The Development of Forest Genetic Breeding and the Application of Genome Selection and CRISPR/Cas9 in Forest Breeding
Abstract
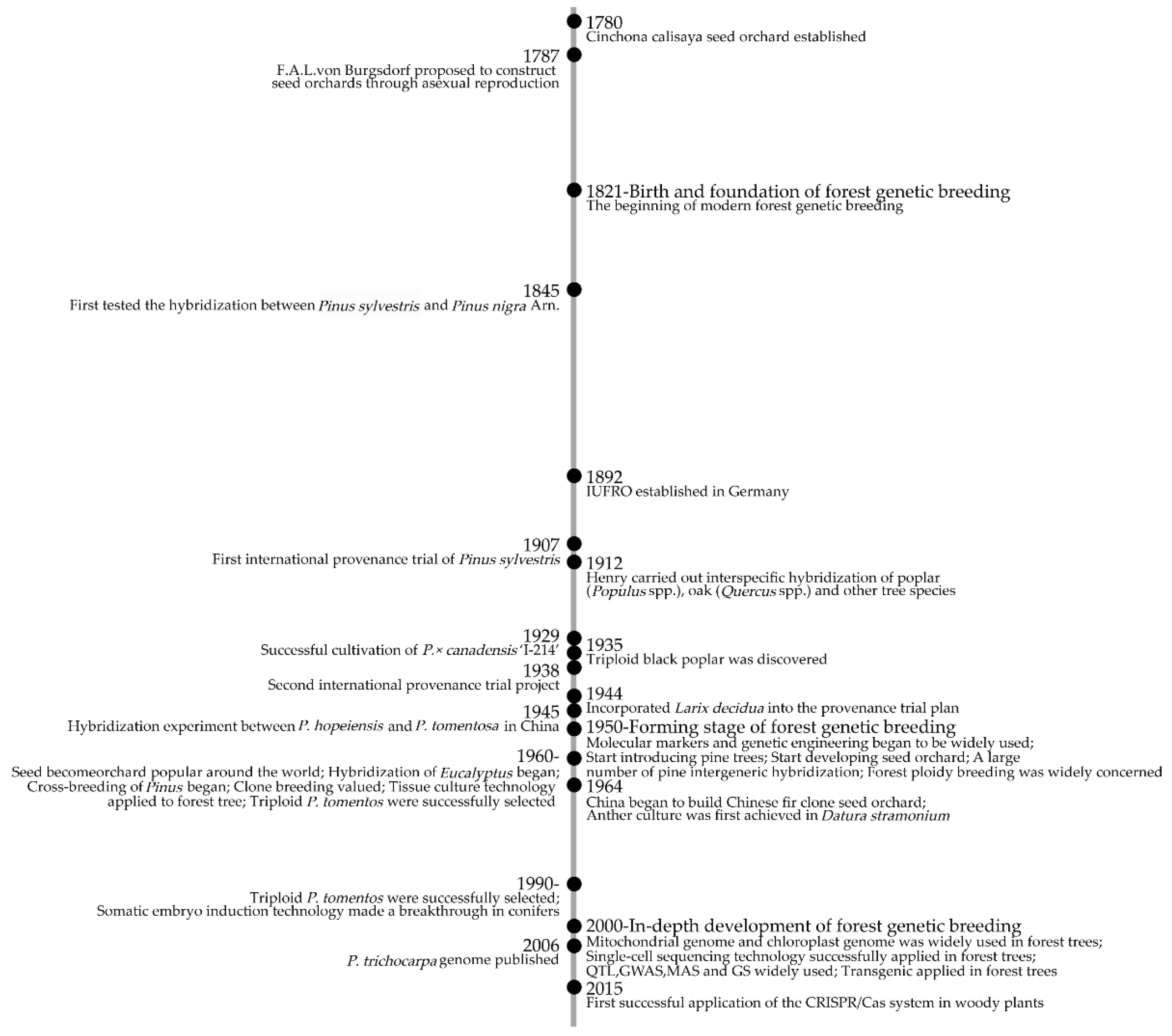
:1. Introduction
2. Birth and Foundation of Forest Genetic Breeding
3. Forming Stage of Forest Genetic Breeding
4. In-Depth Development of Forest Genetic Breeding
5. Application of Genome Selection in Forest Genetic Breeding
6. Application of CRISPR/Cas9 Technology in Forest Tree Breeding
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langlet, O. Two hundred years genecology. Taxon 1971, 20, 653–721. [Google Scholar] [CrossRef]
- Adams, W.T. Forest Genetics; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Langlet, O. A cline or not a cline? A question of scots pine. Silvae Genet. 1959, 8, 13–22. [Google Scholar]
- Huxley, J. Clines: An auxiliary taxonomic principle. Nature 1938, 142, 219–220. [Google Scholar] [CrossRef]
- Veen, B. General remarks on provenance research in forestry. Euphytica 1954, 3, 89–96. [Google Scholar] [CrossRef]
- Chen, X.Y.; Shen, X.H. Forest Breeding; Chinese Forestry Publishing House: Beijing, China, 2005. [Google Scholar]
- Lindgren, D.; Karlsson, B.; Andersson, B.; Prescher, F. The Swedish Seed Orchard Program for Scots pine and Norway spruce. In Proceedings of the Seed Orchard Conference, Umeå, Sweden, 26–28 September 2007; pp. 142–154. [Google Scholar]
- Toda, R. A brief review and conclusions of the discussion on seed orchards. Silvae Genet. 1963, 13, 1–4. [Google Scholar]
- Johnson, L.P.V. A descriptive list of natural and artificial interspecific hybrids in North American forest-tree genera. Can. Med. Assoc. J. 1929, 21, 441. [Google Scholar] [CrossRef]
- Zobel, B.; Talbert, J. Applied Forest Tree Improvement; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Du, C.; Teissier, E. Breeding strategies with poplars in Europe. For. Ecol. Manag. 1984, 8, 23–39. [Google Scholar] [CrossRef]
- White, T.L.; Adams, W.T.; Neale, D.B. Forest Genetics; Cabi: Wallingford, UK, 2007. [Google Scholar]
- Nilsson-Ehle, H.; Tidn, S.P. Production of forest trees with increased chromosome number and increased timber yield. Sven. Papp Tidn 1938, 2, 299–330. [Google Scholar]
- Ye, P.Z.; Gao, Z.D.; Chen, Y.M.; Chen, J.; Wang, Z.R.; Chen, Y.W.; Wu, H.J.; Wu, S.P. Summary report on distant hybrid breeding experiment among poplar species. J. Nanjing For. Univ. (Nat. Sci. Ed.) 1959, 2, 25–42. [Google Scholar] [CrossRef]
- Bergstr, M.I. On the progeny of diploid × triploid Populus tremula: With special reference to the occurrence of tetraploidy. Hereditas 1940, 26, 191–201. [Google Scholar] [CrossRef]
- Wasshausen, L.D. Eucalypt Domestication and Breeding; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Peter, F. Growing exotic forests. For. Sci. 1988, 1, 252–253. [Google Scholar] [CrossRef]
- Ruotsalainen, S. Increased forest production through forest tree breeding. Scand. J. For. Res. 2014, 29, 333–344. [Google Scholar] [CrossRef]
- Balocchi, C.E. Realised versus operational gain: The role of propagation strategies. FRI Bull. 1997, 253–255. [Google Scholar]
- Kleinschmit, J. A programme for large-scale cutting propagation of Norway spruce. N. Z. J. For. Sci. 1974, 4, 359–366. [Google Scholar]
- Shen, X.H. Seed Orchard Technique; Beijing Science and Technology Press: Beijing, China, 1992. [Google Scholar]
- Li, B.; Mckeand, S.; Weir, R. Tree improvement and sustainable forestry—Impact of two cycles of loblolly pine breeding in the USA. Int. J. For. Genet. 1999, 64, 229–234. [Google Scholar]
- Williams, C.G. Accelerated short-term genetic testing for loblolly-pine families. Can. J. For. Res. 1988, 18, 1085–1089. [Google Scholar] [CrossRef]
- Hasan, O.; Reid, J.B. Reid reduction of generation time in Eucalyptus globulus. Plant Growth Regul. 1995, 17, 53–60. [Google Scholar] [CrossRef]
- Greenwood, M.S.; Adams, G.W.; Gillespie, M. Stimulation of flowering by grafted black spruce and white spruce: A comparative study of the effects of gibberellin A4/7, cultural treatments, and environment. Can. J. For. Res. 1991, 21, 395–400. [Google Scholar] [CrossRef]
- Gardner, R.A.W.; Little, K.M.; Arbuthnot, A. Wood and fibre productivity potential of promising new eucalypt species for coastal Zululand, South Africa. Aust. For. 2007, 70, 37–47. [Google Scholar] [CrossRef]
- Xu, W.Y.; Huang, D.S.; Ma, C.G.; Tu, H.F.; Liu, Q.Z. Sexual hybridization of poplar. For. Sci. 1956, 2, 215–225. [Google Scholar]
- Potts, B.M.; Dungey, H.S. Interspecific hybridization of Eucalyptus: Key issues for breeders and geneticists. New For. 2004, 27, 115–138. [Google Scholar] [CrossRef]
- Brisola, S.H. Demarco stem anatomical analysis of Eucalyptus grandis, E. urophylla and E. grandis x urophylla: Wood development and its industrial importance. Sci. For. 2011, 39, 317–330. [Google Scholar]
- Denison, N.P.; Quaile, D.R. The applied clonal eucalypt programme in mondi forests. J. S. Afr. For. Assoc. 1987, 142, 60–67. [Google Scholar] [CrossRef]
- Weisgerber, H. 25 years of forest tree breeding in Hesse. Allg. Forstz. 1980, 66, 665. [Google Scholar]
- Suryanarayana, N. Cytomorphological studies in triploid mulberry evolved by diploidization of female gamete cells. Cytologia 1989, 54, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Mashkina, O.S. Method of obtaining diploid pollen of woody species. Lesoved 1989, 1, 19–25. [Google Scholar]
- Einspahr, D.W. Production and utilization of triploid hybrid aspen. Iowa State J. Res. 1984, 58, 401–409. [Google Scholar]
- Kang, X. Research progress and prospect of triploid breeding of forest trees. Sci. Sin. Vitae 2003, 25, 5. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, F.L. Studies on chromosome doubling and triploid breeding of white poplar (I)—The techniques of the pollen chromosome doubling. J. Beijing For. Univ. 1992, 14, 52–58. [Google Scholar]
- Kang, X.Y. Research progress and prospect of triploid breeding of forest trees. Sci. Sin. Vitae 2020, 50, 136–143. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, Z.T.; Lin, H.B.; Kang, X.Y. Studies on allotriploid breeding of Populus tomentosa B301 clones. Sci. Silvae Sin. 1995, 31, 499–505. [Google Scholar]
- Guha, S.; Maheshwari, S.C. In vitro production of embryos from anthers of Datura. Nature 1964, 204, 497. [Google Scholar] [CrossRef]
- Talbert, C.B.; Ritchie, G.A.; Gupta, P. Conifer Vegetative Propagation: An Overview from a Commercialization Perspective; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Jishen, S. The challenges to forestry biotechnology and tree breeding in the 21th century. Rev. China Agric. Sci. Technol. 2000, 24, 36–41. [Google Scholar] [CrossRef]
- Hakman, I.; Arnold, S.V. Plantlet regeneration through somatic embryogenesis in Picea abies (Norway Spruce). J. Plant Physiol. 1985, 121, 149–158. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Cyr, D.; Polonenko, D.R. Somatic embryogenesis tissue culture for the propagation of conifer seedlings: A technology comes of age. Tree Plant. Notes 1996, 47, 48–57. [Google Scholar]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- Nystedt, B.; Street, N.R.; Wetterbom, A.; Zuccolo, A.; Lin, Y.; Scofield, D.G.; Vezzi, F.; Delhomme, N.; Giacomello, S.; Alexeyenko, A. The Norway spruce genome sequence and conifer genome evolution. Nature 2013, 497, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Myburg, A.A.; Grattapaglia, D.; Tuskan, G.A.; Hellsten, U.; Hayes, R.D.; Grimwood, J.; Jenkins, J.; Lindquist, E.; Tice, H.; Bauer, D.; et al. The genome of Eucalyptus grandis. Nature 2014, 510, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Salojärvi, J.; Smolander, O.; Nieminen, K.; Rajaraman, S.; Safronov, O.; Safdari, P.; Lamminmäki, A.; Immanen, J.; Lan, T.; Tanskanen, J. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017, 49, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Wuyun, T.N.; Wang, L.; Liu, H.; Wang, X.; Zhang, L.; Bennetzen, J.L.; Li, T.; Yang, L.; Liu, P.; Du, L. The hardy rubber tree genome provides insights into the evolution of polyisoprene biosynthesis. Mol. Plant 2018, 3, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Hao, Z.; Guang, X.; Zhao, C.; Wang, P.; Xue, L.; Zhu, Q.; Yang, L.; Sheng, Y.; Zhou, Y. Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Nat. Plants 2019, 5, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; Li, J.; Bo, W.; Yang, W.; Zuccolo, A.; Giacomello, S.; Chen, X.; Han, F.; Yang, J.; Song, Y. The Chinese pine genome and methylome unveil key features of conifer evolution. Cell 2022, 185, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, L.; Xu, C.; Qi, L.; Wu, Z.; Li, J.; Chen, H.; Wu, Y.; Fu, T.; Zhu, H.; et al. Chromosome-scale genome assembly of areca palm (Areca catechu). Mol. Ecol. Resour. 2021, 21, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Park, S.; Kim, S. Primers for complete chloroplast genome sequencing in Magnolia. Appl. Plant Sci. 2019, 7, e11286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Liu, Y.; Zhang, Q.; Nie, X.; Sun, Y.; Zhang, Z.; Li, H.; Fang, K.; Wang, G.; Huang, H.; et al. Hybrid de novo genome assembly of Chinese chestnut (Castanea mollissima). GigaScience 2019, 8, giz112. [Google Scholar] [CrossRef]
- Rahman, A.Y.; Usharraj, A.O.; Misra, B.B.; Thottathil, G.P.; Jayasekaran, K.; Feng, Y.; Hou, S.; Ong, S.Y.; Ng, F.L.; Lee, L.S.; et al. Draft genome sequence of the rubber tree Hevea brasiliensis. BMC Genom. 2013, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Kui, L.; Majeed, A.; Dong, Y. Reference-grade Taxus genome unleashes its pharmacological potential. Trends Plant Sci. 2022, 1, 10–12. [Google Scholar] [CrossRef]
- Liu, W.; Guo, W.; Chen, S.; Xu, H.; Zhao, Y.; Chen, S.; You, X. A high-quality reference genome sequence and genetic transformation system of Aralia elata. Front. Plant Sci. 2022, 13, 538. [Google Scholar] [CrossRef]
- Ithnin, M.; Vu, W.T.; Shin, M.; Suryawanshi, V.; Sherbina, K.; Zolkafli, S.H.; Serdari, N.M.; Amiruddin, M.D.; Abdullah, N.; Mustaffa, S.; et al. Genomic diversity and genome-wide association analysis related to yield and fatty acid composition of wild American oil palm. Plant Sci. 2021, 304, 110731. [Google Scholar] [CrossRef]
- Mishra, B.; Ulaszewski, B.; Meger, J.; Aury, J.; Bodénès, C.; Lesur-Kupin, I.; Pfenninger, M.; Da Silva, C.; Gupta, D.K.; Guichoux, E.; et al. A chromosome-level genome assembly of the European beech (Fagus sylvatica) reveals anomalies for organelle DNA integration, repeat content and distribution of SNPs. Front. Genet. 2022, 12, 691058. [Google Scholar] [CrossRef] [PubMed]
- Mosca, E.; Cruz, F.; Gómez-Garrido, J.; Bianco, L.; Rellstab, C.; Brodbeck, S.; Csilléry, K.; Fady, B.; Fladung, M.; Fussi, B.; et al. A reference genome sequence for the European silver fir (Abies alba Mill.): A community-generated genomic resource. G3 Genes Genomes Genet. 2019, 9, 2039–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, D.; Wu, T.; Xiao, L.; Ma, T.; Fang, W.; Dong, R.; Cao, F. Chromosomal-level assembly of Juglans sigillata genome using Nanopore, BioNano, and Hi-C analysis. GigaScience 2020, 9, giaa006. [Google Scholar] [CrossRef] [PubMed]
- Sowjanya, P.R.; Shilpa, P.; Patil, G.P.; Babu, D.K.; Sharma, J.; Sangnure, V.R.; Mundewadikar, D.M.; Natarajan, P.; Marathe, A.R.; Reddy, U.K.; et al. Reference quality genome sequence of Indian pomegranate cv. ‘Bhagawa’ (Punica granatum L.). Front. Plant Sci. 2022, 13, 2678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, H.; Li, Y.; Chen, Y.; Liu, G.; Ye, M.; Yu, C.; Lian, B.; Zhong, F.; Jiang, Y.; et al. Genome sequencing and phylogenetic analysis of allotetraploid Salix matsudana Koidz. Hortic. Res. 2020, 7, 201. [Google Scholar] [CrossRef]
- Babu, B.K.; Rani, K.L.M.; Sahu, S.; Mathur, R.K.; Kumar, P.N.; Ravichandran, G.; Anitha, P.; Bhagya, H.P. Development and validation of whole genome-wide and genic microsatellite markers in oil palm (Elaeis guineensis Jacq.): First microsatellite database (OpSatdb). Sci. Rep. 2019, 9, 1899. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lee, M.; Wan, Z.Y.; Ye, B.; Alfiko, Y.; Rahmadsyah, R.; Purwantomo, S.; Song, Z.; Suwanto, A.; Yue, G.H. Chromosome-level reference genome provides insights into divergence and stress adaptation of the African oil palm. Genom. Proteom. Bioinform. 2022, in press. [CrossRef]
- Gagalova, K.K.; Warren, R.L.; Coombe, L.; Wong, J.; Nip, K.M.; Yuen, M.M.S.; Whitehill, J.G.A.; Celedon, J.M.; Ritland, C.; Taylor, G.A.; et al. Spruce giga-genomes: Structurally similar yet distinctive with differentially expanding gene families and rapidly evolving genes. Plant J. 2022, 111, 1469–1485. [Google Scholar] [CrossRef]
- Shen, T.; Qi, H.; Luan, X.; Xu, W.; Yu, F.; Zhong, Y.; Xu, M. The chromosome-level genome sequence of the camphor tree provides insights into Lauraceae evolution and terpene biosynthesis. Plant Biotechnol. J. 2022, 20, 244–246. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Xi, H.; Kwon, W.; Kwon, M. The complete chloroplast and mitochondrial genomes of Hyunsasi tree, Populus alba × Populus glandulosa (Salicaceae). Mitochondr. DNA Part B Resour. 2019, 4, 2521–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Li, W.; Xie, X.; Zhang, X.; Sun, C.; Xu, J.; Lu, Y. The complete chloroplast genome of Hemiptelea davidii (Ulmaceae), the only species of the genus Hemiptelea. Mitochondr. DNA Part B Resour. 2019, 4, 229–230. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Li, X. The complete chloroplast genome sequence of Podocarpus macrophyllus (Podocarpaceae) and phylogenetic analysis. Mitochondr. DNA Part B Resour. 2022, 7, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Dong, X.; Yang, J.; Guo, C.; Cao, B.; Guo, Y.; Hu, G. Complete chloroplast genome of Clethra fargesii Franch., an original sympetalous plant from central China: Comparative analysis, adaptive evolution, and phylogenetic relationships. Forests 2021, 12, 441. [Google Scholar] [CrossRef]
- Shao, Y.; Shi, Z.; Wang, Z.; Wang, W.; Chen, Y.; Wen, Q. The complete chloroplast genome of Abies ernestii Rehder (Pinaceae) and its phylogenetic implications. Mitochondr. DNA Part B Resour. 2022, 7, 1497–1503. [Google Scholar] [CrossRef]
- Ren, R.; Li, X. Characterization of the complete chloroplast genome of Salix linearistipularis (Franch.) Hao 1936. Mitochondr. DNA Part B Resour. 2021, 6, 2764–2766. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.C.; Yang, T.J. The complete chloroplast genome sequence of Dendropanax morbifera (Leveille). Mitochondr. DNA Part A 2016, 27, 2923–2924. [Google Scholar] [CrossRef]
- Deng, X.; Jiang, Z.; Huang, J.; Zhang, X. Characterization of the complete chloroplast genome of sugar maple (Acer saccharum). Mitochondr. DNA Part B Resour. 2019, 5, 21–22. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; Shu, X.; Zhang, M.; Wang, T.; Zhang, F.; Wang, N.; Wang, Z. Characterization of the complete chloroplast genome of Populus deltoides Zhonglin 2025. Mitochondr. DNA Part B Resour. 2020, 5, 3723–3724. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Wang, J.; Shen, H.; Ai, B.; Gao, W.; Zhang, C.; Fei, Q.; Yuan, D.; Wu, Z.; et al. Chloroplast genomes in Populus (Salicaceae): Comparisons from an intensively sampled genus reveal dynamic patterns of evolution. Sci. Rep. 2021, 11, 9471. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.; Li, Y.; Zhang, L. Complete chloroplast genome of Engelhardtia fenzlii (Juglandaceae). Mitochondr. DNA Part B Resour. 2021, 6, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.G.; Zhang, J.G.; Zhang, J.P. The complete chloroplast genome of Catalpa. Mitochondr. DNA Part B 2020, 5, 1800–1801. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, Z.; Yan, T. The complete chloroplast genome sequence of Elaeocarpus decipiens (Elaeocarpaceae). Mitochondr. DNA Part B Resour. 2021, 6, 3130–3131. [Google Scholar] [CrossRef] [PubMed]
- Jackman, S.D.; Warren, R.L.; Gibb, E.A.; Vandervalk, B.P.; Mohamadi, H.; Chu, J.; Raymond, A.; Pleasance, S.; Coope, R.; Wildung, M.R.; et al. Organellar genomes of white spruce (Picea glauca): Assembly and annotation. Genome Biol. Evol. 2016, 8, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Yang, J.; Hu, H.; Xia, R.; Li, Y.; Su, J.; Li, Q.; Liu, Y.; Qin, L. A high level of chloroplast genome sequence variability in the Sawtooth Oak Quercus acutissima. Int. J. Biol. Macromol. 2020, 152, 340–348. [Google Scholar] [CrossRef]
- Conde, D.; Triozzi, P.M.; Pereira, W.J.; Schmidt, H.W.; Balmant, K.M.; Knaack, S.A.; Redondo-López, A.; Roy, S.; Dervinis, C.; Kirst, M. Single-nuclei transcriptome analysis of the shoot apex vascular system differentiation in Populus. Development 2022, 149, dev200632. [Google Scholar] [CrossRef]
- Turner, G.W.; Parrish, A.N.; Zager, J.J.; Fischedick, J.T.; Lange, B.M. Assessment of flux through oleoresin biosynthesis in epithelial cells of loblolly pine resin ducts. J. Exp. Bot. 2019, 70, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dai, X.; Huang, X.; Xu, M.; Wang, Q.; Yan, X.; Sederoff, R.R.; Li, Q. Single-cell RNA sequencing reveals a high-resolution cell atlas of xylem in Populus. J. Integr. Plant Biol. 2021, 63, 1906–1921. [Google Scholar] [CrossRef]
- Xie, J.; Li, M.; Zeng, J.; Li, X.; Zhang, D. Single-cell RNA sequencing profiles of stem-differentiating xylem in poplar. Plant Biotechnol. J. 2022, 20, 417–419. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Peng, A.; Cui, J.; Zhao, M.; Pan, Y.; Zhang, M.; Tian, K.; Schwab, W.; Song, C. Single-cell transcriptome atlas reveals developmental trajectories and a novel metabolic pathway of catechin esters in tea leaves. Plant Biotechnol. J. 2022, 20, 2089–2106. [Google Scholar] [CrossRef]
- Tang, W.; Tang, A.Y. Biological significance of RNA-seq and single-cell genomic research in woody plants. J. For. Res. 2019, 30, 1555–1568. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Bae, E.; Lee, H.; Ko, J. Current understanding of the genetics and molecular mechanisms regulating wood formation in plants. Genes 2022, 13, 1181. [Google Scholar] [CrossRef] [PubMed]
- Sulis, D.B.; Wang, J.P. Regulation of lignin biosynthesis by post-translational protein modifications. Front. Plant Sci. 2020, 11, 914. [Google Scholar] [CrossRef]
- Mohamed, I.A.A.; Shalby, N.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Salah, A.; Rady, M.M.; Jie, K.; Wang, B.; et al. RNA-seq analysis revealed key genes associated with salt tolerance in rapeseed germination through carbohydrate metabolism, hormone, and MAPK signaling pathways. Ind. Crop. Prod. 2022, 176, 114262. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, D.; Lu, W.; Zong, X.; Niu, Y.; Guo, X.; Ma, Y.; Qiang, W.; Su, H.; Zhang, S.; et al. PeMPK7 is induced in an ROS-dependent manner and confers poplar para-hydroxybenzoic acid stress resistance through the removal of ROS. Ind. Crop. Prod. 2022, 182, 114861. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Miguel, C.; Chaves, I.; António, C. Mass spectrometry-based forest tree metabolomics. Mass Spectrom. Rev. 2021, 40, 126–157. [Google Scholar] [CrossRef]
- Hill, R.A.; Wong Bajracharya, J.; Anwar, S.; Coles, D.; Wang, M.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Martin, F.; Anderson, I.C.; et al. Abscisic acid supports colonization of Eucalyptus grandis roots by the mutualistic ectomycorrhizal fungus Pisolithus microcarpus. New Phytol. 2022, 233, 966–982. [Google Scholar] [CrossRef]
- Vergara, A.; Haas, J.C.; Aro, T.; Stachula, P.; Street, N.R.; Hurry, V. Norway spruce deploys tissue-specific responses during acclimation to cold. Plant Cell Environ. 2022, 45, 427–445. [Google Scholar] [CrossRef]
- Modesto, I.; Inácio, V.; Van de Peer, Y.; Miguel, C.M. MicroRNA-mediated post-transcriptional regulation of Pinus pinaster response and resistance to pinewood nematode. Sci. Rep. 2022, 12, 5160. [Google Scholar] [CrossRef]
- Haas, J.C.; Vergara, A.; Serrano, A.R.; Mishra, S.; Hurry, V.; Street, N.R. Candidate regulators and target genes of drought stress in needles and roots of Norway spruce. Tree Physiol. 2021, 41, 1230–1246. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Q.; Gao, J.; Wu, Y.; Feng, Z.; Huang, J.; Zou, H.; Zhu, X.; Chen, Y.; Yu, C.; et al. Identify of fast-growing related genes especially in height growth by combining QTL analysis and transcriptome in Salix matsudana (Koidz). Front. Genet. 2021, 12, 596749. [Google Scholar] [CrossRef] [PubMed]
- Laoué, J.; Depardieu, C.; Gérardi, S.; Lamothe, M.; Bomal, C.; Azaiez, A.; Gros-Louis, M.; Laroche, J.; Boyle, B.; Hammerbacher, A.; et al. Combining QTL mapping and transcriptomics to decipher the genetic architecture of phenolic compounds metabolism in the conifer White Spruce. Front. Plant Sci. 2021, 12, 675108. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.; Butler, J.B.; Ammitzboll, H.; Weller, J.L.; Vaillancourt, R.E.; Potts, B.M. Genetic control of the operculum and capsule morphology of Eucalyptus globulus. Ann. Bot. 2022, 130, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Bentley, N.; Grauke, L.J.; Ruhlman, E.; Klein, R.R.; Kubenka, K.; Wang, X.; Klein, P. Linkage mapping and QTL analysis of pecan (Carya illinoinensis) full-siblings using genotyping-by-sequencing. Tree Genet. Genomes 2020, 16, 83. [Google Scholar] [CrossRef]
- Hernández, M.A.; Butler, J.B.; Ammitzboll, H.; Freeman, J.S.; Reilly-Wapstra, J.O.; Vaillancourt, R.E.; Potts, B.M. Genetic variation in fire recovery and other fire-related traits in a global eucalypt species. Tree Genet. Genomes 2022, 18, 42. [Google Scholar] [CrossRef]
- Freeman, J.S.; Slavov, G.T.; Butler, J.B.; Frickey, T.; Graham, N.J.; Klápště, J.; Lee, J.; Telfer, E.J.; Wilcox, P.; Dungey, H.S. High density linkage maps, genetic architecture, and genomic prediction of growth and wood properties in Pinus radiata. BMC Genom. 2022, 23, 731. [Google Scholar] [CrossRef]
- Du, C.; Sun, P.; Cheng, X.; Zhang, L.; Wang, L.; Hu, J. QTL mapping of drought-related traits in the hybrids of Populus deltoides ‘Danhong’×Populus simonii ‘Tongliao1’. BMC Plant Biol. 2022, 22, 238. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Z.; Cheng, Y.; Li, S.; Shao, P.; Yu, Q.; Wang, J.; Xu, G.; Zhang, X.; Liu, J.; et al. Comparative population genomics dissects the genetic basis of seven domestication traits in jujube. Hortic. Res. 2020, 7, 89. [Google Scholar] [CrossRef]
- Santini, F.; Kefauver, S.C.; Araus, J.L.; Resco, D.D.V.; Martin, G.S.; Grivet, D.; Voltas, J. Bridging the genotype-phenotype gap for a Mediterranean pine by semi-automatic crown identification and multispectral imagery. New Phytol. 2021, 229, 245–258. [Google Scholar] [CrossRef]
- Mähler, N.; Schiffthaler, B.; Robinson, K.M.; Terebieniec, B.K.; Vučak, M.; Mannapperuma, C.; Bailey, M.E.S.; Jansson, S.; Hvidsten, T.R.; Street, N.R. Leaf shape in Populus tremula is a complex, omnigenic trait. Ecol. Evol. 2020, 10, 11922–11940. [Google Scholar] [CrossRef]
- Sawitri; Tani, N.; Na’iem, M.; Widiyatno; Indrioko, S.; Uchiyama, K.; Suwa, R.; Ng, K.K.S.; Lee, S.L.; Tsumura, Y. Potential of genome-wide association studies and genomic selection to improve productivity and quality of commercial timber species in tropical rainforest, a case study of Shorea platyclados. Forests 2020, 11, 239. [Google Scholar] [CrossRef] [Green Version]
- Francisco, F.R.; Aono, A.H.; Da Silva, C.C.; Gonçalves, P.S.; Scaloppi, E.J., Jr.; Le Guen, V.; Fritsche-Neto, R.; Souza, L.M.; Souza, A.P.D. Unravelling rubber tree growth by integrating GWAS and biological network-based approaches. Front. Plant Sci. 2021, 12, 2719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zan, Y.; Zhou, L.; Karlsson, B.; Tuominen, H.; García-Gil, M.R.; Wu, H.X. Genetic architecture behind developmental and seasonal control of tree growth and wood properties in Norway spruce. Front. Plant Sci. 2022, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zan, Y.; Milesi, P.; Zhou, L.; Chen, J.; Li, L.; Cui, B.; Niu, S.; Westin, J.; Karlsson, B.; et al. Leveraging breeding programs and genomic data in Norway spruce (Picea abies L. Karst) for GWAS analysis. Genome Biol. 2021, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Ingvarsson, P.K. Integrating genome-wide association mapping of additive and dominance genetic effects to improve genomic prediction accuracy in Eucalyptus. Plant Genome 2022, 15, e20208. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jiang, Y.; Yang, Z.; Chen, S.; El-Kassaby, Y.A.; Chen, H. Marker-assisted selection in C. oleifera hybrid population. Silvae Genet. 2020, 69, 63–72. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Ueno, S.; Hasegawa, Y.; Tadama, T.; Watanabe, M.; Saito, R.; Hirayama, S.; Iwai, J.; Konno, Y. Marker-assisted selection of trees with male sterility 1 in Cryptomeria japonica D. Don. Forests 2020, 11, 734. [Google Scholar] [CrossRef]
- Liu, J.; Sniezko, R.A.; Sissons, R.; Krakowski, J.; Alger, G.; Schoettle, A.W.; Williams, H.; Zamany, A.; Zitomer, R.A.; Kegley, A. Association mapping and development of marker-assisted selection tools for the resistance to White Pine blister rust in the Alberta limber Pine populations. Front. Plant Sci. 2020, 11, 557672. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Tsuruta, M.; Ueno, S.; Kawakami, K.; Bamba, Y.; Moriguchi, Y. An improved and simplified propagation system for Pollen-Free Sugi (Cryptomeria japonica) via somatic embryogenesis. Front. Plant Sci. 2022, 13, 825340. [Google Scholar] [CrossRef]
- Cao, S.; Duan, H.; Sun, Y.; Hu, R.; Wu, B.; Lin, J.; Deng, W.; Li, Y.; Zheng, H. Genome-wide association study with growth-related traits and secondary metabolite contents in red- and white-heart Chinese fir. Front. Plant Sci. 2022, 13, 922007. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Ueno, S.; Hirayama, S.; Kaneeda, T.; Moriguchi, Y. Somatic embryogenesis and plant regeneration from Sugi (Japanese Cedar, Cryptomeria japonica D. Don, Cupressaceae) seed families by marker assisted selection for the male sterility allele ms1. Plants 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.G.; Lebedeva, T.N.; Chernodubov, A.I.; Shestibratov, K.A. Genomic selection for forest tree improvement: Methods, achievements and perspectives. Forests 2020, 11, 1190. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Silva-Junior, O.B.; Resende, R.T.; Cappa, E.P.; Müller, B.S.; Tan, B.; Isik, F.; Ratcliffe, B.; El-Kassaby, Y.A. Quantitative genetics and genomics converge to accelerate forest tree breeding. Front. Plant Sci. 2018, 9, 1693. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wang, C.; Xiao, D.; Liang, Y.; Wang, Y. Advances and perspectives of transgenic technology and biotechnological application in forest trees. Front. Plant Sci. 2021, 12, 786328. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tang, A.Y. Transgenic woody plants for biofuel. J. For. Res. 2014, 25, 225–236. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Oguchi, T.; Akatsuka, N.; Matsunaga, E.; Kawaoka, A.; Yamada, A.; Ozeki, Y.; Watanabe, K.N.; Kikuchi, A. Development and evaluation of novel salt-tolerant Eucalyptus trees by molecular breeding using an RNA-Binding-Protein gene derived from common ice plant (Mesembryanthemum crystallinum L.). Plant Biotechnol. J. 2019, 17, 801–811. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Qin, R.; Zhao, Q.; Li, X.; Wang, C.; Cao, Q.; Zhang, H.; Zhang, L. Genetic transformation of LoHDZ2 and analysis of its function to enhance stress resistance in Larix olgensis. Sci. Rep. 2022, 12, 12831. [Google Scholar] [CrossRef]
- Jia, H.; Chen, J.; Zhang, L.; Zhang, L. The first report on transgenic hairy root induction from the stem of tung tree (Vernicia fordii). Plants 2022, 11, 1315. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zou, J.; Peng, R.; Yu, Q.; Liu, G.; Jiang, J. Selection of elite lines of BpGH3.5-transgenic Betula platyphylla using growth adaptability analysis. J. For. Res. 2022, 33, 1891–1901. [Google Scholar] [CrossRef]
- Ma, M.; Chen, X.; Yin, Y.; Fan, R.; Li, B.; Zhan, Y.; Zeng, F. DNA methylation silences exogenous gene expression in transgenic birch progeny. Front. Plant Sci. 2020, 11, 523748. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Dong, Y.; Li, Y.; Huang, Y.; Ren, M.; Yang, M.; Wang, J. Dynamic monitoring of the impact of insect-resistant transgenic poplar field stands on arthropod communities. For. Ecol. Manag. 2022, 505, 119921. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Y.; Ren, Y.; Wang, S.; Li, Y.; Du, K.; Lin, X.; Yang, M. Niches and seasonal changes, rather than transgenic events, affect the microbial community of Populus × euramericana ‘Neva’. Front. Microbiol. 2022, 12, 805261. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Dong, Y.; Yu, X.; Yao, L.; Li, D.; Wang, J.; Yang, M. Assessment of environmental microbial effects of insect-resistant transgenic Populus × euramericana cv. ‘74/76′ based on high-throughput sequencing. Acta Physiol. Plant. 2020, 42, 167. [Google Scholar] [CrossRef]
- Zuo, L.; Yang, R.; Zhen, Z.; Liu, J.; Huang, L.; Yang, M. A 5-year field study showed no apparent effect of the Bt transgenic 741 poplar on the arthropod community and soil bacterial diversity. Sci. Rep. 2018, 8, 1956. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Zhou, X.; Dong, Y.; Zhang, J.; Wang, J.; Yang, M. Exogenous gene expression and insect resistance in dual Bt toxin Populus × euramericana ‘Neva’ transgenic plants. Front. Plant Sci. 2021, 12, 660226. [Google Scholar] [CrossRef]
- Tran, N.T.; Oguchi, T.; Matsunaga, E.; Kawaoka, A.; Watanabe, K.N.; Kikuchi, A. Evaluation of potential impacts on biodiversity of the salt-tolerant transgenic Eucalyptus camaldulensis harboring an RNA chaperonic RNA-Binding-Protein gene derived from common ice plant. Transgen. Res. 2021, 30, 23–34. [Google Scholar] [CrossRef]
- Lu, M.Z.; Hu, J.J. Research and application of transgenic poplars in China. China For. Sci. Technol. 2006, 20, 1–4. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Li, Y.; Sun, L.; Chai, Y.; Chen, H.; Nie, H.; Huang, C. CRISPR/Cas9 technology and its utility for crop improvement. Int. J. Mol. Sci. 2022, 23, 10442. [Google Scholar] [CrossRef]
- Qiao, H.; Wu, J.; Zhang, X.; Luo, J.; Wang, H.; Ming, D. The advance of CRISPR-Cas9-based and NIR/CRISPR-Cas9-based imaging system. Front. Chem. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 2005, 151, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Šmarda, P.; Horová, L.; Knápek, O.; Dieck, H.; Dieck, M.; Ražná, K.; Hrubík, P.; Orlóci, L.; Papp, L.; Veselá, K. Multiple haploids, triploids, and tetraploids found in modern-day “living fossil” Ginkgo biloba. Hortic. Res. 2018, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nghiem, Q.C.; Griffin, A.R.; Harwood, C.E.; Harbard, J.L.; Le, S.; Price, A.; Koutoulis, A. Occurrence of polyploidy in populations of Acacia dealbata in south-eastern Tasmania and cytotypic variation in reproductive traits. Aust. J. Bot. 2018, 66, 152–160. [Google Scholar] [CrossRef]
- Mason, A.S. Polyploidy and Hybridization for Crop Improvement; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ahuja, M.R. Polyploidy in gymnosperms: Revisited. Silvae Genet. 2005, 54, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Anamthawat-Jónsson, K.; Karlsdóttir, L.; Thórsson, A.T.; Jóhannsson, M.H. Naturally occurring triploid birch hybrids from woodlands in Iceland are partially fertile. New For. 2021, 52, 659–678. [Google Scholar] [CrossRef]
- Farhat, P.; Takvorian, N.; Avramidou, M.; Garraud, L.; Adams, R.P.; Siljak-Yakovlev, S.; Kharrat, M.B.D.; Robert, T. First evidence for allotriploid hybrids between Juniperus thurifera and J. sabina in a sympatric area in the French Alps. Ann. For. Sci. 2020, 77, 93. [Google Scholar] [CrossRef]
- Šmíd, J.; Douda, J.; Krak, K.; Mandák, B. Analyses of hybrid viability across a hybrid zone between two alnus species using microsatellites and cpDNA markers. Genes 2020, 11, 770. [Google Scholar] [CrossRef]
- Vašková, D.; Kolarčik, V. Breeding systems in diploid and polyploid Hawthorns (Crataegus): Evidence from experimental pollinations of C. monogyna, C. subsphaerica, and natural hybrids. Forests 2019, 10, 1059. [Google Scholar] [CrossRef] [Green Version]
- Serapiglia, M.J.; Gouker, F.E.; Hart, J.F.; Unda, F.; Mansfield, S.D.; Stipanovic, A.J.; Smart, L.B. Ploidy level affects important biomass traits of novel shrub willow (Salix) hybrids. BioEnergy Res. 2015, 8, 259–269. [Google Scholar] [CrossRef]
- Nghiem, Q.C.; Griffin, A.R.; Harwood, C.E.; Harbard, J.L.; Huy, T.H.; Koutoulis, A. Seed development following reciprocal crossing among autotetraploid and diploid Acacia mangium and diploid A. auriculiformis. Aust. J. Bot. 2016, 64, 20–31. [Google Scholar] [CrossRef]
- Wang, L.; Tu, M.; Li, J.; Sun, S.; Song, H.; Xu, Z.; Chen, D.; Liang, G. Photosynthetic efficiency and glyco-metabolism changes in artificial triploid loquats contribute to heterosis manifestation. Int. J. Mol. Sci. 2022, 23, 11337. [Google Scholar] [CrossRef]
- Wang, J.; Kang, X.Y.; Li, D.L.; Chen, H.W.; Zhang, P.D. Induction of diploid eggs with colchicine during embryo sac development in Populus. Silvae Genet. 2010, 59, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Wang, P.; Yang, J.; Kang, X. Induction of unreduced megaspores in Eucommia ulmoides by high temperature treatment during megasporogenesis. Euphytica 2016, 212, 515–524. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, Z.; Xiong, T.; Lan, J.; Han, Q.; Li, Y.; Kang, X. Megaspore chromosome doubling in Eucalyptus urophylla ST Blake induced by colchicine treatment to produce triploids. Forests 2018, 9, 728. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.; Li, G.; Long, Q.; He, L.; Kang, X. Microsporogenesis and induction of unreduced pollen with high temperatures in rubber tree clone RRIM 600. Forests 2017, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Kang, X.Y.; Wang, S.D.; Zhang, Z.H.; Chen, H.W. Triploid induction in Populus alba x P. glandulosa by chromosome doubling of female gametes. Silvae Genet. 2008, 57, 37. [Google Scholar] [CrossRef]
- Carlson, C.H.; Choi, Y.; Chan, A.P.; Town, C.D.; Smart, L.B. Nonadditive gene expression is correlated with nonadditive phenotypic expression in interspecific triploid hybrids of willow (Salix spp.). G3 Genes Genomes Genet. 2022, 12, jkab436. [Google Scholar] [CrossRef]
- Thorsson, A.T.; Palsson, S.; Sigurgeirsson, A.; Anamthawat-Jonsson, K. Morphological variation among Betula nana (diploid), B. pubescens (tetraploid) and their triploid hybrids in Iceland. Ann. Bot. 2007, 99, 1183–1193. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, L.; Qi, Q.; Du, K.; Han, Q.; Kang, X. Study of variation in the growth, photosynthesis, and content of secondary metabolites in Eucommia triploids. Trees 2019, 33, 817–826. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Yang, W.; Li, X.; Hou, L.; Li, M.; Pang, X.; Li, Y. Hybrid triploid induced by megaspore chromosome doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and its characteristics. Forests 2021, 12, 112. [Google Scholar] [CrossRef]
- Li, H.; Gan, J.; Xiong, H.; Mao, X.; Li, S.; Zhang, H.; Hu, G.; Liu, C.; Fu, J. Production of triploid germplasm by inducing 2n pollen in Longan. Horticulturae 2022, 8, 437. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Solberg, T.R.; Shepherd, R.; Woolliams, J.A. A fast algorithm for BayesB type of prediction of genome-wide estimates of genetic value. Genet. Sel. Evol. 2009, 41, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.; de Koning, D. Does genomic selection have a future in plant breeding? Trends Biotechnol. 2013, 31, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; De Los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Endelman, J.B. Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant Genome 2011, 4, 255–258. [Google Scholar] [CrossRef]
- Wimmer, V.; Albrecht, T.; Auinger, H.; Schön, C. Synbreed: A framework for the analysis of genomic prediction data using R. Bioinformatics 2012, 28, 2086–2087. [Google Scholar] [CrossRef] [Green Version]
- Desta, Z.A.; Ortiz, R. Genomic selection: Genome-wide prediction in plant improvement. Trends Plant Sci. 2014, 19, 592–601. [Google Scholar] [CrossRef]
- Pérez, P.; de Los Campos, G. Genome-wide regression and prediction with the BGLR statistical package. Genetics 2014, 198, 483–495. [Google Scholar] [CrossRef]
- Wang, C.; Prakapenka, D.; Wang, S.; Pulugurta, S.; Runesha, H.B.; Da, Y. GVCBLUP: A computer package for genomic prediction and variance component estimation of additive and dominance effects. BMC Bioinform. 2014, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.A.; Buckler, E.S.; Zhang, Z. GAPIT: Genome association and prediction integrated tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias-Pazaran, G. Genome-assisted prediction of quantitative traits using the R package sommer. PLoS ONE 2016, 11, e156744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kainer, D.; Stone, E.A.; Padovan, A.; Foley, W.J.; Külheim, C. Accuracy of genomic prediction for foliar terpene traits in Eucalyptus polybractea. G3 Genes Genomes Genet. 2018, 8, 2573–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Fernando, R.; Cheng, H. Interpretable artificial neural networks incorporating Bayesian alphabet models for genome-wide prediction and association studies. G3 2021, 11, b228. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Bernardo, R. Genome wide selection in oil palm: Increasing selection gain per unit time and cost with small populations. Theor. Appl. Genet. 2008, 116, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Grattapaglia, D.; Resende, M.D. Genomic selection in forest tree breeding. Tree Genet. Genomes 2011, 7, 241–255. [Google Scholar] [CrossRef]
- Isik, F. Genomic selection in forest tree breeding: The concept and an outlook to the future. New For. 2014, 45, 379–401. [Google Scholar] [CrossRef]
- Kwong, Q.B.; Ong, A.L.; Teh, C.K.; Chew, F.T.; Tammi, M.; Mayes, S.; Kulaveerasingam, H.; Yeoh, S.H.; Harikrishna, J.A.; Appleton, D.R. Genomic selection in commercial perennial crops: Applicability and improvement in oil palm (Elaeis guineensis Jacq.). Sci. Rep. 2017, 7, 2872. [Google Scholar] [CrossRef] [Green Version]
- Cros, D.; Tchounke, B.; Nkague-Nkamba, L. Training genomic selection models across several breeding cycles increases genetic gain in oil palm in silico study. Mol. Breed. 2018, 38, 89. [Google Scholar] [CrossRef]
- Singh, R.; Ong-Abdullah, M.; Low, E.L.; Manaf, M.A.A.; Rosli, R.; Nookiah, R.; Ooi, L.C.; Ooi, S.E.; Chan, K.; Halim, M.A. Oil palm genome sequence reveals divergence of interfertile species in old and new worlds. Nature 2013, 500, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Grattapaglia, D.; Martins, G.S.; Ferreira, K.Z.; Sundberg, B.; Ingvarsson, P.K. Evaluating the accuracy of genomic prediction of growth and wood traits in two Eucalyptus species and their F1 hybrids. BMC Plant Biol. 2017, 17, 110. [Google Scholar] [CrossRef]
- Resende, M.D.; Resende, M.F., Jr.; Sansaloni, C.P.; Petroli, C.D.; Missiaggia, A.A.; Aguiar, A.M.; Abad, J.M.; Takahashi, E.K.; Rosado, A.M.; Faria, D.A. Genomic selection for growth and wood quality in Eucalyptus: Capturing the missing heritability and accelerating breeding for complex traits in forest trees. New Phytol. 2012, 194, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Cappa, E.P.; de Lima, B.M.; Da Silva, O.B., Jr.; Garcia, C.C.; Mansfield, S.D.; Grattapaglia, D. Improving genomic prediction of growth and wood traits in Eucalyptus using phenotypes from non-genotyped trees by single-step GBLUP. Plant Sci. 2019, 284, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Grattapaglia, D.; Wu, H.X.; Ingvarsson, P.K. Genomic relationships reveal significant dominance effects for growth in hybrid Eucalyptus. Plant Sci. 2018, 267, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.S.; Neves, L.G.; de Almeida Filho, J.E.; Resende, M.F.; Muñoz, P.R.; Dos Santos, P.E.; Kirst, M.; Grattapaglia, D. Genomic prediction in contrast to a genome-wide association study in explaining heritable variation of complex growth traits in breeding populations of Eucalyptus. BMC Genom. 2017, 18, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavamanikumar, S.; Arnold, R.J.; Luo, J.; Thumma, B.R. Genomic studies reveal substantial dominant effects and improved genomic predictions in an open-pollinated breeding population of Eucalyptus pellita. G3 Genes Genomes Genet. 2020, 10, 3751–3763. [Google Scholar] [CrossRef]
- Rambolarimanana, T.; Ramamonjisoa, L.; Verhaegen, D.; Leong Pock Tsy, J.; Jacquin, L.; Cao-Hamadou, T.; Makouanzi, G.; Bouvet, J. Performance of multi-trait genomic selection for Eucalyptus robusta breeding program. Tree Genet. Genomes 2018, 14, 71. [Google Scholar] [CrossRef]
- Suontama, M.; Klápště, J.; Telfer, E.; Graham, N.; Stovold, T.; Low, C.; McKinley, R.; Dungey, H. Efficiency of genomic prediction across two Eucalyptus nitens seed orchards with different selection histories. Heredity 2019, 122, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Klápště, J.; Dungey, H.S.; Telfer, E.J.; Suontama, M.; Graham, N.J.; Li, Y.; McKinley, R. Marker selection in multivariate genomic prediction improves accuracy of low heritability traits. Front. Genet. 2020, 11, 499094. [Google Scholar] [CrossRef]
- Cappa, E.P.; El-Kassaby, Y.A.; Muñoz, F.; Garcia, M.N.; Villalba, P.V.; Klápště, J.; Poltri, S.N.M. Genomic-based multiple-trait evaluation in Eucalyptus grandis using dominant DArT markers. Plant Sci. 2018, 271, 27–33. [Google Scholar] [CrossRef]
- Mphahlele, M.M.; Isik, F.; Mostert-O’Neill, M.M.; Reynolds, S.M.; Hodge, G.R.; Myburg, A.A. Expected benefits of genomic selection for growth and wood quality traits in Eucalyptus grandis. Tree Genet. Genomes 2020, 16, 49. [Google Scholar] [CrossRef]
- Ballesta, P.; Maldonado, C.; Pérez-Rodríguez, P.; Mora, F. SNP and haplotype-based genomic selection of quantitative traits in Eucalyptus globulus. Plants 2019, 8, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán, R.; Isik, F.; Zapata-Valenzuela, J.; Balocchi, C.; Valenzuela, S. Genomic predictions of breeding values in a cloned Eucalyptus globulus population in Chile. Tree Genet. Genomes 2017, 13, 74. [Google Scholar] [CrossRef]
- Jurcic, E.J.; Villalba, P.V.; Pathauer, P.S.; Palazzini, D.A.; Oberschelp, G.P.; Harrand, L.; Garcia, M.N.; Aguirre, N.C.; Acuña, C.V.; Martínez, M.C. Single-step genomic prediction of Eucalyptus dunnii using different identity-by-descent and identity-by-state relationship matrices. Heredity 2021, 127, 176–189. [Google Scholar] [CrossRef]
- Ballesta, P.; Bush, D.; Silva, F.F.; Mora, F. Genomic predictions using low-density SNP markers, pedigree and GWAS information: A case study with the non-model species Eucalyptus cladocalyx. Plants 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, H.; Nakamura, Y.; Kaneko, T.; Isobe, S.; Sakai, H.; Kato, T.; Hibino, T.; Sasamoto, S.; Watanabe, A.; Yamada, M. Survey of the genetic information carried in the genome of Eucalyptus camaldulensis. Plant Biotechnol. 2011, 28, 471–480. [Google Scholar] [CrossRef]
- Cros, D.; Mbo-Nkoulou, L.; Bell, J.M.; Oum, J.; Masson, A.; Soumahoro, M.; Tran, D.M.; Achour, Z.; Le Guen, V.; Clement-Demange, A. Within-family genomic selection in rubber tree (Hevea brasiliensis) increases genetic gain for rubber production. Ind. Crop. Prod. 2019, 138, 111464. [Google Scholar] [CrossRef]
- Souza, L.M.; Francisco, F.R.; Gonçalves, P.S.; Scaloppi, E.J., Jr.; Le Guen, V.; Fritsche-Neto, R.; Souza, A.P. Genomic selection in rubber tree breeding: A comparison of models and methods for managing G× E interactions. Front. Plant Sci. 2019, 10, 1353. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Yang, M.; Fang, Y.; Luo, Y.; Gao, S.; Xiao, X.; An, Z.; Zhou, B.; Zhang, B.; Tan, X. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat. Plants 2016, 2, 16073. [Google Scholar] [CrossRef] [Green Version]
- Lau, N.; Makita, Y.; Kawashima, M.; Taylor, T.D.; Kondo, S.; Othman, A.S.; Shu-Chien, A.C.; Matsui, M. The rubber tree genome shows expansion of gene family associated with rubber biosynthesis. Sci. Rep. 2016, 6, 28594. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Baison, J.; Pan, J.; Karlsson, B.; Andersson, B.; Westin, J.; García-Gil, M.R.; Wu, H.X. Accuracy of genomic selection for growth and wood quality traits in two control-pollinated progeny trials using exome capture as the genotyping platform in Norway spruce. BMC Genom. 2018, 19, 946. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.; Doerksen, T.; Clément, S.; MacKay, J.; Bousquet, J. Accuracy of genomic selection models in a large population of open-pollinated families in white spruce. Heredity 2014, 113, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulieu, J.; Doerksen, T.K.; MacKay, J.; Rainville, A.; Bousquet, J. Genomic selection accuracies within and between environments and small breeding groups in white spruce. BMC Genom. 2014, 15, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, P.; Nadeau, S.; Azaiez, A.; Gérardi, S.; Deslauriers, M.; Perron, M.; Isabel, N.; Beaulieu, J.; Bousquet, J. Genomic prediction for hastening and improving efficiency of forward selection in conifer polycross mating designs: An example from white spruce. Heredity 2020, 124, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulieu, J.; Nadeau, S.; Ding, C.; Celedon, J.M.; Azaiez, A.; Ritland, C.; Laverdière, J.P.; Deslauriers, M.; Adams, G.; Fullarton, M. Genomic selection for resistance to spruce budworm in white spruce and relationships with growth and wood quality traits. Evol. Appl. 2020, 13, 2704–2722. [Google Scholar] [CrossRef]
- Fuentes-Utrilla, P.; Goswami, C.; Cottrell, J.E.; Pong-Wong, R.; Law, A.; Hara, S.W.A.; Lee, S.J.; Woolliams, J.A. QTL analysis and genomic selection using RADseq derived markers in Sitka spruce: The potential utility of within family data. Tree Genet. Genomes 2017, 13, 33. [Google Scholar] [CrossRef]
- Lenz, P.R.; Nadeau, S.; Mottet, M.J.; Perron, M.; Isabel, N.; Beaulieu, J.; Bousquet, J. Multi-trait genomic selection for weevil resistance, growth, and wood quality in Norway spruce. Evol. Appl. 2020, 13, 76–94. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, B.; El-Dien, O.G.; Cappa, E.P.; Porth, I.; Klápště, J.; Chen, C.; El-Kassaby, Y.A. Single-step BLUP with varying genotyping effort in open-pollinated Picea glauca. G3 Genes Genomes Genet. 2017, 7, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Isik, F.; Bartholomé, J.; Farjat, A.; Chancerel, E.; Raffin, A.; Sanchez, L.; Plomion, C.; Bouffier, L. Genomic selection in maritime pine. Plant Sci. 2016, 242, 108–119. [Google Scholar] [CrossRef]
- Resende, M., Jr.; Muñoz, P.; Acosta, J.J.; Peter, G.F.; Davis, J.M.; Grattapaglia, D.; Resende, M.; Kirst, M. Accelerating the domestication of trees using genomic selection: Accuracy of prediction models across ages and environments. New Phytol. 2012, 193, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Resende, M., Jr.; Munoz, P.; Resende, M.D.; Garrick, D.J.; Fernando, R.L.; Davis, J.M.; Jokela, E.J.; Martin, T.A.; Peter, G.F.; Kirst, M. Accuracy of genomic selection methods in a standard data set of loblolly pine (Pinus taeda L.). Genetics 2012, 190, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Valenzuela, J.; Whetten, R.W.; Neale, D.; McKeand, S.; Isik, F. Genomic estimated breeding values using genomic relationship matrices in a cloned population of loblolly pine. G3 Genes Genomes Genet. 2013, 3, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukrainetz, N.K.; Mansfield, S.D. Assessing the sensitivities of genomic selection for growth and wood quality traits in lodgepole pine using Bayesian models. Tree Genet. Genomes 2020, 16, 14. [Google Scholar] [CrossRef]
- Li, Y.; Klápště, J.; Telfer, E.; Wilcox, P.; Graham, N.; Macdonald, L.; Dungey, H.S. Genomic selection for non-key traits in radiata pine when the documented pedigree is corrected using DNA marker information. BMC Genom. 2019, 20, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calleja-Rodriguez, A.; Pan, J.; Funda, T.; Chen, Z.; Baison, J.; Isik, F.; Abrahamsson, S.; Wu, H.X. Evaluation of the efficiency of genomic versus pedigree predictions for growth and wood quality traits in Scots pine. BMC Genom. 2020, 21, 796. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.C.; Balmant, K.M.; Resende, M.F., Jr.; Kirst, M.; de Los Campos, G. Accelerating forest tree breeding by integrating genomic selection and greenhouse phenotyping. Plant Genome 2020, 13, e20048. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, J.; Zhou, G.; Yue, Z.; Hu, Q.; Chen, Y.; Liu, B.; Qiu, Q.; Wang, Z.; Zhang, J. Genomic insights into salt adaptation in a desert poplar. Nat. Commun. 2013, 4, 2797. [Google Scholar] [CrossRef] [Green Version]
- Rincent, R.; Charpentier, J.; Faivre-Rampant, P.; Paux, E.; Le Gouis, J.; Bastien, C.; Segura, V. Phenomic selection is a low-cost and high-throughput method based on indirect predictions: Proof of concept on wheat and poplar. G3 Genes Genomes Genet. 2018, 8, 3961–3972. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, A.M.; Westbrook, J.W.; Zhang, Q.; Johnson, H.A.; Saielli, T.M.; Scrivani, J.A.; Fitzsimmons, S.F.; Collins, K.; Perkins, M.T.; Craddock, J.H. Frozen in time: Rangewide genomic diversity, structure, and demographic history of relict American chestnut populations. Mol. Ecol. 2022, 31, 4640–4655. [Google Scholar] [CrossRef]
- Thistlethwaite, F.R.; Ratcliffe, B.; Klápště, J.; Porth, I.; Chen, C.; Stoehr, M.U.; El-Kassaby, Y.A. Genomic prediction accuracies in space and time for height and wood density of Douglas-fir using exome capture as the genotyping platform. BMC Genom. 2017, 18, 930. [Google Scholar] [CrossRef] [Green Version]
- Street, N.R. Genomics of forest trees—ScienceDirect. Adv. Bot. Res. 2019, 89, 1–37. [Google Scholar] [CrossRef]
- Iwata, H.; Minamikawa, M.F.; Kajiya-Kanegae, H.; Ishimori, M.; Hayashi, T. Genomics-assisted breeding in fruit trees. Breed. Sci. 2016, 66, 100–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, B.J.; Pryce, J.; Chamberlain, A.J.; Bowman, P.J.; Goddard, M.E. Genetic architecture of complex traits and accuracy of genomic prediction: Coat colour, milk-fat percentage, and type in Holstein cattle as contrasting model traits. PLoS Genet. 2010, 6, e1001139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Invited review: Genomic selection in dairy cattle: Progress and challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhou, H.; Wu, Y.; Li, X.; Zhao, J.; Zuo, T.; Zhang, X.; Zhang, Y.; Liu, S.; Shen, Y. The impact of genetic relationship and linkage disequilibrium on genomic selection. PLoS ONE 2015, 10, e132379. [Google Scholar] [CrossRef] [Green Version]
- Habier, D.; Tetens, J.; Seefried, F.; Lichtner, P.; Thaller, G. The impact of genetic relationship information on genomic breeding values in German Holstein cattle. Genet. Sel. Evol. 2010, 42, 5. [Google Scholar] [CrossRef]
- Ma, P.; Huang, J.; Gong, W.; Li, X.; Gao, H.; Zhang, Q.; Ding, X.; Wang, C. The impact of genomic relatedness between populations on the genomic estimated breeding values. J. Anim. Sci. Biotechnol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Pliura, A.; Zhang, S.Y.; Bousquet, J.; MacKay, J. Age trends in genotypic variation of wood density and its intra-ring components in young poplar hybrid crosses. Ann. For. Sci. 2006, 63, 673–685. [Google Scholar] [CrossRef]
- Osorio, L.F.; White, T.L.; Huber, D.A. Age trends of heritabilities and genotype-by-environment interactions for growth traits and wood density from clonal trials of Eucalyptus grandis Hill ex Maiden. Silvae Genet. 2001, 50, 108–116. [Google Scholar]
- Dhillon, G.P.S.; Singh, A.; Sidhu, D.S. Variation, inheritance and correlation of growth characteristics of Populus deltoides Bartr. at various ages in the central-plain region of Punjab, India. For. Stud. China 2010, 12, 126–130. [Google Scholar] [CrossRef]
- Ahmar, S.; Ballesta, P.; Ali, M.; Mora-Poblete, F. Achievements and challenges of genomics-assisted breeding in forest trees: From marker-assisted selection to genome editing. Int. J. Mol. Sci. 2021, 22, 10583. [Google Scholar] [CrossRef]
- Khan, S.H. Genome-editing technologies: Concept, pros, and cons of various genome-editing techniques and bioethical concerns for clinical application. Mol. Ther.-Nucl. Acids 2019, 16, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, N. Targeted genome editing of sweet orange using Cas9/sgRNA. PLoS ONE 2014, 9, e93806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; LeBlanc, C.; Irish, V.F.; Jacob, Y. Rapid and efficient CRISPR/Cas9 gene editing in Citrus using the YAO promoter. Plant Cell Rep. 2017, 36, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Orbovic, V.; Jones, J.B.; Wang, N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sg RNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4: DCsLOB1.3 infection. Plant Biotechnol. J. 2016, 14, 1291–1301. [Google Scholar] [CrossRef]
- Zhou, X.; Jacobs, T.B.; Xue, L.; Harding, S.A.; Tsai, C. Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial Populus reveals 4-coumarate: CoA ligase specificity and redundancy. New Phytol. 2015, 208, 298–301. [Google Scholar] [CrossRef]
- Fan, D.; Liu, T.; Li, C.; Jiao, B.; Li, S.; Hou, Y.; Luo, K. Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Sci. Rep. 2015, 5, 12217. [Google Scholar] [CrossRef] [Green Version]
- Odipio, J.; Alicai, T.; Ingelbrecht, I.; Nusinow, D.A.; Bart, R.; Taylor, N.J. Efficient CRISPR/Cas9 genome editing of phytoene desaturase in cassava. Front. Plant Sci. 2017, 8, 1780. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. CRISPR–Cas9 strikes out in cassava. Nat. Biotechnol. 2019, 37, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhang, L.; Fu, Q.; Xu, Z. Identification and expression analysis of cytokinin metabolic genes IPTs, CYP735A and CKXs in the biofuel plant Jatropha curcas. PeerJ 2018, 6, e4812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.W.; Zhang, Y.; Zhang, R. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Xin, S.; Dai, X.; Yang, X.; Huang, H.; Hua, Y. Efficient genome editing of rubber tree (Hevea brasiliensis) protoplasts using CRISPR/Cas9 ribonucleoproteins. Ind. Crop. Prod. 2020, 146, 112146. [Google Scholar] [CrossRef]
- Ye, S.; Chen, G.; Kohnen, M.V.; Wang, W.; Cai, C.; Ding, W.; Wu, C.; Gu, L.; Zheng, Y.; Ma, X. Robust CRISPR/Cas9 mediated genome editing and its application in manipulating plant height in the first generation of hexaploid Ma bamboo (Dendrocalamus latiflorus Munro). Plant Biotechnol. J. 2020, 18, 1501. [Google Scholar] [CrossRef]
- Bruegmann, T.; Deecke, K.; Fladung, M. Evaluating the Efficiency of gRNAs in CRISPR/Cas9 mediated genome editing in Poplars. Int. J. Mol. Sci. 2019, 20, 3623. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, Y.; Wang, X.; Han, X.; An, Y.; Lin, S.; Shen, C.; Wen, J.; Liu, C.; Yin, W. Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020, 227, 407–426. [Google Scholar] [CrossRef]
- Tang, X.; Wang, C.; Chai, G.; Wang, D.; Xu, H.; Liu, Y.; He, G.; Liu, S.; Zhang, Y.; Kong, Y. Ubiquitinated DA1 negatively regulates vascular cambium activity through modulating the stability of WOX4 in Populus. Plant Cell 2022, 34, 3364–3382. [Google Scholar] [CrossRef]
- Ma, J.; Wan, D.; Duan, B.; Bai, X.; Bai, Q.; Chen, N.; Ma, T. Genome sequence and genetic transformation of a widely distributed and cultivated poplar. Plant Biotechnol. J. 2019, 17, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Li, S.; Miao, D.; Huang, S.; Guo, B.; Li, S.; An, X. Investigation of PtSGT1 and PtSGT4 function in cellulose biosynthesis in Populus tomentosa using CRISPR/Cas9 technology. Int. J. Mol. Sci. 2021, 22, 13200. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Yu, H.; Qin, S.; Li, Y.; Alam, A.; Xu, C.; Fan, D.; Zhang, Q.; Wang, Y.; Zhu, W. Brassinosteroid overproduction improves lignocellulose quantity and quality to maximize bioethanol yield under green-like biomass process in transgenic poplar. Biotechnol. Biofuels 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhao, X.; Ran, L.; Li, C.; Fan, D.; Luo, K. PtoMYB156 is involved in negative regulation of phenylpropanoid metabolism and secondary cell wall biosynthesis during wood formation in poplar. Sci. Rep. 2017, 7, srep41209. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Fu, X.; Liu, R.; Guo, L.; Ran, L.; Li, C.; Tian, Q.; Jiao, B.; Wang, B.; Luo, K. PtoMYB170 positively regulates lignin deposition during wood formation in poplar and confers drought tolerance in transgenic Arabidopsis. Tree Physiol. 2017, 37, 1713–1726. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Li, C.; Ma, X.; Luo, K. PtrMYB57 contributes to the negative regulation of anthocyanin and proanthocyanidin biosynthesis in poplar. Plant Cell Rep. 2017, 36, 1263–1276. [Google Scholar] [CrossRef]
- Fan, D.; Wang, X.; Tang, X.; Ye, X.; Ren, S.; Wang, D.; Luo, K. Histone H3K9 demethylase JMJ25 epigenetically modulates anthocyanin biosynthesis in poplar. Plant J. 2018, 96, 1121–1136. [Google Scholar] [CrossRef] [Green Version]
- Jiao, B.; Zhao, X.; Lu, W.; Guo, L.; Luo, K. The R2R3 MYB transcription factor MYB189 negatively regulates secondary cell wall biosynthesis in Populus. Tree Physiol. 2019, 39, 1187–1200. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Xu, D.; Yang, C.; Li, C.; Luo, K. Molecular cloning and characterization of a brassinosteriod biosynthesis-related gene PtoDWF4 from Populus tomentosa. Tree Physiol. 2018, 38, 1424–1436. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, L.; Ma, X.; Zhao, X.; Jiao, B.; Li, C.; Luo, K. The WRKY transcription factors PtrWRKY18 and PtrWRKY35 promote Melampsora resistance in Populus. Tree Physiol. 2017, 37, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Zhang, Y.; Li, Q.; Liu, S.; He, F.; An, Y.; Zhou, Y.; Liu, C.; Yin, W.; Xia, X. PdGNC confers drought tolerance by mediating stomatal closure resulting from NO and H2O2 production via the direct regulation of PdHXK1 expression in Populus. New Phytol. 2021, 230, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Elorriaga, E.; Klocko, A.L.; Ma, C.; Strauss, S.H. Variation in mutation spectra among CRISPR/Cas9 mutagenized poplars. Front. Plant Sci. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhr, M.; Paulat, M.; Awwanah, M.; Brinkkötter, M.; Teichmann, T. CRISPR/Cas9-mediated knockout of Populus BRANCHED1 and BRANCHED2 orthologs reveals a major function in bud outgrowth control. Tree Physiol. 2018, 38, 1588–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Sanchez, J.M.; Triozzi, P.M.; Alique, D.; Geng, F.; Gao, M.; Jaeger, K.E.; Wigge, P.A.; Allona, I.; Perales, M. LHY2 integrates night-length information to determine timing of poplar photoperiodic growth. Curr. Biol. 2019, 29, 2402–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; An, Y.; Zhou, Y.; Liu, C.; Yin, W.; Xia, X. Comparative transcriptome analyses define genes and gene modules differing between two Populus genotypes with contrasting stem growth rates. Biotechnol. Biofuels 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.M.; Schmidt, H.W.; Dervinis, C.; Kirst, M.; Conde, D. Simple, efficient and open-source CRISPR/Cas9 strategy for multi-site genome editing in Populus tremula× alba. Tree Physiol. 2021, 41, 2216–2227. [Google Scholar] [CrossRef]
- Bewg, W.P.; Harding, S.A.; Engle, N.L.; Vaidya, B.N.; Zhou, R.; Reeves, J.; Horn, T.W.; Joshee, N.; Jenkins, J.W.; Shu, S. Multiplex knockout of trichome-regulating MYB duplicates in hybrid poplar using a single gRNA. Plant Physiol. 2022, 189, 516–526. [Google Scholar] [CrossRef]
- Maurya, J.P.; Singh, R.K.; Miskolczi, P.C.; Prasad, A.N.; Jonsson, K.; Wu, F.; Bhalerao, R.P. Branching regulator BRC1 mediates photoperiodic control of seasonal growth in hybrid aspen. Curr. Biol. 2020, 30, 122–126. [Google Scholar] [CrossRef]
- Nayeri, S.; Baghban Kohnehrouz, B.; Ahmadikhah, A.; Mahna, N. CRISPR/Cas9-mediated P-CR domain-specific engineering of CESA4 heterodimerization capacity alters cell wall architecture and improves saccharification efficiency in poplar. Plant Biotechnol. J. 2022, 20, 1197–1212. [Google Scholar] [CrossRef]
- Jiang, Y.; Tong, S.; Chen, N.; Liu, B.; Bai, Q.; Chen, Y.; Bi, H.; Zhang, Z.; Lou, S.; Tang, H. The PalWRKY77 transcription factor negatively regulates salt tolerance and abscisic acid signaling in Populus. Plant J. 2021, 105, 1258–1273. [Google Scholar] [CrossRef]
- Jang, H.; Bae, E.; Kim, M.; Park, S.; Choi, N.; Pyo, S.; Lee, C.; Jeong, H.; Lee, H.; Choi, Y. CRISPR-Knockout of CSE gene improves saccharification efficiency by reducing lignin content in hybrid poplar. Int. J. Mol. Sci. 2021, 22, 9750. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Choi, H.; Choi, J.W.; Lee, H.; Kim, S.; Ko, J.; Choi, Y. Efficient knockout of the phytoene desaturase gene in a hybrid poplar (Populus alba× Populus glandulosa) using the CRISPR/Cas9 system with a single gRNA. Transgenic Res. 2021, 30, 837–849. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhou, Y.; Han, X.; Shen, C.; Wang, S.; Liu, C.; Yin, W.; Xia, X. The GATA transcription factor GNC plays an important role in photosynthesis and growth in poplar. J. Exp. Bot. 2020, 71, 1969–1984. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Fan, C.; Li, X.; Li, Y.; Hu, J.; Li, C.; Luo, K. LACCASE14 is required for the deposition of guaiacyl lignin and affects cell wall digestibility in poplar. Biotechnol. Biofuels 2020, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Leite Montalvão, A.P.; Kersten, B.; Kim, G.; Fladung, M.; Müller, N.A. ARR17 controls dioecy in Populus by repressing B-class MADS-box gene expression. Philos. Trans. R. Soc. B 2022, 377, 20210217. [Google Scholar] [CrossRef]
- de Vries, L.; Brouckaert, M.; Chanoca, A.; Kim, H.; Regner, M.R.; Timokhin, V.I.; Sun, Y.; De Meester, B.; Van Doorsselaere, J.; Goeminne, G. CRISPR-Cas9 editing of CAFFEOYL SHIKIMATE ESTERASE 1 and 2 shows their importance and partial redundancy in lignification in Populus tremula× P. alba. Plant Biotechnol. J. 2021, 19, 2221–2234. [Google Scholar] [CrossRef]
- Takata, N.; Awano, T.; Nakata, M.T.; Sano, Y.; Sakamoto, S.; Mitsuda, N.; Taniguchi, T. Populus NST/SND orthologs are key regulators of secondary cell wall formation in wood fibers, phloem fibers and xylem ray parenchyma cells. Tree Physiol. 2019, 39, 514–525. [Google Scholar] [CrossRef]
- Azeez, A.; Busov, V. CRISPR/Cas9-mediated single and biallelic knockout of poplar STERILE APETALA (PopSAP) leads to complete reproductive sterility. Plant Biotechnol. J. 2021, 19, 23. [Google Scholar] [CrossRef]
- Harding, S.A.; Tuma, T.T.; Aulakh, K.; Ortega, M.A.; Ci, D.; Ou, Y.; Tsai, C. Tonoplast sucrose trafficking modulates starch utilization and water deficit behavior in poplar leaves. Plant Cell Physiol. 2022, 63, 1117–1129. [Google Scholar] [CrossRef]
- André, D.; Marcon, A.; Lee, K.C.; Goretti, D.; Zhang, B.; Delhomme, N.; Schmid, M.; Nilsson, O. FLOWERING LOCUS T paralogs control the annual growth cycle in Populus trees. Curr. Biol. 2022, 13, 2988–2996. [Google Scholar] [CrossRef]
- Wang, W.; Talide, L.; Viljamaa, S.; Niittylä, T. Aspen growth is not limited by starch reserves. Curr. Biol. 2022, 32, 3619–3627. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Yu, J.; Wang, Z.; Sun, Y.; Li, S.; Lin, Y.J.; Chiang, V.L.; Li, W.; Wang, J.P. Transcriptional reprogramming of xylem cell wall biosynthesis in tension wood. Plant Physiol. 2021, 186, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Y.J.; Wang, P.; Zhang, B.; Li, M.; Chen, S.; Shi, R.; Tunlaya-Anukit, S.; Liu, X.; Wang, Z. The AREB1 transcription factor influences histone acetylation to regulate drought responses and tolerance in Populus trichocarpa. Plant Cell 2019, 31, 663–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Mao, Y.; Guo, Y.; Gao, J.; Liu, X.; Li, S.; Lin, Y.J.; Chen, H.; Wang, J.P.; Chiang, V.L. MYB transcription factor161 mediates feedback regulation of secondary wall-associated NAC-Domain1 family genes for wood formation. Plant Physiol. 2020, 184, 1389–1406. [Google Scholar] [CrossRef]
- Liu, H.; Gao, J.; Sun, J.; Li, S.; Zhang, B.; Wang, Z.; Zhou, C.; Sulis, D.B.; Wang, J.P.; Chiang, V.L. Dimerization of PtrMYB074 and PtrWRKY19 mediates transcriptional activation of PtrbHLH186 for secondary xylem development in Populus trichocarpa. New Phytol. 2022, 234, 918. [Google Scholar] [CrossRef]
- Xu, W.; Cheng, H.; Zhu, S.; Cheng, J.; Ji, H.; Zhang, B.; Cao, S.; Wang, C.; Tong, G.; Zhen, C. Functional understanding of secondary cell wall cellulose synthases in Populus trichocarpa via the Cas9/gRNA-induced gene knockouts. New Phytol. 2021, 231, 1478–1495. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, X.; Lam, P.; Zhang, K.; Tobimatsu, Y.; Liu, C. Monolignol acyltransferase for lignin p-hydroxybenzoylation in Populus. Nat. Plants 2021, 7, 1288–1300. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene CsLOB 1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Xu, J.; Orbović, V.; Zhang, Y.; Wang, N. Editing citrus genome via SaCas9/sgRNA system. Front. Plant Sci. 2017, 8, 2135. [Google Scholar] [CrossRef]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF 4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.; Stürchler, A.; Anjanappa, R.B.; Zaidi, S.S.; Hirsch-Hoffmann, M.; Gruissem, W.; Vanderschuren, H. Linking CRISPR-Cas9 interference in cassava to the evolution of editing-resistant geminiviruses. Genome Biol. 2019, 20, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zeijl, A.; Wardhani, T.A.; Seifi Kalhor, M.; Rutten, L.; Bu, F.; Hartog, M.; Linders, S.; Fedorova, E.E.; Bisseling, T.; Kohlen, W. CRISPR/Cas9-mediated mutagenesis of four putative symbiosis genes of the tropical tree Parasponia andersonii reveals novel phenotypes. Front. Plant Sci. 2018, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Walawage, S.L.; Zaini, P.A.; Mubarik, M.S.; Martinelli, F.; Balan, B.; Caruso, T.; Leslie, C.A.; Dandekar, A.M. Deploying genome editing tools for dissecting the biology of nut trees. Front. Sustain. Food Syst. 2019, 3, 100. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhao, J.; Gao, Y.; Zhao, R.; Zhang, J.; Kong, L. Efficient multi-sites genome editing and plant regeneration via somatic embryogenesis in Picea glauca. Front. Plant Sci. 2021, 12, 2198. [Google Scholar] [CrossRef]
- Nanasato, Y.; Mikami, M.; Futamura, N.; Endo, M.; Nishiguchi, M.; Ohmiya, Y.; Konagaya, K.; Taniguchi, T. CRISPR/Cas9-mediated targeted mutagenesis in Japanese cedar (Cryptomeria japonica D. Don). Sci. Rep. 2021, 11, 16186. [Google Scholar] [CrossRef] [PubMed]
- Pavese, V.; Moglia, A.; Corredoira, E.; Martínez, M.T.; Torello Marinoni, D.; Botta, R. First report of CRISPR/Cas9 gene editing in Castanea sativa Mill. Front. Plant Sci. 2021, 12, 728516. [Google Scholar] [CrossRef]
- Elorriaga, E.; Klocko, A.L.; Ma, C.; Du Plessis, M.; An, X.; Myburg, A.A.; Strauss, S.H. Genetic containment in vegetatively propagated forest trees: CRISPR disruption of LEAFY function in Eucalyptus gives sterile indeterminate inflorescences and normal juvenile development. Plant Biotechnol. J. 2021, 19, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
| Genus | Species | Reference |
|---|---|---|
| Elaeis | E. guineensis | [175,176] |
| E. oleifera | [177] | |
| Eucalyptus | E. grandis × E. urophylla | [178,179,180,181] |
| E. pellita F. Muell. | [182,183] | |
| E. robusta Sm. | [184] | |
| E. benthamii Maiden & Cambage. | [182] | |
| E. nitens (H.Deane & Maiden) Maiden. | [185,186] | |
| E. urophylla × E. grandis | [178] | |
| E. grandis | [187,188] | |
| E. globulus Labill. | [189,190] | |
| E. dunni Maiden | [191] | |
| E. cladocalyx F. Muell. | [192] | |
| E. camaldulensis | [193] | |
| E. grandis | [47] | |
| E. polybractea | [170] | |
| Hevea | H. brasiliensis | [55,194,195,196,197] |
| Picea | P. abies | [198] |
| P. glauca | [199,200,201,202] | |
| P. sitchensis | [203] | |
| P. mariana (Mill.) Britton. | [198,204,205] | |
| Pinus | P. pinaster | [206] |
| P. taeda | [207,208,209] | |
| P. contorta Douglas ex Loudon | [210] | |
| P. radiata D. Don | [186,211] | |
| P. sylvestris Thunb. | [212] | |
| Populus | P. deltoides W. Bartram ex Marshall | [213] |
| P. trichocarpa | [44] | |
| P. euphratica | [214] | |
| P. nigra L | [215] | |
| Castanea | C. dentata | [216] |
| Pseudotsuga | P. menziesii (Mirb.) Franco | [217] |
| Genus | Species | Genes | Phenotype | Reference |
|---|---|---|---|---|
| Populus | P. canescens P. tremula | SOC1, FUL, NFP-like genes, TOZ19 | [245] | |
| P. alba × P. glandulosa | PdNF-YB21 | Drought resistance | [246] | |
| P. alba × P. glandulosa | PagDA1 | Promoting xylem formation | [247] | |
| P. alba var. pyramidalis | Hyg | [248] | ||
| P. tomentosa | PtSGT1, PtSGT4 | Regulating cellulose synthesis in cell wall | [249] | |
| P. tomentosa Carr. clone 741 | PtoDET2 | Xylem development and reduced wall thickness | [250] | |
| P. tomentosa Carr. clone 741 | MYB115 | Reduced proanthocyanidin accumulation | [251] | |
| P. tomentosa Carr. clone 741 | PtoMYB156 | Negative regulation of secondary wall formation | [252] | |
| P. tomentosa Carr. clone 741 | PtoMYB170 | Regulates lignin deposition | [253] | |
| P. tomentosa Carr. clone 741 | PtrMYB57 | Increased anthocyanins and procyanidins | [254] | |
| P. tomentosa Carr. clone 741 | JMJ25 | Increased anthocyanin accumulation | [255] | |
| P. tomentosa Carr. clone 741 | MYB189 | Regulating secondary cell-wall biosynthesis | [256] | |
| P. tomentosa Carr. clone 741 | PtoDWF4 | Reduced xylem development | [257] | |
| P. tomentosa Carr. clone 741 | PtrWRKY18, PtrWRKY35 | Melampsora resistance | [258] | |
| P. tremula × P. alba clone INRA 717-IB4 | GNC | Drought stress tolerance | [259] | |
| P. tremula × P. alba clone INRA 717-IB4 | 4CL1, 4CL2 | Decreased lignin content, discoloration of stems | [237] | |
| P. tremula × P. alba clone INRA 717-IB4 | LEAFY | [260] | ||
| P. tremula × P. alba clone INRA 717-IB4 | BRANCHED1, BRANCHED2 | Bud outgrowth control | [261] | |
| P. tremula × P. alba clone INRA 717-IB4 | LHY2 | Photoperiodic growth | [262] | |
| P. tremula × P. alba clone INRA 717-IB4 | PeuBELL15 | Improved accumulation of glucan and lignin | [263] | |
| P. tremula × P. alba clone INRA 717-IB4 | SHR | Affecting endoderm single-cell layer | [264] | |
| P. tremula × P. alba clone INRA 717-IB4 | MYB186, MYB138, MYB38 | Non-glandular trichomes | [265] | |
| P. tremula× P. tremuloides clone T89 | BRC1 | Photoperiodic control of seasonal growth | [266] | |
| P. alba | PalCESA4 | Affecting cellulose content | [267] | |
| P. alba var. pyramidalis | PalWRKY77 | Salt resistance | [268] | |
| P. alba x P. glandulosa | CSE | Increased lignocellulose biomass | [269] | |
| P. alba x P. glandulosa | PagPDS1 | Chlorophyll biosynthesis | [270] | |
| P. tomentosa | GATA19 | Photosynthesis and growth | [271] | |
| P. tomentosa | PtoLAC14 | Integrated enhancement on biomass enzymatic saccharification | [272] | |
| P. tomentosa Carr. clone 741 | PtoPDS | Chlorophyll biosynthesis | [238] | |
| P. tremula | ARR17 | Regulating gender | [273] | |
| P. tremula × P. alba | CSE1, CSE2 | Reduced lignin and increased cellulose | [274] | |
| P. tremula L. × P. tremuloides Michx. | VNS | Secondary cell-wall thinning | [275] | |
| P. tremula × P. alba | PopSAP | Impaired growth, complete sterility with no initiation of inflorescences | [276] | |
| P. tremula × P. alba | PtaSUT4 | Orchestration of ROS, antioxidant, starch utilization, and RWC dynamics | [277] | |
| P.tremula × P. tremuloides | FT1, FT2 | Yearly growth cycle | [278] | |
| P.tremula × P. tremuloides | PGM | Starch biosynthesis | [279] | |
| P. trichocarpa | PtrHSFB3-1, PtrMYB092 | Reduced lignin and increased cellulose | [280] | |
| P. trichocarpa | PtrADA2b-3 | Drought resistance | [281] | |
| P. trichocarpa | PtrMYB161 | Wood Formation | [282] | |
| P. trichocarpa | PtrMYB074, PtrWRKY19 | Strong drought-tolerant | [283] | |
| P. trichocarpa | PtrCesA4, PtrCes7A/B or 8A/B | Cellulose biosynthesis | [284] | |
| P. trichocarpa L. | PHBMT1 | Controlling the formation of p-hydroxybenzoylated lignin structures | [285] | |
| Citrus | C. sinensis cv. Valencia | CsPDS | Chlorophyll biosynthesis | [233] |
| C. sinensis Osbeck | CsLOB1 | Canker resistance | [234] | |
| C. paradisi | CsLOB1 | Canker resistance | [236,286] | |
| Poncirus | P. trifoliate L. × C. sinensis L. Osb | PDS | Chlorophyll biosynthesis | [235] |
| P. trifoliate L. × C. sinensis L. Osb | Cs7g03360 | Leaf development | [287] | |
| Manihot | M. esculenta | MePDS | Chlorophyll biosynthesis | [239] |
| M. esculenta cv. 60444 | nCBP-1, nCBP-2 | Biotic stress response | [288] | |
| M. esculenta | AC2, AC3 | Biotic stress response | [289] | |
| Hevea | H. brasiliensis | FT, TFL1 | Early flowering | [243] |
| Jatropha | J. curcas | JcCYP735A | Growth and flowering regulation | [241] |
| Parasponia | P. andersonii | PanHK4, PanEIN2, PanNSP1, PanNSP2 | Nodule formation, layer activity, plant sex | [290] |
| Bambusa | B. oldhamii | PDS | Chlorophyll biosynthesis | [242] |
| Dendrocalamus | D. latiflorus Munro | DlmPSY1-A, DlmPSY1-B, DlmPSY1-C, GRG1 | Whitening, increased plant height | [244] |
| Juglans | J. regia L. | JrPDS | Chlorophyll biosynthesis | [291] |
| Picea | P. glauca | DXS1 | Albino somatic embryo (SE) plants | [292] |
| Cryptomeria | C. japonica | CjChlI | Whitening | [293] |
| Castanea | C. sativa Mill | PDS | Chlorophyll biosynthesis | [294] |
| Eucalyptus | Eucalyptus | FT | No statistical difference in seedling vegetative growth rate or leaf morphology | [295] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Tian, Y.; Sun, Y.; Li, Y. The Development of Forest Genetic Breeding and the Application of Genome Selection and CRISPR/Cas9 in Forest Breeding. Forests 2022, 13, 2116. https://doi.org/10.3390/f13122116
Zhao Y, Tian Y, Sun Y, Li Y. The Development of Forest Genetic Breeding and the Application of Genome Selection and CRISPR/Cas9 in Forest Breeding. Forests. 2022; 13(12):2116. https://doi.org/10.3390/f13122116
Chicago/Turabian StyleZhao, Ye, Yanting Tian, Yuhan Sun, and Yun Li. 2022. "The Development of Forest Genetic Breeding and the Application of Genome Selection and CRISPR/Cas9 in Forest Breeding" Forests 13, no. 12: 2116. https://doi.org/10.3390/f13122116
APA StyleZhao, Y., Tian, Y., Sun, Y., & Li, Y. (2022). The Development of Forest Genetic Breeding and the Application of Genome Selection and CRISPR/Cas9 in Forest Breeding. Forests, 13(12), 2116. https://doi.org/10.3390/f13122116






