Partitioning of Ambrosia Beetle Diversity on Teak Plantations in Java, Sumbawa, and Sulawesi Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Identification of Ambrosia Beetles
2.3. Data Analyses
3. Results
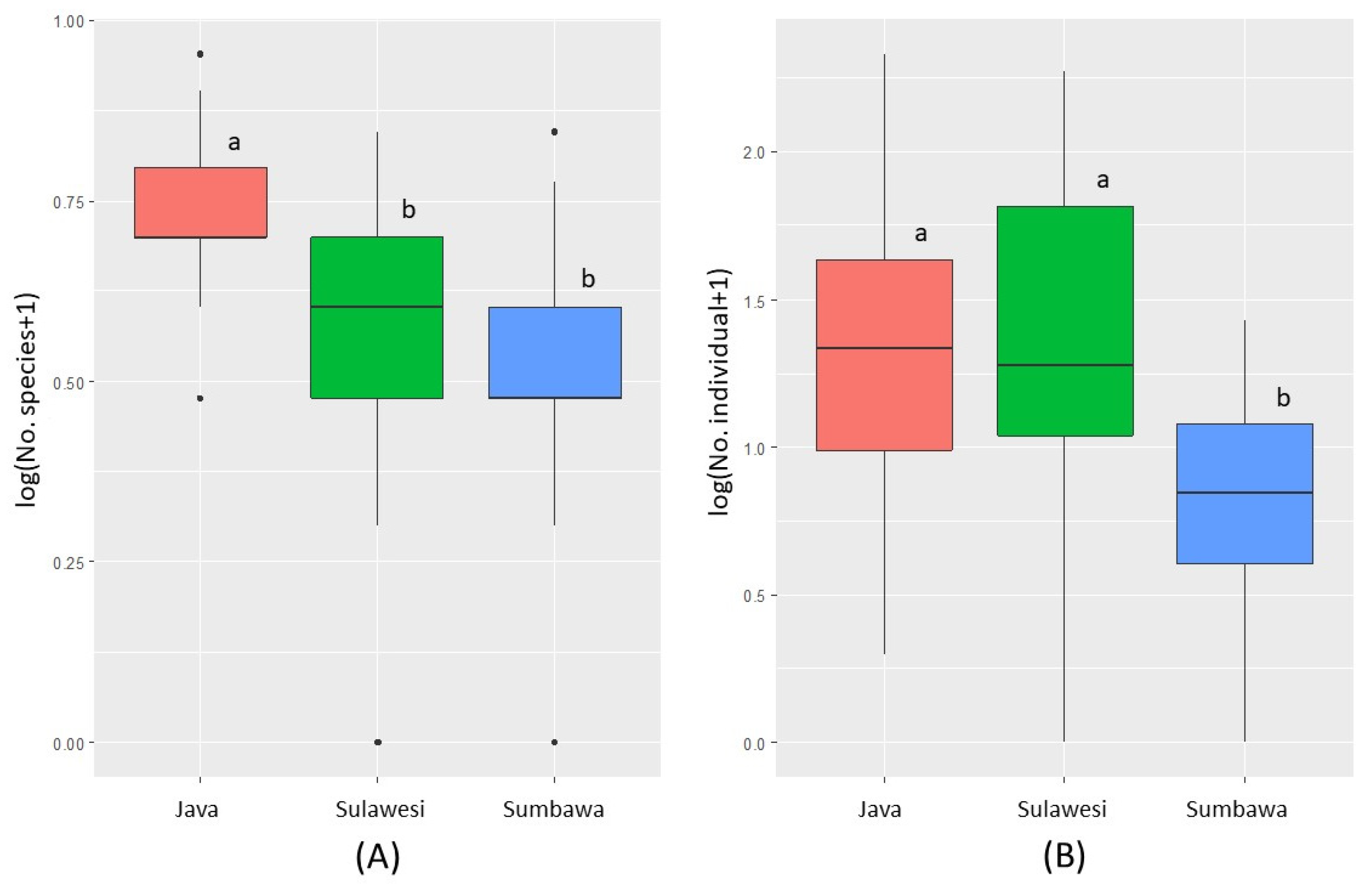
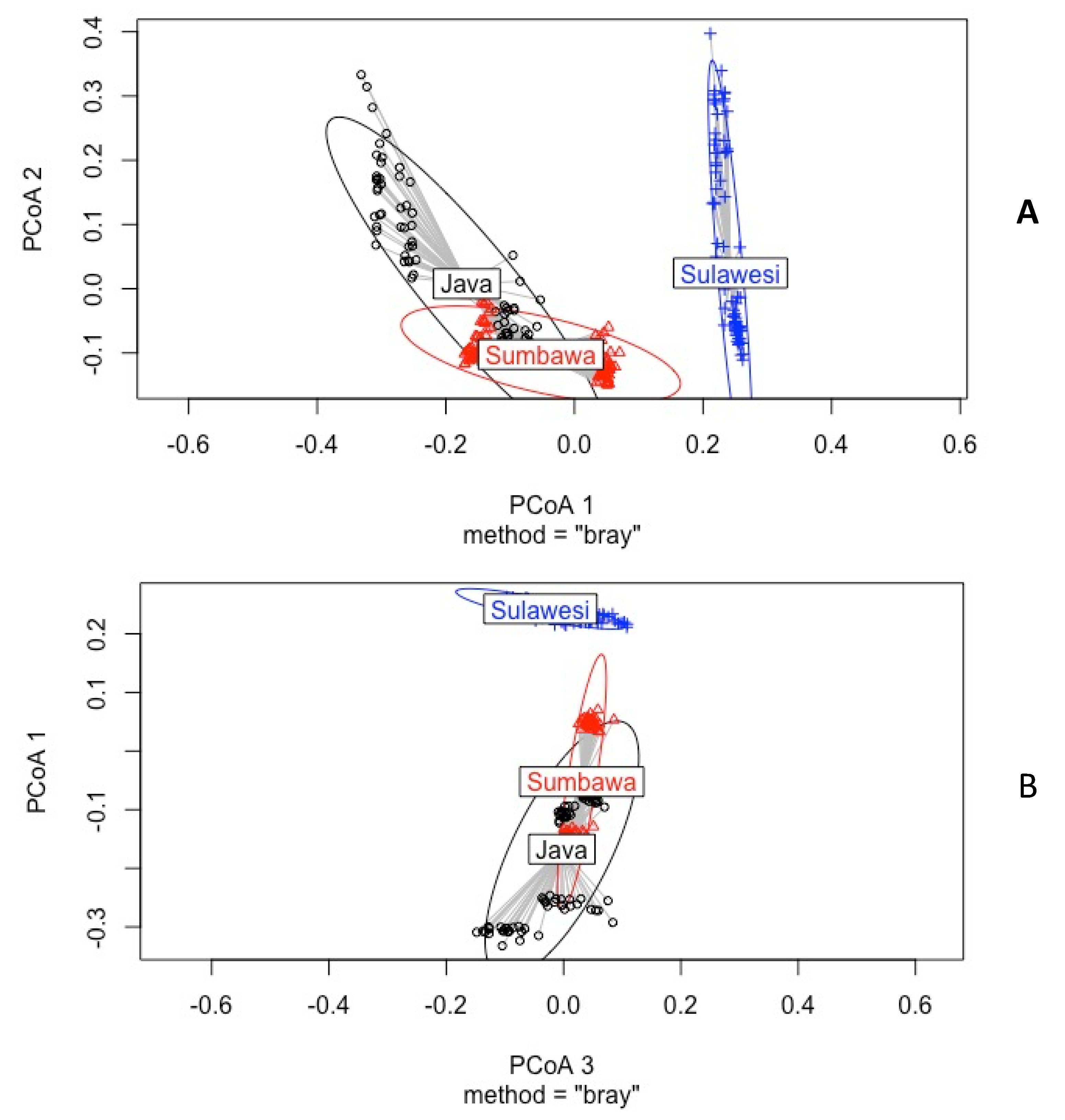
3.1. Diversity and Composition of Ambrosia Beetles
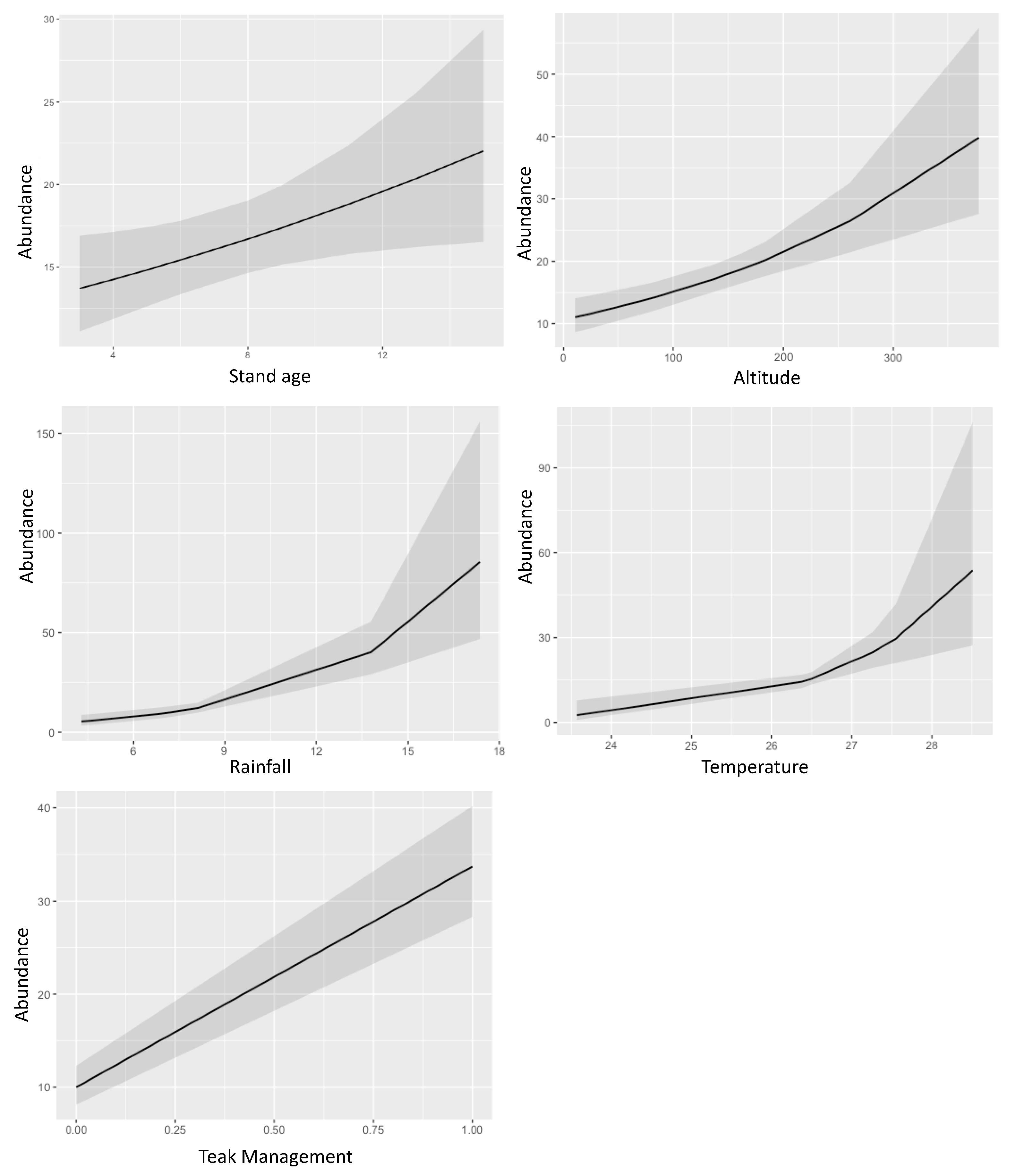
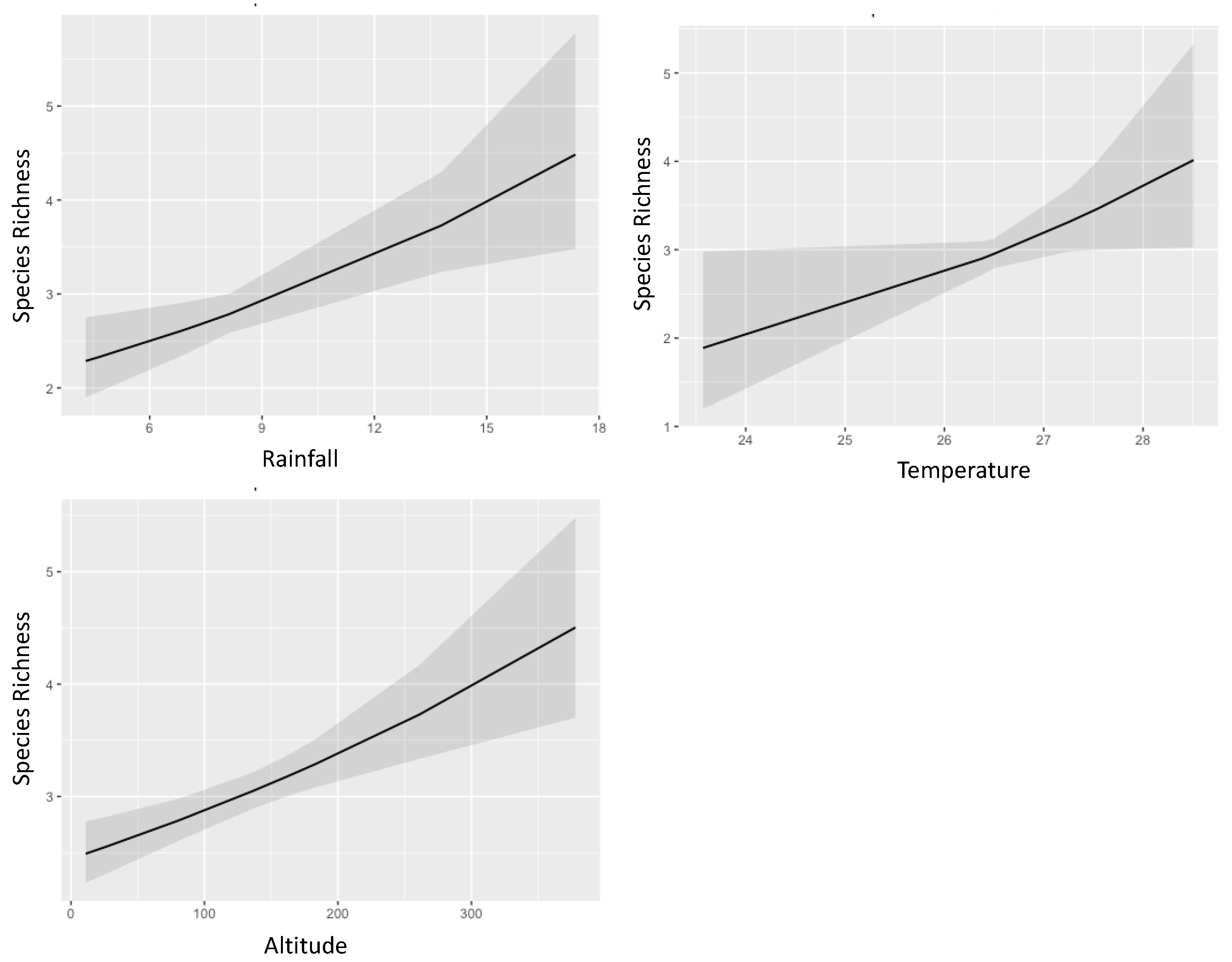
3.2. Ambrosia Beetles Diversity Partitioning on Teak Plantation
3.3. Partitioning the Beta Diversity and Its Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricklefs, R.E. Disintegration of the Ecological Community. Am. Nat. 2008, 172, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Soininen, J.; McDonald, R.; Hillebrand, H. The Distance Decay of Similarity in Ecological Communities. Ecography 2007, 30, 3–12. [Google Scholar] [CrossRef]
- Legendre, P.; Borcard, D.; Peres-Neto, P.R. Analyzing Beta Diversity: Partitioning the Spatial Variation of Community Composition Data. Ecol. Monogr. 2005, 75, 435–450. [Google Scholar] [CrossRef]
- Brooks, D.; Mclennan, D. The Nature of Diversity: An Evolutionary Voyage of Discovery; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J. The Relationships between Local and Regional Species Richness and Spatial Turnover. Glob. Ecol. Biogeogr. 2002, 11, 363–375. [Google Scholar] [CrossRef]
- Tack, A.J.M.; Ovaskainen, O.; Harrison, P.J.; Roslin, T. Competition as a Structuring Force in Leaf Miner Communities. Oikos 2009, 118, 809–818. [Google Scholar] [CrossRef]
- Wilson, D.S. Complex Interactions in Metacommunities, with Implications for Biodiversity and Higher Levels of Selection. Ecology 1992, 73, 1984–2000. [Google Scholar] [CrossRef]
- Tarno, H.; Setiawan, Y.; Putri, R.A.A.; Nardo, A.; Tsamarah, F.G.; Asri, J.; Wang, J. Effect of Pine Forest Management on the Diversity of Ambrosia Beetles (Curculionidae: Platypodinae and Scolytinae) in East Java, Indonesia. Diversity 2022, 14, 484. [Google Scholar] [CrossRef]
- Reed, S.; Muzika, R.-M. The Influence of Forest Stand and Site Characteristics on the Composition of Exotic Dominated Ambrosia Beetle Communities (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2010, 39, 1482–1491. [Google Scholar] [CrossRef]
- Yotkham, S.; Suttiprapan, P.; Likhitrakarn, N.; Sulin, C.; Srisuka, W. Biodiversity and Spatiotemporal Variation of Longhorn Beetles (Coleoptera: Cerambycidae) in Tropical Forest of Thailand. Insects 2021, 12, 45. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Lakatos, F. Bark and Wood Boring Insects-Past, Present, and the Future Knowledge We Need. Insects 2021, 12, 28. [Google Scholar] [CrossRef]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The Evolution of Agriculture in Beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and Diversity of Bark and Ambrosia Beetles; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Wood, S.L.; Bright, D.E. A Catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index. Volume B. Gt. Basin Nat. Mem. 1992, 13, 835–1557. [Google Scholar]
- Haack, R.A. Exotic Bark- and Wood-Boring Coleoptera in the United States: Recent Establishments and Interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Allen, E.A.; Humble, L.M. Nonindigenous Species Introductions: A Threat to Canada’s Forests and Forest Economy. Can. J. Plant Pathol. 2002, 24, 103–110. [Google Scholar] [CrossRef]
- Forsse, E.; Solbreck, C. Migration in the Bark Beetle Ips typographus L.: Duration, Timing and Height of Flight. Z. Angew. Entomol. 2009, 100, 47–57. [Google Scholar] [CrossRef]
- Safranyik, L.; Carroll, A.L.; Régnière, J.; Langor, D.W.; Riel, W.G.; Shore, T.L.; Peter, B.; Cooke, B.J.; Nealis, V.G.; Taylor, S.W. Potential for Range Expansion of Mountain Pine Beetle into the Boreal Forest of North America. Can. Entomol. 2010, 142, 415–442. [Google Scholar] [CrossRef]
- Sallé, A.; Arthofer, W.; Lieutier, F.; Stauffer, C.; Kerdelhué, C. Phylogeography of a Host-Specific Insect: Genetic Structure of Ips Typographus in Europe Does Not Reflect Past Fragmentation of Its Host. Biol. J. Linn. Soc. 2007, 90, 239–246. [Google Scholar] [CrossRef]
- Verhaegen, D.; Fofana, I.; Logossa, Z.; Ofori, D. What Is the Genetic Origin of Teak (Tectona grandis L.) Introduced in Africa and in Indonesia? Tree Genet. Genomes 2010, 6, 717–733. [Google Scholar] [CrossRef]
- Prasetyo, E.; Widiyatno, W.; Indrioko, S.; Na’iem, M.; Matsui, T.; Matsuo, A.; Suyama, Y.; Tsumura, Y. Genetic Diversity and the Origin of Commercial Plantation of Indonesian Teak on Java Island. Tree Genet. Genomes 2020, 16, 34. [Google Scholar] [CrossRef]
- Setiawan, Y.; Rachmawati, R.; Tarno, H. Diversity of Ambrosia Beetles (Coleoptera: Scolytidae) on Teak Forest in Malang District, East Java, Indonesia. J. Biol. Divers. 2018, 19, 1783–1790. [Google Scholar] [CrossRef]
- Tarno, H.; Setiawan, Y.; Kusuma, C.B.; Fitriyah, M.; Hudan, A.N.; Yawandika, A.P.; Nasution, H.A.; Saragih, R.; Bagasta, A.P.Y.; Wang, Z.; et al. Diversity and Species Composition of Bark and Ambrosia Beetles Captured Using Ethanol Baited Traps on Different Hosts in East Java, Indonesia. Zool. Stud. 2021, 60, 1–9. [Google Scholar] [CrossRef]
- Pachas, A.N.A.; Sakanphet, S.; Midgley, S.; Dieters, M. Teak (Tectona grandis) Silviculture and Research: Applications for Smallholders in Lao PDR. Aust. For. 2019, 82 (Suppl. S1), 94–105. [Google Scholar] [CrossRef]
- Ugalde Arias, L.A. Teak: New Trends in Silviculture, Commercialization and Wood Utilization; International Forestry and Agroforestry: Bogor, Indonesia, 2013; ISBN 9968-47-716-8. [Google Scholar]
- Fettig, C.J.; Klepzig, K.D.; Billings, R.F.; Munson, A.S.; Nebeker, T.E.; Negrón, J.F.; Nowak, J.T. The Effectiveness of Vegetation Management Practices for Prevention and Control of Bark Beetle Infestations in Coniferous Forests of the Western and Southern United States. For. Ecol. Manage. 2007, 238, 24–53. [Google Scholar] [CrossRef]
- Zeng, Y.; Kidd, K.; Eckhardt, L. The Effect of Thinning and Clear-Cut on Changes in the Relative Abundance of Root-Feeding Beetle (Coleoptera:Curculionidae) in Pinus Taeda Plantations in Central Alabama and Georgia. Pest Manag. Sci. 2014, 70, 915–921. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Waring, R.H.; Pitman, G.B. Thinning Lodgepole Pine Increases Tree Vigor and Resistance to Mountain Pine Beetle. For. Sci. 1983, 29, 204–211. [Google Scholar]
- Declerck, S.A.J.; Coronel, J.S.; Legendre, P.; Brendonck, L. Scale Dependency of Processes Structuring Metacommunities of Cladocerans in Temporary Pools of High-Andes Wetlands. Ecography 2011, 34, 296–305. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Lande, R. Statistics and Partitioning of Species Diversity, and Similarity among Multiple Communities. Oikos 1996, 76, 5–13. [Google Scholar] [CrossRef]
- Veech, J.; Summerville, K.; Crist, T.; Gering, J. The Additive Partitioning of Species Diversity: Recent Revival of an Old Idea. Oikos 2002, 99, 3–9. [Google Scholar] [CrossRef]
- Baselga, A.; Lobo, J.M.; Svenning, J.-C.; Araújo, M.B. Global Patterns in the Shape of Species Geographical Ranges Reveal Range Determinants. J. Biogeogr. 2012, 39, 760–771. [Google Scholar] [CrossRef]
- Bush, A.; Harwood, T.; Hoskins, A.; Mokany, K.; Ferrier, S. Current Uses of Beta-Diversity in Biodiversity Conservation: A Response to Socolar et Al. Trends Ecol. Evol. 2016, 31, 337–338. [Google Scholar] [CrossRef]
- Gering, J.; Crist, T.; Veech, J. Additive Partitioning of Species Diversity across Multiple Spatial Scales: Implications for Regional Conservation of Biodiversity. Conserv. Biol. 2003, 17, 488–499. [Google Scholar] [CrossRef]
- Davies, J.G.; Stork, N.E.; Brendell, M.J.D.; Hine, S.J. Beetle Species Diversity and Faunal Similarity in Venezuelan Rainforest Tree Canopies. In Canopy Arthropods; Stork, N.E., Adis, J., Didham, R.K., Eds.; Chapman & Hall: London, UK, 1997; pp. 85–103. [Google Scholar]
- Gorzlancyk, A.; Held, D.; Ranger, C.; Barwary, Z.; Kim, D.-J. Capture of Cnestus mutilatus, Xylosandrus crassiusculus, and Other Scolytinae (Coleoptera: Curculionidae) in Response to Green Light Emitting Diodes, Ethanol, and Conophthorin. Fla. Entomol. 2014, 97, 301–303. [Google Scholar] [CrossRef]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I.; Hulcr, J.; Redford, A.J. Southeast Asian Ambrosia Beetle ID. Available online: http://idtools.org/id/wbb/sea-ambrosia/about_index.php (accessed on 7 May 2022).
- Crist, T.O.; Veech, J.A.; Gering, J.C.; Summerville, K.S. Partitioning Species Diversity across Landscapes and Regions: A Hierarchical Analysis of Alpha, Beta, and Gamma Diversity. Am. Nat. 2003, 162, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: Interpolation and Extrapolation for Species Diversity. 2020. Available online: https://cran.r-project.org/web/packages/iNEXT/iNEXT.pdf (accessed on 8 November 2022).
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Baselga, A.; Orme, D.; Villeger, S.; Bortoli, D.; Leprieur, F. Betapart: Partitioning Beta Diversity Into Turnover and Nestedness Components. R Package Version 1.3. Available online: https://cran.r-project.org/web/packages/betapart/index.html (accessed on 24 July 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-3. 2018. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 8 November 2022).
- R Core Development Team. R: A Language and Environment for Statistical Computing; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2. [Google Scholar] [CrossRef]
- Kalshoven, L.G.E. Studies on the Biology of Indonesian Scolytoidea. Tijdschr. Voor Entomol. 1958, 101, 157–180. [Google Scholar]
- Wood, S.L. The Bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae), a Taxonomic Monograph. Gt. Basin Nat. Mem. 1982, 6, 1–1359. [Google Scholar]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I. A Monograph of the Xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. Zookeys 2020, 983, 1–442. [Google Scholar] [CrossRef]
- Urvois, T.; Auger-Rozenberg, M.A.; Roques, A.; Rossi, J.P.; Kerdelhue, C. Climate Change Impact on the Potential Geographical Distribution of Two Invading Xylosandrus Ambrosia Beetles. Sci. Rep. 2021, 11, 1339. [Google Scholar] [CrossRef]
- Atkinson, T.H.; Foltz, J.L.; Wilkinson, R.C.; Mizell, R.F. Granulate Ambrosia Beetle, Xylosandrus Crassiusculus (Motschulsky) (Insecta: Coleoptera: Curculionidae: Scolytinae). IFAS Extention 2011, 2011, 2–5. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Faccoli, M. Bark Beetles and Pinhole Borers (Curculionidae, Scolytinae, Platypodinae) Alien to Europe. Zookeys 2010, 56, 227–251. [Google Scholar] [CrossRef]
- Vega, F.; Infante, F.; Johnson, A. The Genus Hypothenemus, with Emphasis on H. Hampei, the Coffee Berry Borer. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier: Amsterdam, The Netherlands, 2015; pp. 427–497. [Google Scholar] [CrossRef]
- Macedo-Reis, L.; Novais, S.; Monteiro, G.; Flechtmann, C.; Neves, F. Spatio-Temporal Distribution of Bark and Ambrosia Beetles in a Brazilian Tropical Dry Forest. J. Insect Sci. 2016, 16, 48. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Lai, S.; Yin, T.; Ji, Y.; Wang, S.; Wang, J.; Hulcr, J. First Record of Euplatypus parallelus (Coleoptera: Curculionidae) in China. Fla. Entomol. 2018, 101, 141–143. [Google Scholar] [CrossRef]
- Tiago Neto, L.J.; Pereira, J.M.; Rodrigues, O.D.; Lima, N.L.; Froio, L.D.L.; Flechtmann, C.A.H. Diversity of Scolytinae, Platypodinae (Curculionidae) and Bostrichidae in Hevea brasiliensis (Willd. Ex A.Juss.) in the State of Goiás, Brazil. Cienc. Florest. 2022, 32, 493–503. [Google Scholar] [CrossRef]
- Beaver, R. The Invasive Neotropical Ambrosia Betle Euplatypus parallelus (Fabricus, 1801) in the Oriental Region and Its Pest Status (Coleoptera:Curculionidae, Platypodinae). Entomol. Mon. Mag. 2013, 149, 143–154. [Google Scholar]
- Tarno, H.; Setiawan, Y.; Rahardjo, B.T.; Wang, J. Evaluation of the Ambrosia Beetles Traps on Pterocarpus indicus in Indonesia. J. Biol. Divers. 2021, 22, 1332–1339. [Google Scholar] [CrossRef]
- Gümüş, M.; Ergün, A. Report of a Pest Risk Analysis for Platypus Parallelus (Fabricus, 1801) for Turkey. EPPO Bull. 2015, 45, 112–118. [Google Scholar] [CrossRef]
- Tarno, H.; Suprapto, H.; Himawan, T. New Record of the Ambrosia Beetle, Treptoplatypus micrurus Schedl. Attack on Sonokembang (Pterocarpus indicus Willd.) in Batu, Indonesia. Agrivita 2015, 37, 220–225. [Google Scholar]
- Hauptman, T.; Pavlin, R.; Grošelj, P.; Jurc, M. Distribution and abundance of the alien Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in different forest stands in central Slovenia. iForest 2019, 12, 451–458. [Google Scholar] [CrossRef]
- Galko, J.; Dzurenko, M.; Ranger, C.M.; Kulfan, J.; Kula, E.; Nikolov, C.; Zubrik, M.; Zach, P. Distribution, habitat preference, and management of the invasive ambrosia beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European forests with an emphasis on the West Carpathians. Forests 2019, 10, 10. [Google Scholar] [CrossRef]
- Flerchinger, G.; Reba, M.; Link, T.; Marks, D. Modeling Temperature and Humidity Profiles within Forest Canopies. Agric. For. Meteorol. 2015, 213, 251–262. [Google Scholar] [CrossRef]
- Holusa, J.; Fiala, T.; Foit, J. Ambrosia Beetles Prefer Closed Canopies: A Case Study in Oak Forests in Central Europe. Forests 2021, 12, 1223. [Google Scholar] [CrossRef]
- Marini, L.; Haack, R.A.; Rabaglia, R.J.; Petrucco, T.E.; Battisti, A.; Faccoli, M. Exploring associations between international trade and environmental factors with establishment patterns of alien Scolytinae. Biol. Invasions 2011, 13, 2275–2288. [Google Scholar]
- Reich, R.M.; Lundquist, J.E.; Acciavatti, R. Influence of climatic conditions and elevation on the spatial distribution and abundance of Trypodendron ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in Alaska. For. Sci. 2014, 60, 308–316. [Google Scholar] [CrossRef]
- Rassati, D.; Faccoli, M.; Battisti, A.; Marini, L. Habitat and climatic preferences drive invasions of non-native ambrosia beetles in deciduous temperate forests. Biol. Invasions 2016, 18, 2809–2821. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Dempsey, T.D.; Klepzig, K.D.; Ayres, M.P. Temperature-dependent effects on mutualistic, antagonistic, and commensalistic interactions among insects, fungi and mites. Community Ecol. 2007, 8, 47–56. [Google Scholar]
- Rice, A.V.; Thormann, M.N.; Langor, D.W. Mountain pine beetle-associated blue stain fungi are differentially adapted to boreal temperatures. For. Pathol. 2008, 38, 113–123. [Google Scholar] [CrossRef]
- Reding, M.E.; Ranger, C.M.; Oliver, J.B.; Schultz, P.B. Monitoring attack and flight activity of Xylosandrus spp. (Coleoptera: Scolytinae Curculionidae:): The influence of temperature on activity. J. Econ. Entomol. 2013, 106, 1780–1787. [Google Scholar] [CrossRef]
- Menocal, O.; Kendra, P.E.; Padilla, A.; Chagas, P.C.; Chagas, E.A.; Crane, J.H.; Carrillo, D. Influence of Canopy Cover and Meteorological Factors on the Abundance of Bark and Ambrosia Beetles (Coleoptera: Curculionidae) in Avocado Orchards Affected by Laurel Wilt. Agronomy 2022, 12, 547. [Google Scholar] [CrossRef]
- Martínez, M.; Cognato, A.I.; Guachambala, M.; Boivin, T. Bark and Ambrosia Beetle (Coleoptera: Curculionidae: Scolytinae) Diversity in Natural and Plantation Forests in Ecuador. Environ. Entomol. 2019, 48, 603–613. [Google Scholar] [CrossRef]
- Coulson, R.N.; Witter, J.A. Forest Entomology: Ecology and Management; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Raffa, K.F.; Grégoire, J.C.; Lindgren, B.S. Natural History and Ecology of Bark Beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F., Hofstetter, R., Eds.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Sanguansub, S.; Buranapanichpan, S.; Beaver, R.; Saowaphak, T.; Tanaka, N.; Kamata, N. Influence of Seasonality and Climate on Captures of Wood-Boring Coleoptera (Bostrichidae and Curculionidae (Scolytinae and Platypodinae) Using Ethanol-Baited Traps in a Seasonal Tropical Forest of Northern Thailand. J. For. Res. 2020, 25, 223–231. [Google Scholar] [CrossRef]
- Hindmarch, T.; Reid, M. Thinning of Mature Lodgepole Pine Stands Increases Scolytid Bark Beetle Abundance and Diversity. Can. J. For. Res. 2001, 31, 1502–1512. [Google Scholar] [CrossRef]
- Dieters, M.; Newby, J.; Cramb, R.; Sexton, G.; McNamara, S.; Johnson, M.M.; Sakanphet, M.S.; Sodarak, M.H.; Kikeo, M.; Savathvong, M.S. Enhancing On-Farm Incomes through Improved Silvicultural Management of Teak in Luang Prabang Province of Lao PDR; ACIAR: Canberra, Australia, 2014; pp. 1–121.
- Tarno, H.; Suprapto, H.; Himawan, T. First Record of Ambrosia Beetle (Euplatypus paralellus Fabricius) Infestation on Sonokembang (Pterocarpus Indicus Willd.) from Malang Indonesia. Agrivita 2014, 36, 189–200. [Google Scholar] [CrossRef]
- Peltonen, M.; Heliövaara, K. Attack Density and Breeding Success of Bark Beetles (Coleoptera, Scolytidae) at Different Distances from Forest-Clearcut Edge. Agric. For. Entomol. 1999, 1, 237–242. [Google Scholar] [CrossRef]
- Sousa, E.; Inacio, M.L. New Aspects of Platypus cylindrus Fab. (Coleoptera: Platypodidae) Life History on Cork Oak Stands in Portugal. In Mediterranean Forest Ecosystems; Lieutier, F., Ghaioule, D., Eds.; INRA Editions: Paris, France, 2005; pp. 147–168. [Google Scholar]
- Ferreira, W.; Hepp, L.; Ligeiro, R.; Macedo, D.; Hughes, R.; Kaufmann, P. Partitioning Taxonomic Diversity of Aquatic Insect Assemblages and Functional Feeding Groups in Neotropical Savanna Headwater Streams. Ecol. Indic. 2017, 72, 365–373. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Roschewitz, I.; Thies, C.; Tscharntke, T. Differential Effects of Landscape and Management on Diversity and Density of Ground-Dwelling Farmland Spiders. J. Appl. Ecol. 2005, 42, 281–287. [Google Scholar] [CrossRef]
- Rassati, D.; Faccoli, M.; Haack, R.A.; Rabaglia, R.J.; Petrucco Toffolo, E.; Battisti, A.; Marini, L. Bark and Ambrosia Beetles Show Different Invasion Patterns in the USA. PLoS ONE 2016, 11, e0158519. [Google Scholar] [CrossRef]
- Hulcr, J.; Novotny, V.; Maurer, B.A.; Cognato, A.I. Low beta diversity of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) in lowland rainforests of Papua New Guinea. Oikos 2008, 117, 214–222. [Google Scholar] [CrossRef]
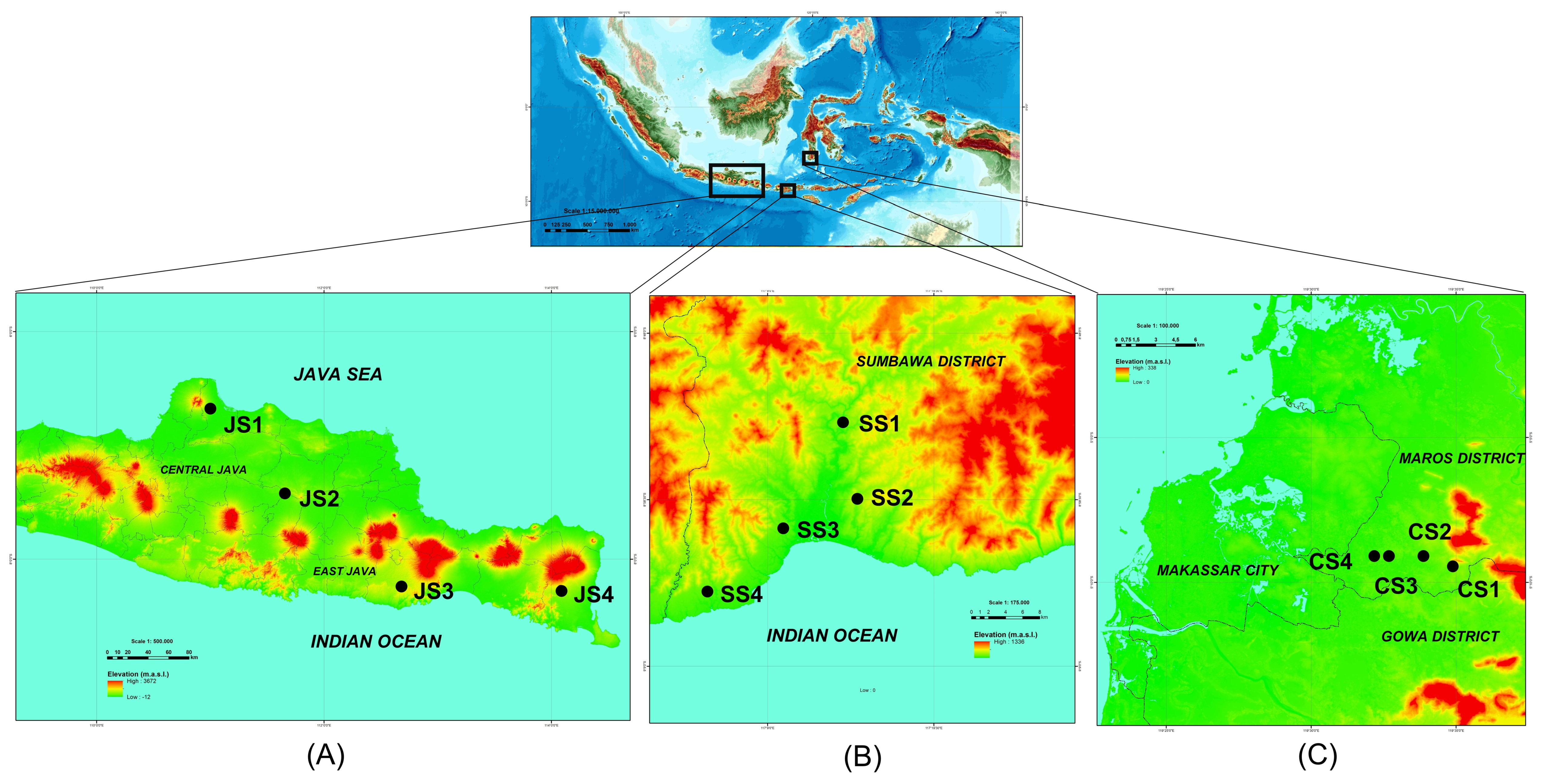



| Island and Sampled Site | Site Code | Stand Age (year) | Altitude (m.a.s.l.) | Mean Temperature (°C) | Mean Humidity (%) | Mean Rainfall (mm) | Teak Management | Longitude; Latitude |
|---|---|---|---|---|---|---|---|---|
| Java Island | ||||||||
| Pati District | JS1 | 13 | 136 | 27.55 | 80.63 | 6.83 | Un-thinned | 6°40′43.6″ S; 110°59′36.7″ E |
| Ngawi District | JS2 | 9 | 162 | 23.57 | 86.75 | 17.37 | Un-thinned | 7°24′47.2″ S; 111°39′34.3″ E |
| Malang District | JS3 | 11 | 378 | 26.46 | 83.77 | 8.12 | Thinned | 8°14′05.9″ S; 112°41′09.0″ E |
| Banyuwangi District | JS4 | 6 | 261 | 28.51 | 79.30 | 4.63 | Thinned | 8°15′10.4″ S; 114°04′49.0″ E |
| Sumbawa Island | ||||||||
| Sumbawa District | SS1 | 5 | 184 | 26.38 | 87.81 | 7.27 | Un-thinned | 8°58′25.0″ S; 117°14′44.2″ E |
| Sumbawa District | SS2 | 3 | 176 | 27.26 | 83.44 | 4.29 | Thinned | 8°53′38.5″ S; 117°13′48.2″ E |
| Sumbawa District | SS3 | 4 | 78 | 26.38 | 87.81 | 7.27 | Un-thinned | 9°00′20.8″ S; 117°10′02.0″ E |
| Sumbawa District | SS4 | 5 | 83 | 27.26 | 83.44 | 4.29 | Un-thinned | 9°04′23.1″ S; 117°05′06.9″ E |
| Sulawesi Island | ||||||||
| Maros District | CS1 | 15 | 27 | 26.5 | 83.75 | 13.79 | Thinned | 5°09′30″ S; 119°34′54″ E |
| Maros District | CS2 | 11 | 21 | 26.5 | 83.75 | 13.79 | Thinned | 5°09′11″ S; 119°33′51″ E |
| Maros District | CS3 | 4 | 11 | 26.5 | 83.75 | 13.79 | Un-thinned | 5°09′09″ S; 119°32′36″ E |
| Maros District | CS4 | 8 | 8 | 26.5 | 83.75 | 13.79 | Un-thinned | 5°09′08″ S; 119°32′15″ E |
| Subfamily and Species | No. of Individual | Total | Occ. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Java Island | Sumbawa Island | Sulawesi Island | ||||||||||||
| JS1 | JS2 | JS3 | JS4 | SS1 | SS2 | SS3 | SS4 | CS1 | CS2 | CS3 | CS4 | |||
| Platypodinae | ||||||||||||||
| Euplatypus parallelus | 4 | 3 | 0 | 16 | 5 | 0 | 3 | 0 | 47 | 16 | 2 | 6 | 102 | 1.66 |
| Scolytinae | ||||||||||||||
| Xylosandrus crassiusculus | 38 | 84 | 1045 | 440 | 45 | 7 | 29 | 8 | 0 | 4 | 1 | 0 | 1701 | 27.64 |
| Xyleborus affinis | 61 | 108 | 14 | 0 | 81 | 44 | 31 | 69 | 1171 | 1151 | 141 | 32 | 2903 | 47.17 |
| Xylosandrus morigerus | 12 | 39 | 32 | 0 | 7 | 0 | 8 | 6 | 2 | 2 | 0 | 0 | 108 | 1.75 |
| Xylosandrus compactus | 2 | 6 | 0 | 87 | 24 | 27 | 24 | 40 | 0 | 0 | 0 | 0 | 210 | 3.41 |
| Premnobius cavipennis | 0 | 1 | 0 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0.80 |
| Eccoptopterus spinosus | 4 | 11 | 0 | 15 | 4 | 0 | 6 | 0 | 1 | 2 | 0 | 0 | 43 | 0.70 |
| Hypothenemus sp. | 17 | 19 | 11 | 162 | 23 | 68 | 6 | 50 | 123 | 77 | 72 | 131 | 759 | 12.33 |
| Xyleborinus exiguus | 0 | 0 | 19 | 47 | 0 | 0 | 4 | 0 | 9 | 37 | 1 | 1 | 118 | 1.92 |
| Euwallacea fornicatus | 0 | 0 | 0 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 84 | 1.36 |
| Arixyleborus sp. | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 31 | 0.50 |
| Diuncus haberkorni | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 |
| Coptodryas sp. | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0.10 |
| Xylosandrus eupatorii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 1 | 3 | 18 | 29 | 0.47 |
| Ambrosiodmus asperatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 0.06 |
| Diuncus quadrispinulosus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.02 |
| Xyleborinus andrewesi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 5 | 0.08 |
| Total | 138 | 271 | 1152 | 899 | 195 | 146 | 111 | 173 | 1360 | 1296 | 224 | 189 | 6154 | 100.00 |
| Model 1 | Model 2 * | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Incidence Rate Ratios | CI | p | Incidence Rate Ratios | CI | p | Incidence Rate Ratios | CI | p |
| (Intercept) | 0.00 | 0.00–0.01 | 0.013 | 0.00 | 0.00–0.01 | <0.001 | 0.00 | 0.00–0.00 | <0.001 |
| Stand age | 1.04 | 1.00–1.08 | 0.027 | 1.04 | 1.00–1.08 | 0.025 | 1.10 | 1.07–1.14 | <0.001 |
| Temperature | 1.86 | 1.30–2.69 | 0.001 | 1.50 | 1.23–1.84 | <0.001 | 2.43 | 1.96–3.05 | <0.001 |
| Humidity | 1.08 | 0.97–1.20 | 0.152 | - | - | - | - | - | - |
| Rainfall | 1.24 | 1.14–1.34 | <0.001 | 1.20 | 1.12–1.29 | <0.001 | 1.35 | 1.25–1.46 | <0.001 |
| Altitude | 1.00 | 1.00–1.01 | <0.001 | 1.00 | 1.00–1.00 | <0.001 | 1.01 | 1.00–1.01 | <0.001 |
| Teak management | 3.38 | 2.52–4.54 | <0.001 | 3.51 | 2.65–4.70 | <0.001 | - | - | - |
| Observations | 240 | 240 | 240 | ||||||
| R2 Nagelkerke | 1.000 | ||||||||
| Model 1 | Model 2 | Model 3 * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Incidence Rate Ratios | CI | p | Incidence Rate Ratios | CI | p | Incidence Rate Ratios | CI | p |
| (Intercept) | 0.09 | 0.00–148.65 | 0.530 | 0.05 | 0.00–58.05 | 0.416 | 0.01 | 0.00–0.07 | <0.001 |
| Stand age | 1.00 | 0.98–1.01 | 0.603 | - | - | - | - | - | - |
| Temperature | 1.17 | 1.01–1.35 | 0.043 | 1.18 | 1.03–1.35 | 0.019 | 1.22 | 1.13–1.31 | <0.001 |
| Humidity | 0.98 | 0.94–1.03 | 0.486 | 0.99 | 0.95–1.03 | 0.565 | - | - | - |
| Rainfall | 1.05 | 1.02–1.09 | 0.002 | 1.05 | 1.02–1.08 | <0.001 | 1.06 | 1.03–1.08 | <0.001 |
| Altitude | 1.00 | 1.00–1.00 | <0.001 | 1.00 | 1.00–1.00 | <0.001 | 1.00 | 1.00–1.00 | <0.001 |
| Teak management | 1.03 | 0.89–1.18 | 0.728 | - | - | - | - | - | - |
| Observations | 240 | 240 | 240 | ||||||
| R2 Nagelkerke | 0.200 | 0.199 | 0.198 | ||||||
| Scale | β-Diversity | Absolute Value | Percentage of Overall β |
|---|---|---|---|
| Landscape | |||
| Java | β-sor | 0.47 | 100.00 |
| β-sim | 0.44 | 92.97 | |
| β-nes | 0.03 | 7.03 | |
| Sumbawa | β-sor | 0.35 | 100.00 |
| β-sim | 0.06 | 16.00 | |
| β-nes | 0.29 | 83.04 | |
| Sulawesi | β-sor | 0.38 | 100.00 |
| β-sim | 0.17 | 44.44 | |
| β-nes | 0.21 | 55.56 | |
| Regional | β-sor | 0.71 | 100.00 |
| β-sim | 0.61 | 85.00 | |
| β-nes | 0.10 | 14.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarno, H.; Setiawan, Y.; Wang, J.; Ito, S.; Mario, M.B.; Kurahman, T.; Suraningwulan, M.; Amaliah, A.A.; Sari, N.I.; Achmad, M.A. Partitioning of Ambrosia Beetle Diversity on Teak Plantations in Java, Sumbawa, and Sulawesi Islands. Forests 2022, 13, 2111. https://doi.org/10.3390/f13122111
Tarno H, Setiawan Y, Wang J, Ito S, Mario MB, Kurahman T, Suraningwulan M, Amaliah AA, Sari NI, Achmad MA. Partitioning of Ambrosia Beetle Diversity on Teak Plantations in Java, Sumbawa, and Sulawesi Islands. Forests. 2022; 13(12):2111. https://doi.org/10.3390/f13122111
Chicago/Turabian StyleTarno, Hagus, Yogo Setiawan, Jianguo Wang, Satoshi Ito, M. Bayu Mario, Taufik Kurahman, Medyanti Suraningwulan, Asri Ainun Amaliah, Nur Indah Sari, and Muhammad Alifuddin Achmad. 2022. "Partitioning of Ambrosia Beetle Diversity on Teak Plantations in Java, Sumbawa, and Sulawesi Islands" Forests 13, no. 12: 2111. https://doi.org/10.3390/f13122111
APA StyleTarno, H., Setiawan, Y., Wang, J., Ito, S., Mario, M. B., Kurahman, T., Suraningwulan, M., Amaliah, A. A., Sari, N. I., & Achmad, M. A. (2022). Partitioning of Ambrosia Beetle Diversity on Teak Plantations in Java, Sumbawa, and Sulawesi Islands. Forests, 13(12), 2111. https://doi.org/10.3390/f13122111





