Melaleuca alternifolia (Maiden & Betche) Cheel Residues Affect the Biomass and Soil Quality of Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Plot Setting
2.3. Biomass Measurement
2.4. Soil Sampling and Analysis
2.5. Soil Quality Evaluation Method
2.5.1. Construct MDS
2.5.2. Weights of Indicators
2.5.3. Indicators Scores
2.5.4. Calculating the SQI
2.6. Statistical Analysis
3. Results
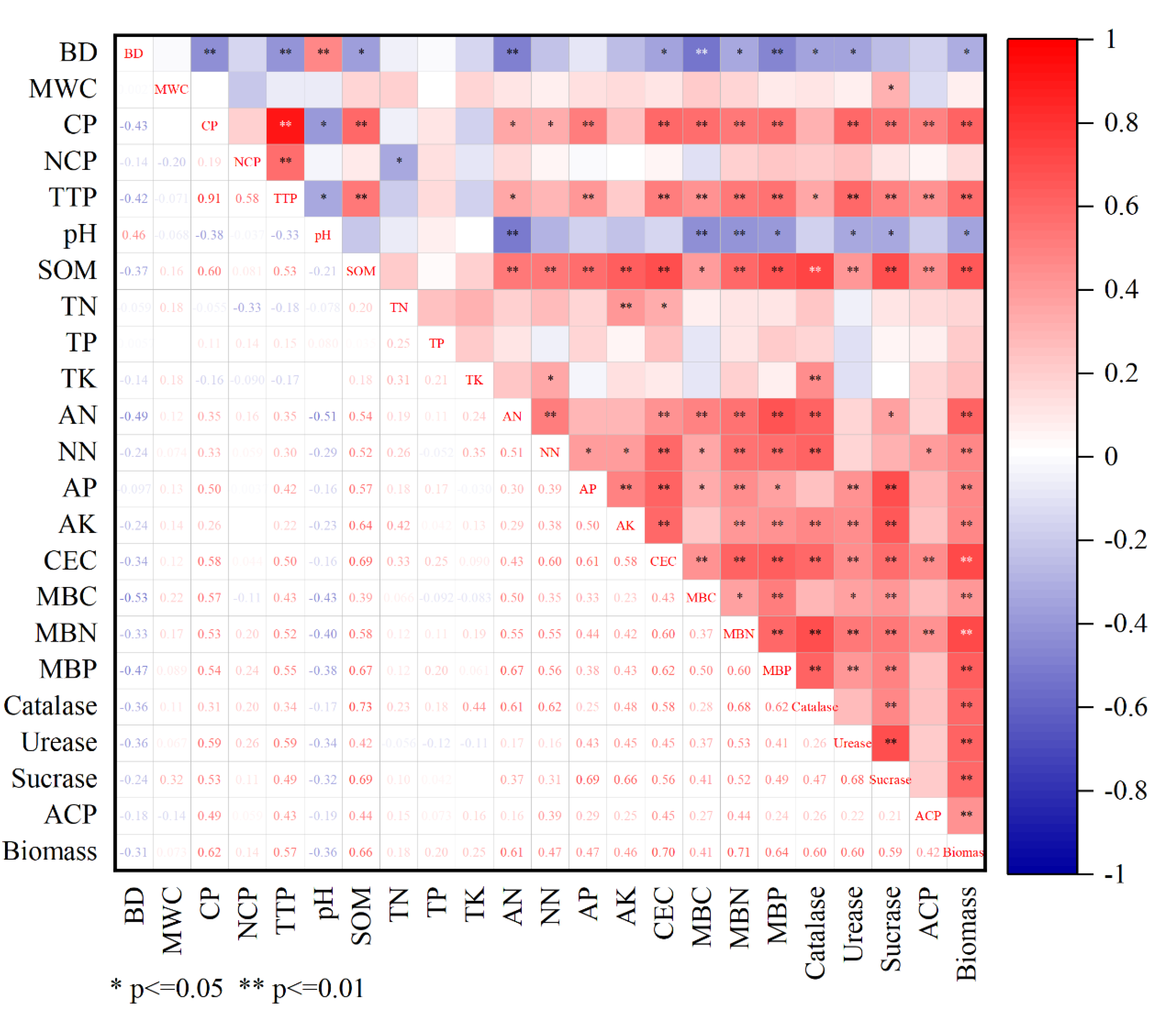
3.1. Soil Physical, Chemical, and Biological Properties of Different Treatments
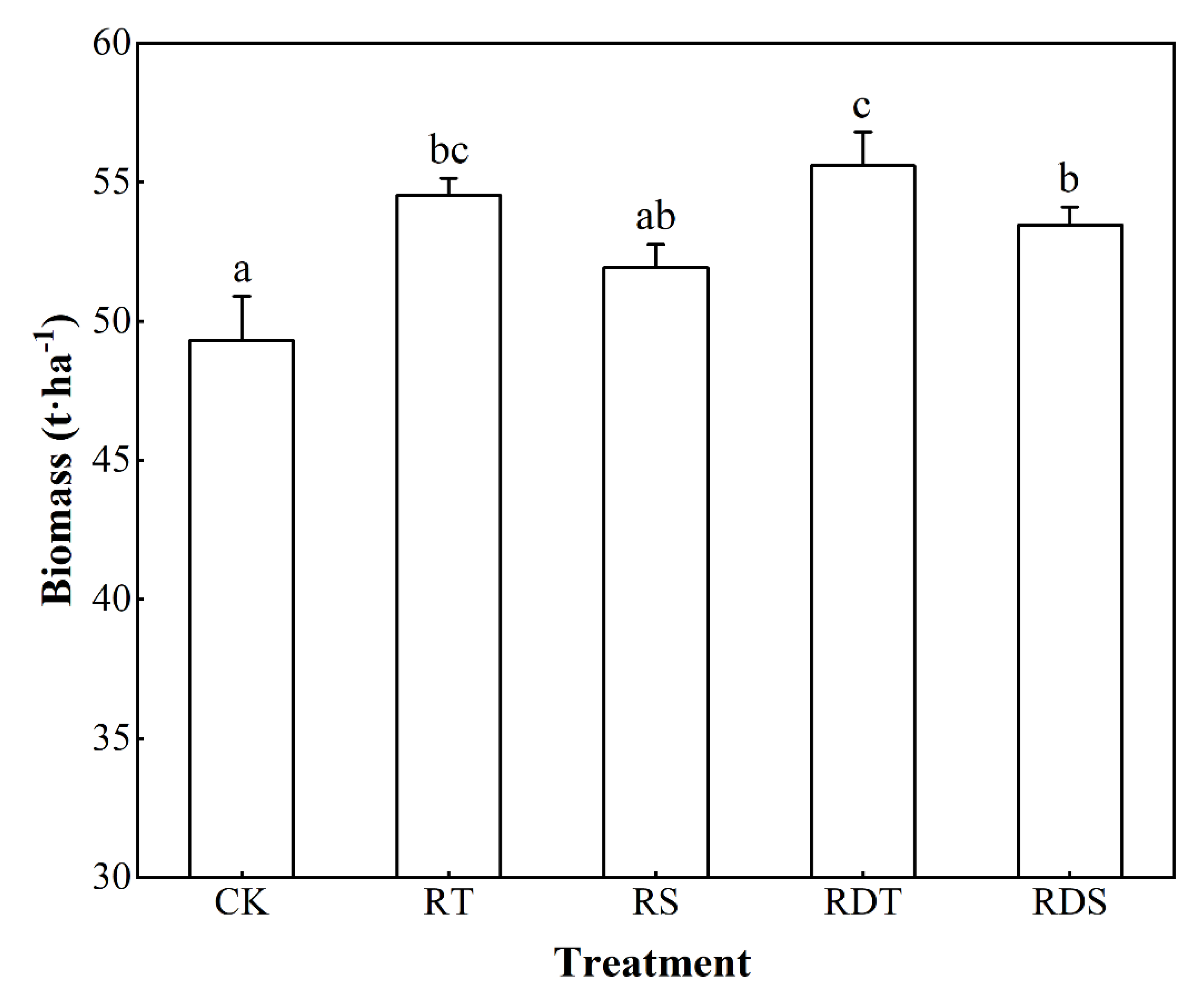
3.2. Biomasses of Melaleuca alternifolia of Different Treatments
3.3. Soil Quality Evaluation
3.3.1. Determining Indicators for IDS
3.3.2. Determining Indicators for MDS
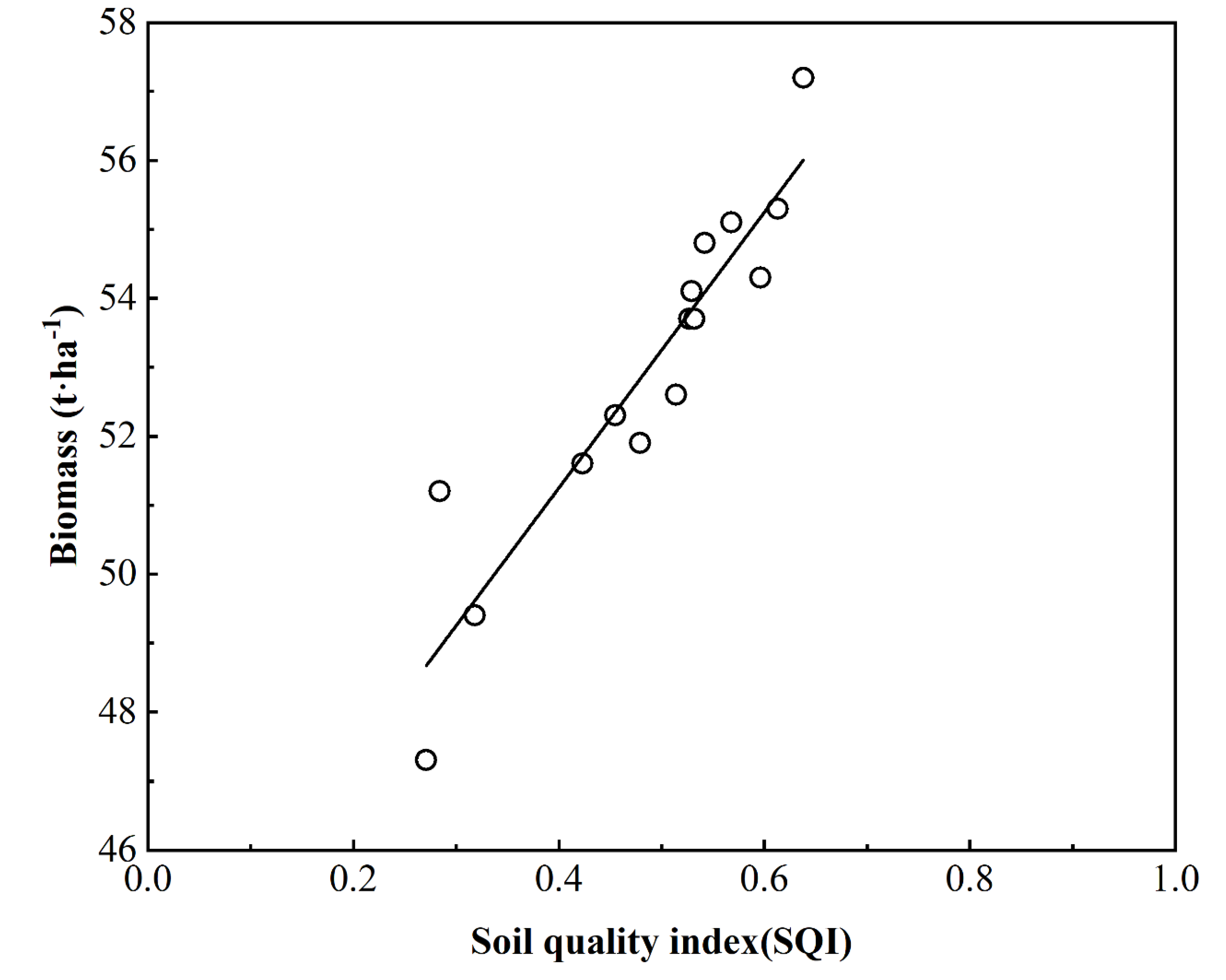
3.3.3. Calculating the SQI
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sá Silva, C.; De Figueiredo, H.M.; Stamford, T.L.M.; Da Silva, L.H.M. Inhibition of Listeria monocytogenes by Melaleuca alternifolia (tea tree) essential oil in ground beef. Int. J. Food Microbiol. 2019, 293, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’Aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models. Cell Death Discov. 2021, 7, 127. [Google Scholar] [CrossRef]
- Zheng, H.; Ouyang, Z.Y.; Wang, X.K.; Miao, H.; Zhao, T.Q.; Peng, T.B. How different reforestation approaches affect red soil properties in southern China. Land Degrad. Dev. 2005, 16, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Yao, X.; Deng, Y.; Lai, Z.; Lin, L.; Liu, J. Effects of stand age on soil respiration in Pinus massoniana plantations in the hilly red soil region of Southern China. CATENA 2019, 178, 313–321. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Mendham, D.S.; Grove, T.S.; O’Connell, A.M.; Sankaran, K.V.; Rance, S.J. Harvest residue effects on soil organic matter, nutrients and microbial biomass in eucalypt plantations in Kerala, India. For. Ecol. Manag. 2014, 328, 140–149. [Google Scholar] [CrossRef]
- Mendham, D.S.; O’connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. For. Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Sankaran, K.V.; Grove, T.S.; Kumaraswamy, S.; Manju, V.S.; Mendham, D.S.; Os’connell, A.M. Export of nutrients in plant biomass following harvest of eucalypt plantations in Kerala, India. J. Sustain. For. 2005, 20, 15–36. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Kurka, A.M.; Mannerkoski, H.; Piirainen, S.; Starr, M. Decomposition and nutrient release from logging residues after clear-cutting of mixed boreal forest. Plant Soil 2004, 263, 53–67. [Google Scholar] [CrossRef]
- Bargali, S.S.; Shukla, K.; Singh, L.; Ghosh, L.; Lakhera, M.L. Leaf litter decomposition and nutrient dynamics in four tree species of dry deciduous forest. Trop. Ecol. 2015, 56, 191–200. [Google Scholar]
- Hernández, J.; Del Pino, A.; Hitta, M.; Lorenzo, M. Management of forest harvest residues affects soil nutrient availability during reforestation of Eucalyptus grandis. Nutr. Cycl. Agroecosyst. 2016, 105, 141–155. [Google Scholar] [CrossRef]
- Nakajima, T.; Lal, R.; Jiang, S. Soil quality index of a crosby silt loam in central Ohio. Soil Tillage Res. 2015, 146, 323–328. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M. Soil quality indicators: Critical tools in ecosystem restoration. Curr. Opin. Environ. Sci. Health 2018, 5, 47–52. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S.K.; Mishra, V.N.; Reddy, G.P.; Bajpai, R.K. Soil quality ranking of a small sample size using AHP. J. Soil Water Conserv. 2017, 16, 339–346. [Google Scholar] [CrossRef]
- Paul, G.C.; Saha, S.; Ghosh, K.G. Assessing the soil quality of Bansloi river basin, eastern India using soil-quality indices (SQIs) and Random Forest machine learning technique. Ecol. Indic. 2020, 118, 106804. [Google Scholar] [CrossRef]
- Şenol, H.; Alaboz, P.; Demir, S.; Dengiz, O. Computational intelligence applied to soil quality index using GIS and geostatistical approaches in semiarid ecosystem. Arab. J. Geosci. 2020, 13, 1235. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Chen, D.; Li, M.; Wei, C. Soil quality assessment of different Camellia oleifera stands in mid-subtropical China. Appl. Soil Ecol. 2017, 113, 29–35. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Wang, X.; Yu, D. Development of biological soil quality indicator system for subtropical China. Soil Tillage Res. 2013, 126, 112–118. [Google Scholar] [CrossRef]
- Chang, X.; Qiu, X.; Liu, X.; Peng, D.; Cheng, S. Soil fertility quality evaluation of pure and mixed Larix principis-rupprechtii forests in Saihanba, Hebei Province of northern China. J. Beijing For. Univ. 2021, 43, 50–59. [Google Scholar] [CrossRef]
- Pang, S.; Ou, Z.; Shen, W.; Hou, Y.; Huang, X.; Zheng, W. Edaphic characteristics of different regeneration patterns in karst mountainous areas of Guangxi. J. Cent. South Univ. For. Technol. 2016, 36, 60–66. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; de Moraes Goncalves, J.L.; Brandani, C.B.; de Vicente Ferraz, A.; Franci, A.F.; Marques, E.R.G.; Junior, J.C.A.; Hubner, A. Forest residue removal decreases soil quality and affects wood productivity even with high rates of fertilizer application. For. Ecol. Manag. 2018, 430, 188–195. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Harwood, C.E. Productivity of acacia and eucalypt plantations in Southeast Asia. 1. Bio-physical determinants of production: Opportunities and challenges. Int. For. Rev. 2014, 16, 225–248. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth-A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; de Moraes Gonçalves, J.L.; Gava, J.L.; de Oliveira Godinho, T.; Melo, E.A.; Bazani, J.H.; Hubner, A.; Junior, J.C.A.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Hardiyanto, E.B.; Sadanandan Nambiar, E.K. Productivity of successive rotations of Acacia mangium plantations in Sumatra, Indonesia: Impacts of harvest and inter-rotation site management. New For. 2014, 45, 557–575. [Google Scholar] [CrossRef]
- Huong, V.D.; Nambiar, E.K.S.; Hai, N.X.; Ha, K.M.; Dang, N.V. Sustainable management of Acacia auriculiformis plantations for wood production over four successive rotations in South Vietnam. Forests 2020, 11, 550. [Google Scholar] [CrossRef]
- Prats, S.A.; Abrantes, J.R.; Crema, I.P.; Keizer, J.J.; de Lima, J.L. Runoff and soil erosion mitigation with sieved forest residue mulch strips under controlled laboratory conditions. For. Ecol. Manag. 2017, 396, 102–112. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, J.; Wan, H.; Hu, X. Review of research on hydrological characteristics of forest litter. Sci. Soil Water Conserv. 2007, 5, 130–134. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, Y.; Zhang, W.; Hu, G.; Xie, H.; Yan, J.; Han, S.; He, H.; Zhang, X. Linkage of microbial residue dynamics with soil organic carbon accumulation during subtropical forest succession. Soil Biol. Biochem. 2017, 114, 114–120. [Google Scholar] [CrossRef]
- He, Z.; Yu, Z.; Huang, Z.; Davis, M.; Yang, Y. Litter decomposition, residue chemistry and microbial community structure under two subtropical forest plantations: A reciprocal litter transplant study. Appl. Soil Ecol. 2016, 101, 84–92. [Google Scholar] [CrossRef]
- Maier, C.A.; Johnsen, K.H.; Dougherty, P.; McInnis, D.; Anderson, P.; Patterson, S. Effect of harvest residue management on tree productivity and carbon pools during early stand development in a loblolly pine plantation. For. Sci. 2012, 58, 430–445. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhou, Y.; Wang, L.; Si, R.; Lu, J.; Jia, Z. Effects of different treatments on decomposition of Larix principis-rupprechtii tending residue. J. Beijing For. Univ. 2021, 43, 15–23. [Google Scholar] [CrossRef]
- Sun, S.; Fan, X.; Ning, J.; Di, X.; Yang, G. Forest surface fuel degradation by efficient cellulose-degrading fungi in field. J. Northeast For. Univ. 2020, 48, 61–65. [Google Scholar] [CrossRef]
- Qiu, X.; Peng, D.; Wang, H.; Wang, Z.; Cheng, S. Minimum data set for evaluation of stand density effects on soil quality in Larix principis-rupprechtii plantations in North China. Ecol. Indic. 2019, 103, 236–247. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- D’Hose, T.; Cougnon, M.; De Vliegher, A.; Vandecasteele, B.; Viaene, N.; Cornelis, W.; Bockstaele, E.V.; Reheul, D. The positive relationship between soil quality and crop production: A case study on the effect of farm compost application. Appl. Soil Ecol. 2014, 75, 189–198. [Google Scholar] [CrossRef]
| Element | C/g·kg−1 | N/g·kg−1 | P/g·kg−1 | K/g·kg−1 |
|---|---|---|---|---|
| Content | 468.3 ± 5.7 | 14.5 ± 1.36 | 0.21 ± 0.04 | 1.96 ± 0.25 |
| Indicator | Method | Reference |
|---|---|---|
| Bulk density (BD, g·cm−3) Mass water-holding capacity (MWC, g·kg−1) | Soil core method | Liu, J.; et al., 2017 [19] |
| Capillary porosity (CP, %) Non-capillary porosity (NCP, %) The total porosity (TTP, %) | Soil core immersion method | Liu, J.; et al., 2017 [19] |
| pH | Potentiometry (soil: H2O = 1:2.5) | Liu, J.; et al., 2017 [19] |
| Soil organic matter (SOM, g·kg−1) | Potassium dichromate oxidation | Li, P.; et al., 2013 [20] |
| Total nitrogen (TN, g·kg−1) | Kjeldahl method | Li, P.; et al., 2013 [20] |
| Total phosphorus (TP, g·kg−1) | HClO4 and HF digestion, acidic molybate–ascorbic acid blue color detection | Li, P.; et al., 2013 [20] |
| Total kalium (TK, g·kg−1) | HClO4 and HF digestion, flame photometer detection | Li, P.; et al., 2013 [20] |
| Ammonia nitrogen (AN, mg·kg−1) | Kcl leaching-indophenol blue colorimetric method | Chang, X.; et al., 2021 [21] |
| Nitrate nitrogen (NN, mg·kg−1) | Phenol disulfonic acid colorimetry | Chang, X.; et al., 2021 [21] |
| Available phosphorus (AP, mg·kg−1) | Mehlich 3 method | Liu, J.; et al., 2017 [19] |
| Available kalium (AK, mg·kg−1) | Mehlich 3 method and flame photometry | Liu, J.; et al., 2017 [19] |
| Cation exchange capacity (CEC, cmol·kg−1) | Ammonium acetate extraction | Liu, J.; et al., 2017 [19] |
| Microbial biomass C (MBC, mg·kg−1) Microbial biomass N (MBN, mg·kg−1) Microbial biomass P (MBP, mg·kg−1) | Chloroform fumigating method | Chang, X.; et al., 2021 [21] |
| Catalase (mg·kg−1·h−1) | Potassium permanganate titration | Chang, X.; et al., 2021 [21] |
| Urease (mg·kg−1·h−1) | Phenol–sodium hypochlorite colorimetry | Chang, X.; et al., 2021 [21] |
| Sucrase (mg·kg−1·h−1) | 3,5-Dinitrosalicylic acid colorimetry | Chang, X.; et al., 2021 [21] |
| Acid phosphatase (ACP, mg·kg−1·h−1) | Phosphoric acid–disodium benzene colorimetry | Chang, X.; et al., 2021 [21] |
| Indicator | CK | RT | RS | RDT | RDS |
|---|---|---|---|---|---|
| BD | 1.254 ± 0.035 a | 1.148 ± 0.059 b | 1.185 ± 0.087 b | 1.188 ± 0.053 b | 1.201 ± 0.046 ab |
| MWC | 30.850 ± 2.421 a | 31.263 ± 1.996 a | 32.263 ± 2.938 a | 31.900 ± 2.314 a | 32.088 ± 1.890 a |
| CP | 29.775 ± 1.161 a | 34.513 ± 1.087 b | 33.200 ± 2.783 b | 34.425 ± 1.305 b | 33.638 ± 1.941 b |
| NCP | 21.088 ± 0.848 a | 21.400 ± 0.984 a | 21.02 5± 1.072 a | 21.763 ± 1.046 a | 21.150 ± 1.870 a |
| TTP | 50.863 ± 1.787 a | 55.913 ± 1.742 b | 54.225 ± 2.792 b | 56.188 ± 1.924 b | 54.788 ± 2.962 b |
| Indicator | CK | RT | RS | RDT | RDS |
|---|---|---|---|---|---|
| pH | 4.606 ± 0.105 a | 4.414 ± 0.068 b | 4.505 ± 0.109 ab | 4.509 ± 0.131 ab | 4.496 ± 0.146 ab |
| SOM | 13.563 ± 1.280 a | 17.863 ± 0.818 c | 15.775 ± 1.146 b | 19.138 ± 1.460 c | 18.608 ± 1.385 c |
| TN | 1.545 ± 0.066 a | 1.574 ± 0.094 a | 1.559 ± 0.080 a | 1.594 ± 0.071 a | 1.578 ± 0.117 a |
| TP | 0.497 ± 0.012 a | 0.499 ± 0.014 ab | 0.515 ± 0.015 b | 0.508 ± 0.017 ab | 0.504 ± 0.015 ab |
| TK | 11.400 ± 0.904 a | 11.763 ± 1.043 a | 11.438 ± 0.676 a | 11.950 ± 1.048 a | 11.713 ± 0.732 a |
| AN | 12.525 ± 1.158 a | 15.788 ± 1.095 b | 14.703 ± 1.138 b | 15.063 ± 0.862 b | 15.213 ± 1.025 b |
| NN | 4.916 ± 0.154 a | 5.656 ± 0.373 b | 5.430 ± 0.223 b | 5.689 ± 0.589 b | 5.795 ± 0.368 b |
| AP | 4.676 ± 0.144 a | 5.004 ± 0.210 ab | 5.014 ± 0.399 b | 5.811 ± 0.296 c | 5.755 ± 0.408 c |
| AK | 67.475 ± 3.197 a | 72.663 ± 5.351 b | 69.225 ± 3.28 ab | 76.363 ± 5.146 c | 76.038 ± 3.189 c |
| CEC | 13.525 ± 0.877 a | 16.388 ± 0.660 b | 16.463 ± 1.150 b | 17.863 ± 0.841 c | 16.875 ± 0.835 b |
| Indicator | CK | RT | RS | RDT | RDS |
|---|---|---|---|---|---|
| MBC | 143.63 ± 10.02 a | 191.13 ± 13.59 c | 181.5 ± 27.28 bc | 173.27 ± 13.48 b | 170.50 ± 7.55 b |
| MBN | 9.413 ± 0.755 a | 13.452 ± 0.738 c | 11.951 ± 1.207 b | 13.14 ± 0.911 bc | 12.82 ± 1.793 bc |
| MBP | 22.171 ± 1.547 a | 26.263 ± 0.901 b | 25.382 ± 1.829 b | 26.049 ± 0.898 b | 25.630 ± 1.195 b |
| Catalase | 1.538 ± 0.089 a | 2.148 ± 0.100 c | 1.866 ± 0.195 b | 2.071 ± 0.147 c | 1.999 ± 0.234 bc |
| Urease | 0.216 ± 0.014 a | 0.241 ± 0.014 c | 0.222 ± 0.013 ab | 0.246 ± 0.010 c | 0.235 ± 0.021 bc |
| Sucrase | 19.91 ± 1.177 a | 23.99 ± 1.211 c | 22.16 ± 1.902 b | 25.63 ± 1.630 c | 25.61 ± 2.141 c |
| ACP | 1.985 ± 0.124 a | 2.138 ± 0.110 ab | 2.111 ± 0.151 ab | 2.220 ± 0.155 b | 2.160 ± 0.189 b |
| PC | PC1 | PC2 |
|---|---|---|
| Eigenvalue | 13.507 | 1.963 |
| Percent | 71.32 | 23.54 |
| Cumulative percent | 71.32 | 94.86 |
| Eigenvector | ||
| MBC | 0.973 | 0.185 |
| BD | −0.973 | −0.222 |
| AN | −0.949 | −0.238 |
| pH | 0.844 | 0.520 |
| MBP | 0.802 | 0.585 |
| Catalase | 0.791 | 0.601 |
| CP | 0.789 | 0.609 |
| MBN | 0.774 | 0.632 |
| TTP | 0.750 | 0.646 |
| AP | 0.056 | 0.989 |
| AK | 0.249 | 0.955 |
| Sucrase | 0.410 | 0.904 |
| SOM | 0.489 | 0.867 |
| ACP | 0.509 | 0.838 |
| CEC | 0.553 | 0.779 |
| NN | 0.615 | 0.760 |
| Urease | 0.533 | 0.752 |
| Indicator | Communality | Weight |
|---|---|---|
| MBC | 0.739 | 0.173 |
| BD | 0.673 | 0.157 |
| AN | 0.633 | 0.148 |
| AP | 0.735 | 0.172 |
| AK | 0.664 | 0.155 |
| Sucrase | 0.831 | 0.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Chen, J.; He, J.; Kang, W. Melaleuca alternifolia (Maiden & Betche) Cheel Residues Affect the Biomass and Soil Quality of Plantation. Forests 2022, 13, 2134. https://doi.org/10.3390/f13122134
Luo H, Chen J, He J, Kang W. Melaleuca alternifolia (Maiden & Betche) Cheel Residues Affect the Biomass and Soil Quality of Plantation. Forests. 2022; 13(12):2134. https://doi.org/10.3390/f13122134
Chicago/Turabian StyleLuo, Hang, Jiao Chen, Jienan He, and Wenxing Kang. 2022. "Melaleuca alternifolia (Maiden & Betche) Cheel Residues Affect the Biomass and Soil Quality of Plantation" Forests 13, no. 12: 2134. https://doi.org/10.3390/f13122134
APA StyleLuo, H., Chen, J., He, J., & Kang, W. (2022). Melaleuca alternifolia (Maiden & Betche) Cheel Residues Affect the Biomass and Soil Quality of Plantation. Forests, 13(12), 2134. https://doi.org/10.3390/f13122134






