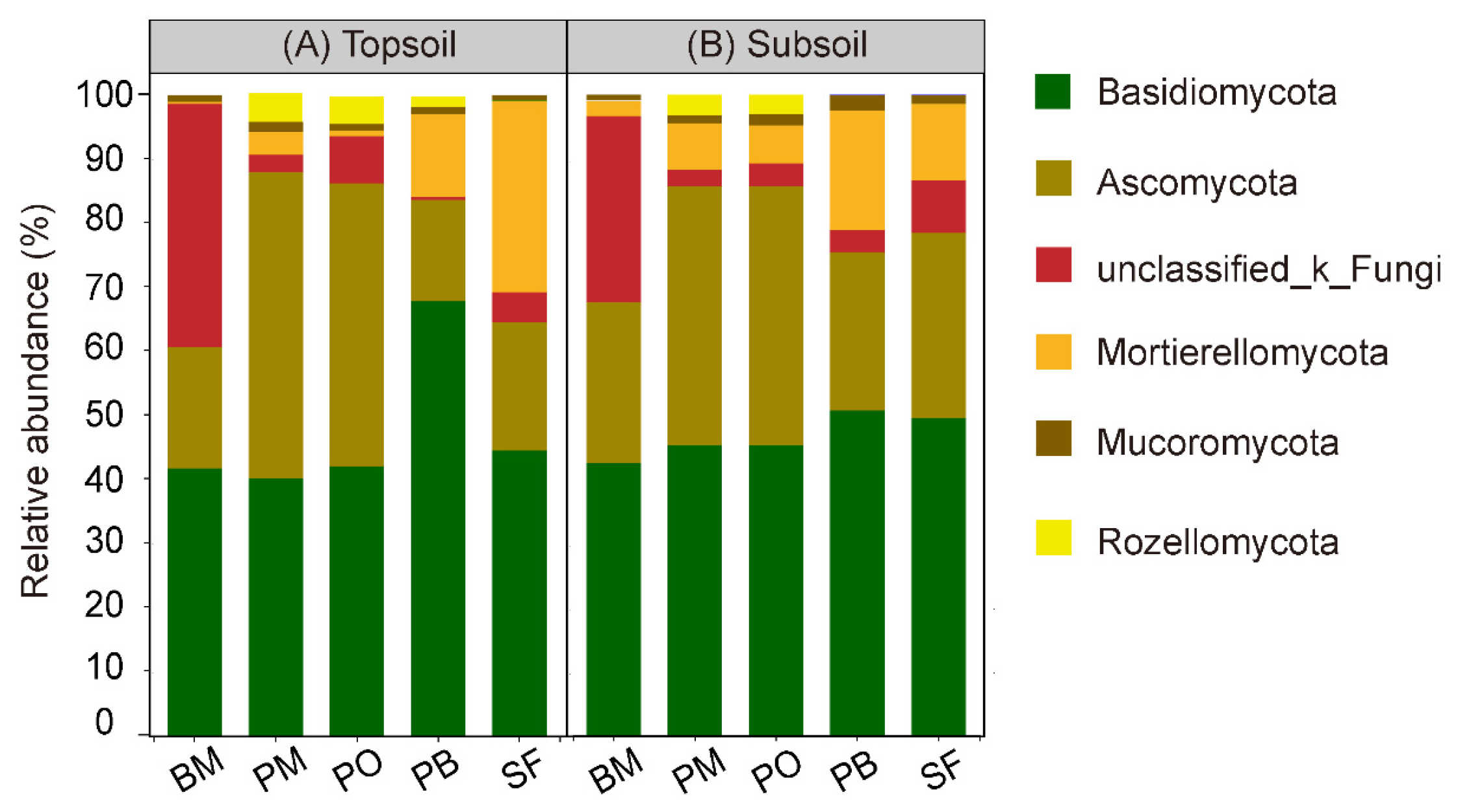
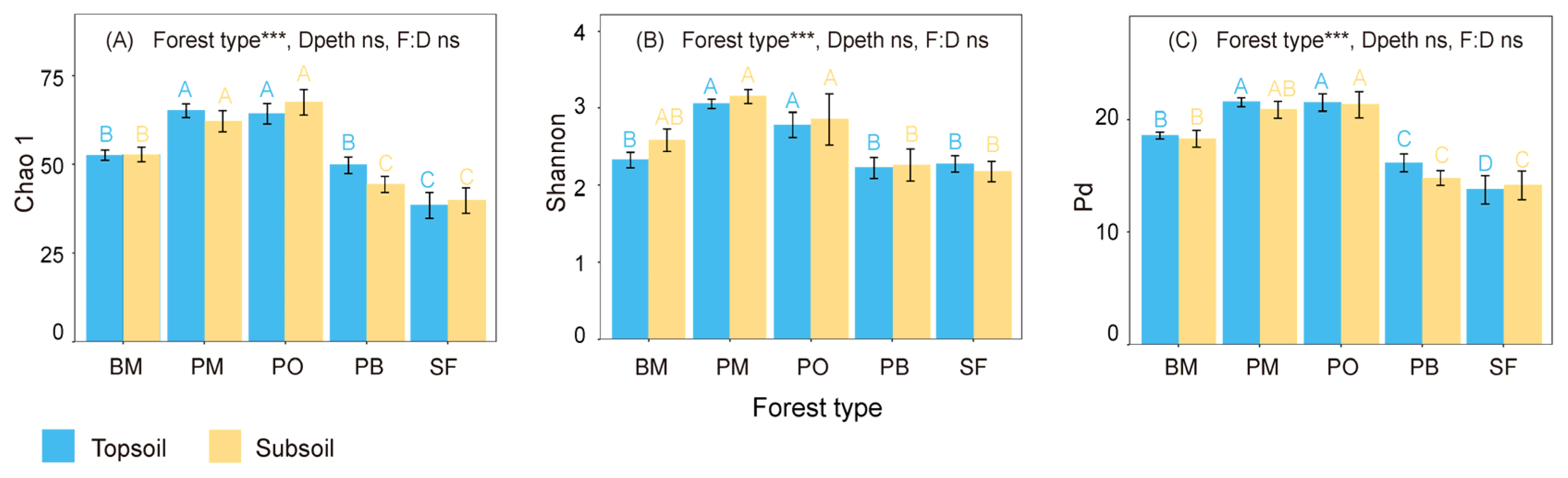
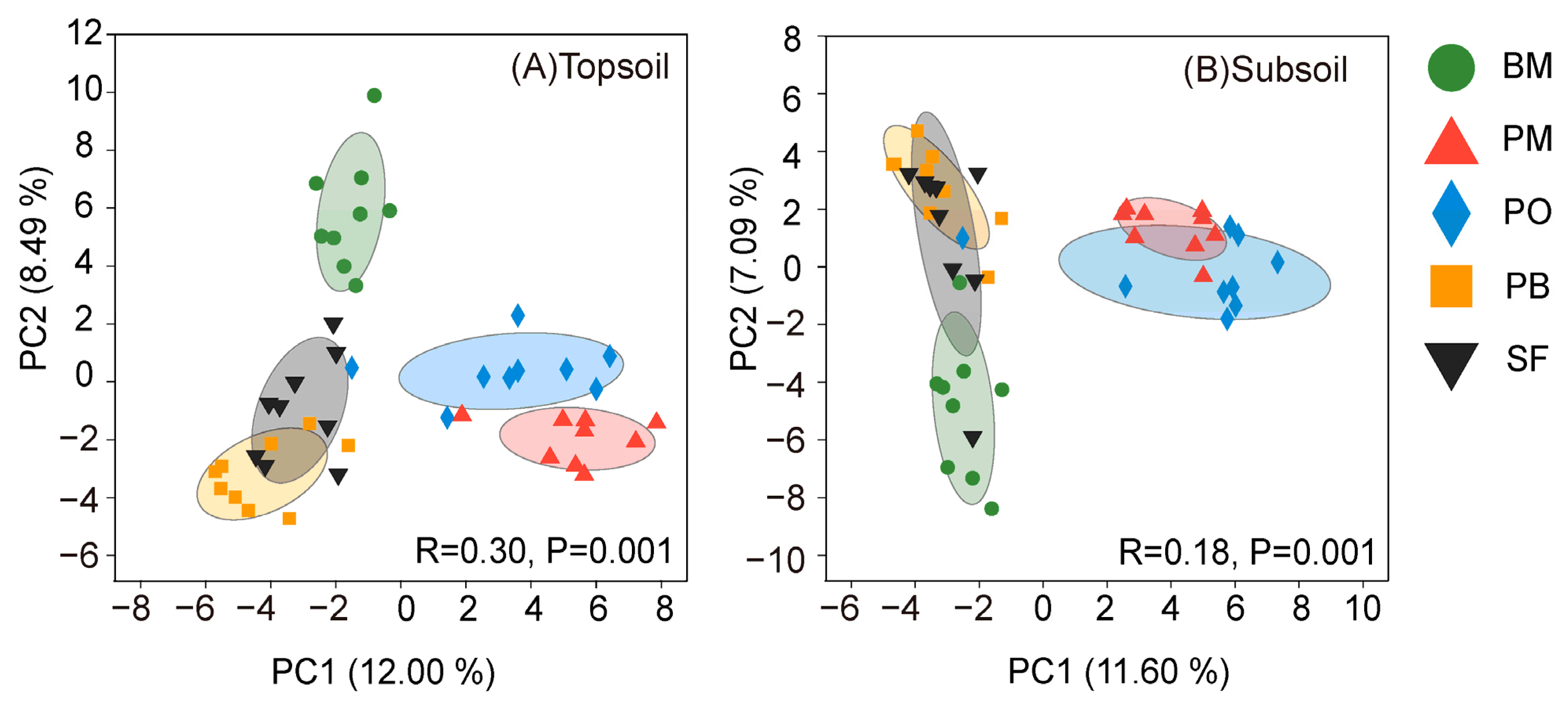
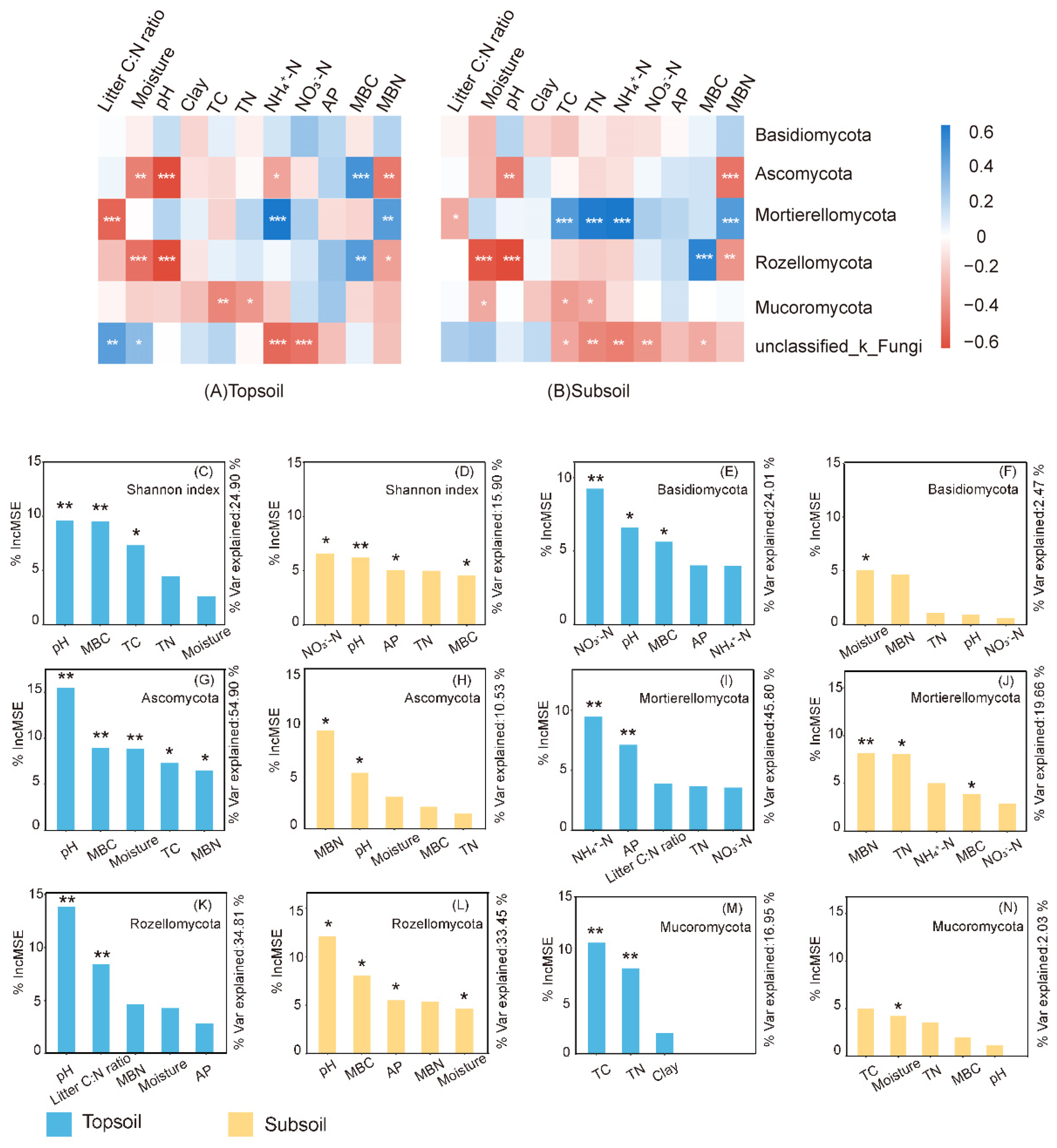
3.1. Soil Fungal Diversity and Composition
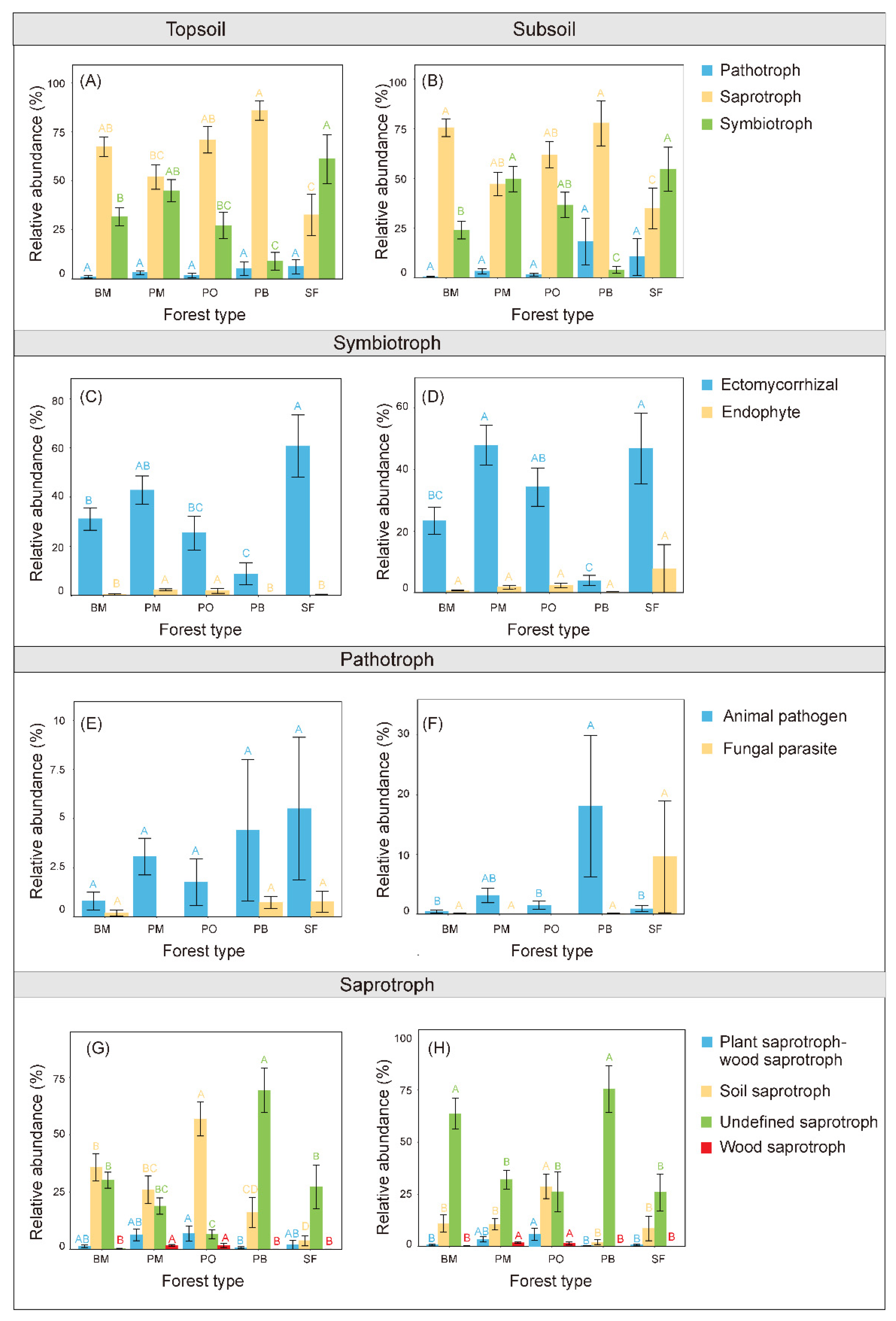
3.2. Soil Fungal Guilds
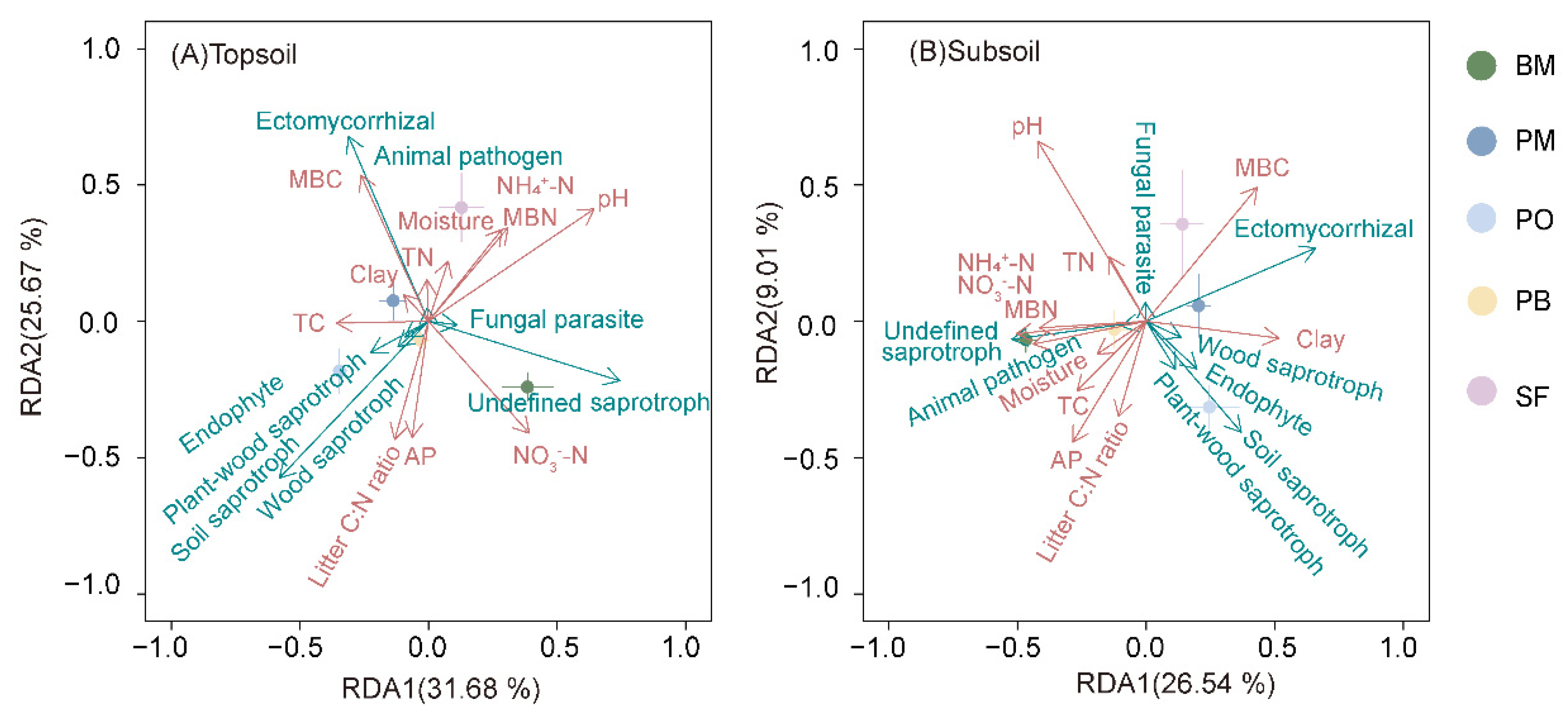
3.3. Relationship between Environmental Predictors and Fungal Communities
4. Discussion
4.1. Multispecies Restoration Altered the Fungal Taxonomic Composition
4.2. Multispecies Restoration Decreased the Fungal Diversity
4.3. Multispecies Restoration Altered the Soil Fungal Guilds
4.4. Linking Litter and Soil Biogeochemistry to Soil Fungal Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Depth | Phyla | Orders | Families | Genera | Species | |
|---|---|---|---|---|---|---|
| BM | Topsoil | 8 | 61 | 112 | 164 | 228 |
| Subsoil | 10 | 77 | 138 | 204 | 273 | |
| PM | Topsoil | 10 | 68 | 143 | 244 | 344 |
| Subsoil | 10 | 85 | 164 | 277 | 375 | |
| PO | Topsoil | 11 | 77 | 141 | 236 | 323 |
| Subsoil | 12 | 83 | 178 | 313 | 441 | |
| PB | Topsoil | 11 | 87 | 189 | 347 | 504 |
| Subsoil | 11 | 89 | 185 | 314 | 457 | |
| SF | Topsoil | 10 | 70 | 140 | 247 | 378 |
| Subsoil | 11 | 78 | 163 | 273 | 415 |
| Alpha Diversity | Forest Type | Depth | F:D | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | F | p | DF | F | p | DF | F | p | |
| Chao 1 | 4 | 33.60 | *** | 1 | 0.17 | ns | 4 | 0.80 | ns |
| Shannon | 4 | 10.76 | *** | 1 | 0.48 | ns | 4 | 0.30 | ns |
| Phylogenetic diversity | 4 | 0.80 | *** | 1 | 0.60 | ns | 4 | 0.28 | ns |
| Fungal Trophic Type | Fungal Guilds | Forest Type | Depth | F:D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | F | p | DF | F | p | DF | F | p | ||
| Symbiotroph | Ectomycorrhizal | 4 | 12.94 | *** | 1 | 0.30 | ns | 4 | 0.85 | ns |
| Endophyte | 4 | 0.75 | ns | 1 | 0.91 | ns | 4 | 0.89 | ns | |
| Total | 4 | 13.85 | *** | 1 | 0.00 | ns | 4 | 0.00 | ns | |
| Pathotroph | Animal pathogen | 4 | 2.08 | ns | 1 | 0.42 | ns | 4 | 1.42 | ns |
| Fungal parasite | 4 | 1.15 | ns | 1 | 0.76 | ns | 4 | 0.92 | ns | |
| Total | 4 | 1.74 | ns | 1 | 0.00 | ns | 4 | 0.00 | ns | |
| Saprotroph | Plant saprotroph-wood saprotroph | 4 | 4.40 | ** | 1 | 1.22 | ns | 4 | 0.18 | ns |
| Soil saprotroph | 4 | 15.71 | *** | 1 | 22.14 | *** | 4 | 3.10 | * | |
| Undefined saprotroph | 4 | 17.36 | *** | 1 | 8.69 | ** | 4 | 1.54 | ns | |
| Wood saprotroph | 4 | 8.36 | *** | 1 | 0.00 | ns | 4 | 0.04 | ns | |
| Total | 4 | 16.70 | *** | 1 | 0.00 | ns | 4 | 0.00 | ns | |
References
- Yang, Y.H.; Shi, Y.; Sun, W.J.; Chang, J.F.; Zhu, J.X.; Chen, L.Y.; Wang, X.; Guo, Y.P.; Zhang, H.T.; Yu, L.F.; et al. Terrestrial carbon sinks in China and around the world and their contribution to carbon neutrality. Sci. China Life Sci. 2022, 65, 861–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Wang, J.; Shi, Z.; Lu, L.; Zeng, J.; Ming, A.; Tang, J.; Yu, H. Effects of tree species mixture on soil organic carbon stocks and greenhouse gas fluxes in subtropical plantations in China. For. Ecol. Manag. 2013, 300, 4–13. [Google Scholar] [CrossRef]
- Hua, F.Y.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.R.; Wang, W.; McEvoy, C.R.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Li, X.; Wu, J. A global meta-analysis of the impacts of tree plantations on biodiversity. Glob. Ecol. Biogeogr. 2022, 31, 576–587. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, L.; Rincón, A.; Lindahl, B.D.; Buée, M. Fungal community of forest soil: Diversity, functions, and services. In Forest Microbiology; Academic Press: New York, NY, USA, 2021; pp. 231–255. [Google Scholar]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramos, J.C.; Cale, J.A.; Cahill Jr, J.F.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2021, 229, 1105–1117. [Google Scholar] [CrossRef]
- Liu, S.; García-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K.; et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022, 6, 900–906. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguan, Z.; Chang, F.; Jia, F.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Wardle, D.A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 2006, 9, 870–886. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, M.; López-García, Á.; Domínguez, M.T.; Kjøller, R.; Navarro-Fernández, C.M.; Rosendahl, S.; Marañón, T. Soil fungal diversity and functionality are driven by plant species used in phytoremediation. Soil Biol. Biochem. 2021, 153, 108102. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.; Cheng, H.; Chang, S.X.; Liang, C.; An, S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Lekberg, Y.; Waller, L.P. What drives differences in arbuscular mycorrhizal fungal communities among plant species? Fungal Ecol. 2016, 24, 135–138. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Cajthaml, T.; Põlme, S.; Hiiesalu, I.; Anslan, S.; Harend, H.; Buegger, F.; Pritsch, K.; Abarenkov, K. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 2016, 10, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, C.; Yi, J.; Doughty, R.; Zhao, F. Contrasting responses of rhizosphere bacteria, fungi and arbuscular mycorrhizal fungi along an elevational gradient in a temperate montane forest of China. Front. Microbiol. 2020, 11, 2042. [Google Scholar] [CrossRef]
- Yang, Y.R.; Ding, J.H.; Zhang, T.L.; Wang, X.X. Phenolic acids detection in soil of Pinus massoniana forest and its effect on shrub and grass germination. Soils 2013, 45, 291–294. [Google Scholar]
- Tu, C.; Lu, Q.; Zhang, Y.; Tian, J.; Gao, Y.; Liu, Y.; Yang, H.; Chen, L.; Zhang, J.; Wang, J.; et al. The soil nematode community indicates the soil ecological restoration of the Pinus massoniana plantation gap replanted with Cinnamomum longipaniculatum. Ecol. Indic. 2022, 136, 108678. [Google Scholar] [CrossRef]
- Amazonas, N.T.; Forrester, D.I.; Oliveira, R.S.; Brancalion, P.H.S. Combining Eucalyptus wood production with the recovery of native tree diversity in mixed plantings: Implications for water use and availability. For. Ecol. Manag. 2018, 418, 34–40. [Google Scholar] [CrossRef]
- Feng, Y.H.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.L.; Zhu, J.X.; Tang, Z.Y.; Zhu, J.L.; Hong, P.B.; Ji, C.J.; et al. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Wang, J.Q.; Lucas-Borja, M.E.; Wang, Z.Y.; Li, X.; Huang, Z.Q. Microbial diversity regulates ecosystem multifunctionality during natural secondary succession. J. Appl. Ecol. 2021, 58, 2833–2842. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanable, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Wyngaard, N.; Cabrera, M.L.; Shober, A.; Kanwar, R. Fertilization strategy can affect the estimation of soil nitrogen mineralization potential with chemical methods. Plant Soil 2018, 432, 75–89. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agriczzolae: Statistical Procedures for Agricultural Research, R Package Version 1.2–1; CRAN Team: Online, 2014.
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan’. Community Ecology Package, Version 2; R Foundation for Statistical Computing: Vienna, Austria, 2013.
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mader, P.; et al. Soil quality-a critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, Z.J.; Zong, Y.Y.; Li, W.Q.; Chen, F.S.; Wang, G.G.; Li, J.J.; Fang, X.M. Mixing with coniferous tree species alleviates rhizosphere soil phosphorus limitation of broad-leaved trees in subtropical plantations. Soil Biol. Biochem. 2022, 175, 108853. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes-ecological, functional and phylogenetic review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R.W. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Odriozola, I.; Navrátilová, D.; Tláskalová, P.; Klinerová, T.; Červenková, Z.; Kohout, P.; Větrovskýa, T.; Čížková, P.; Starý, M.; Baldrian, P. Predictors of soil fungal biomass and community composition in temperate mountainous forests in Central Europe. Soil Biol. Biochem. 2021, 161, 108366. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Tamura, M.; Suseela, V.; Simpson, M.; Powell, B.; Tharayil, N. Plant litter chemistry alters the content and composition of organic carbon associated with soil mineral and aggregate fractions in invaded ecosystems. Glob. Chang. Biol. 2017, 23, 4002–4018. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Dick, D.L.; Gardner, T.G.; Frene, J.P.; Heitman, J.L.; Sucre, E.B.; Leggett, Z.H. Forest floor manipulation effects on the relationship between aggregate stability and ectomycorrhizal fungi. For. Ecol. Manag. 2022, 505, 119873. [Google Scholar] [CrossRef]
- Hu, J.; Yang, A.; Wang, J.; Zhu, A.; Dai, J.; Wong, M.H.; Lin, X. Arbuscular mycorrhizal fungal species composition, propagule density, and soil alkaline phosphatase activity in response to continuous and alternate no-tillage in Northern China. Catena 2015, 133, 215–220. [Google Scholar] [CrossRef]
- Leski, T.; Rudawska, M.; Kujawska, M.; Stasińska, M.; Janowski, D.; Karliński, L.; Wilgan, R. Both forest reserves and managed forests help maintain ectomycorrhizal fungal diversity. Biol. Conserv. 2019, 238, 108206. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Bahnmann, B.; Mašínová, T.; Halvorsen, R.; Davey, M.L.; Sedlak, P.; Tomšovský, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Doulcier, G.; Eldridge, D.J.; Stouffer, D.B.; Maestre, F.T.; Wang, J.; Powell, J.R.; Jeffries, T.C.; Singh, B.K. Increases in aridity lead to drastic shifts in the assembly of dryland complex microbial networks. Land Degrad. Dev. 2019, 31, 346–355. [Google Scholar] [CrossRef]
- Gan, H.; Li, X.; Wang, Y.; Lü, P.; Ji, N.; Yao, H.; Guo, L. Plants Play Stronger Effects on Soil Fungal than Bacterial Communities and Co-occurrence network structures in a subtropical tree diversity experiment. Microbiol. Spectr. 2022, 10, e00134-22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, S.; Shen, H.; Zhao, M.; Xu, L.; Xing, A.; Fang, J. Soil extracellular enzyme activity and stoichiometry in China’s forests. Funct. Ecol. 2020, 34, 1461–1471. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bomito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, J.; Li, T.; Liao, Y. Response of soil fungal communities to continuous cropping of flue-cured tobacco. Sci. Rep. 2020, 10, 19911. [Google Scholar] [CrossRef]
- Zak, D.R.; Pellitier, P.T.; Argiroff, W.A.; Castillo, B.; James, T.Y.; Nave, L.E.; Averill, C.; Beidler, K.V.; Bhatnagar, J.; Blesh, J.; et al. Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytol. 2019, 223, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Carteron, A.; Beigas, M.; Joly, S.; Turner, B.L.; Laliberté, E. Temperate forests dominated by arbuscular or ectomycorrhizal fungi are characterized by strong shifts from saprotrophic to mycorrhizal fungi with increasing soil depth. Microb. Ecol. 2021, 82, 377–390. [Google Scholar] [CrossRef] [PubMed]

| Phylum | H Statistic | p-Value | ||
|---|---|---|---|---|
| Topsoil | Subsoil | Topsoil | Subsoil | |
| Basidiomycota | 12.43 | 1.61 | * | ns |
| Ascomycota | 28.12 | 10.08 | *** | * |
| Mortierellomycota | 33.57 | 16.02 | *** | ** |
| Mucoromycotina | 6.05 | 5.49 | ns | ns |
| Rozellomycota | 28.37 | 35.54 | *** | *** |
| Lc:n | Moisture | pH | Clay | TC | TN | NH4+-N | NO3−-N | AP | MBC | MBN | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chao 1 | 0.26 | −0.24 | −0.69 *** | −0.10 | −0.06 | −0.27 | −0.30 * | 0.00 | 0.48 *** | −0.10 | −0.45 ** | |
| Topsoil | Shannon | 0.07 | −0.25 | −0.50 *** | −0.08 | −0.15 | −0.08 | −0.21 | −0.14 | 0.27 | 0.19 | −0.34 * |
| Pd | 0.29 | −0.18 | −0.64 *** | −0.09 | −0.04 | −0.27 | −0.39 ** | −0.15 | 0.42 ** | −0.11 | −0.49 *** | |
| Chao 1 | 0.23 | −0.11 | −0.03 | 0.20 | −0.11 | −0.31 * | −0.25 | −0.03 | 0.23 | −0.03 | −0.26 | |
| Subsoil | Shannon | 0.06 | −0.22 | 0.07 | 0.04 | −0.22 | −0.30 * | −0.45 ** | −0.14 | 0.04 | −0.04 | −0.34 * |
| Pd | 0.24 | −0.08 | −0.06 | 0.20 | −0.15 | −0.32 * | −0.35 * | −0.10 | 0.14 | −0.02 | −0.34 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhou, L.; Zou, B.; Wang, S.; Zheng, Y.; Huang, Z.; He, J.-Z. Soil Fungal Diversity and Functionality Changes Associated with Multispecies Restoration of Pinus massoniana Plantation in Subtropical China. Forests 2022, 13, 2075. https://doi.org/10.3390/f13122075
Wu L, Zhou L, Zou B, Wang S, Zheng Y, Huang Z, He J-Z. Soil Fungal Diversity and Functionality Changes Associated with Multispecies Restoration of Pinus massoniana Plantation in Subtropical China. Forests. 2022; 13(12):2075. https://doi.org/10.3390/f13122075
Chicago/Turabian StyleWu, Linfang, Luhong Zhou, Bingzhang Zou, Sirong Wang, Yong Zheng, Zhiqun Huang, and Ji-Zheng He. 2022. "Soil Fungal Diversity and Functionality Changes Associated with Multispecies Restoration of Pinus massoniana Plantation in Subtropical China" Forests 13, no. 12: 2075. https://doi.org/10.3390/f13122075
APA StyleWu, L., Zhou, L., Zou, B., Wang, S., Zheng, Y., Huang, Z., & He, J.-Z. (2022). Soil Fungal Diversity and Functionality Changes Associated with Multispecies Restoration of Pinus massoniana Plantation in Subtropical China. Forests, 13(12), 2075. https://doi.org/10.3390/f13122075





