Abstract
In many spruce stands, trees are frequently attacked by the pathogen Heterobasidion parviporum, albeit without visible symptoms in the crown. In the present work, the results of the presence of stem rot, assessed by PICUS Sonic Tomography, and the fungal biota on trees and stumps in eight plots in the Puszcza Borecka Forest are described. The plots were located in stands on original forest soil (4) and on post-agricultural soil (4), where around a stump with H. parviporum symptoms (signs of internal rot and basidiocarps), 30 trees were selected and examined for internal rot. Wood samples were collected from two selected trees for fungal molecular analysis. A total of 79 fungal taxa were found, including 57 taxa in plots on post-agricultural soil and 45 on forest soil. There were 395 fungal records on stumps and 22 records on trees, therein, from the inner parts of felled trunks. Significant differences in the Chao-1 diversity index indicate that the origin of the soil—post-agricultural or forest soil—influenced the alpha diversity of the fungal communities in the forests studied. The values of the Shannon and Simpson indices show that the two communities were similar in terms of species numbers. The presence of basidiomata of H. parviporum and two species of Armillaria (mainly A. cepistipes) in samples on all plots is striking, although Armillaria spp. was detected more frequently. Most of the species identified were typical saprotrophs, although rare species were also found, such as Entoloma byssisedum, Onnia tomentosa, Physisporinus vitreus, Postia ptychogaster, and Ramaria apiculata. The presence of H. parviporum in the inner woody parts was confirmed by PCR analysis, and decay was detected even up to a stem height of 6 m. Armillaria was the dominant genus in the studied stands and plays a significant and underestimated role in heartwood decay of old spruce trees in Puszcza Borecka Forest.
1. Introduction
In spruce (Picea abies (L.) H. Karst.) stands, the pathogen Heterobasidion parviporum (Niemela & Korhonen) is an important wood destroyer [1,2,3,4]. Together with H. annosum (Fr.) Bref. and Armillaria solidipes Peck = A. ostoyae Romagn. (Herink), it is one of the main causal agents of diseases in harvested forests in the Northern Hemisphere [1,5,6,7]. Wood-inhabiting fungi, both pathogens and saprotrophs, decompose cellulose and lignin in the cell walls through oxidoreduction enzymes secreted extracellularly during substrate colonization [8]. The decomposition of wood leads to the release of D-glucose and its oxygenation, binding water and carbon dioxide [9,10,11,12]. These processes produce large amounts of energy and various metabolites, including nitrogen compounds, that can be used by other organisms [13,14,15,16,17,18]. As a result of the infection of live spruce trees by H. parviporum and the associated persistent decomposition of the heartwood, also after the felling of the trees [19,20], attempts to detect the presence of wood rot on standing trees at an early stage have been made, albeit with varying degrees of success [21,22,23]. For example, computed tomography allows the assessment of both changes in tree structure and the extent of wood loss caused by H. parviporum [24,25,26].
Decomposing tissue appears to provide favorable conditions for the colonization by other fungi along with natural succession [27,28]. Fungi that decompose spruce wood have been largely described in terms of their distribution at different sites [29], the infectivity of root residues [30], the genetic aspects of infection [31,32], or root endophytes as potential biocontrol agents [33], among other issues. However, the biota accompanying H. parviporum in decaying wood [34,35] have rarely been recorded, with only 95 search results in Google Scholar.
Since H. parviporum is a major cause of infection and death of mature trees in spruce stands in many countries [36,37,38], the aim of the present study was to determine the proportion of this pathogen in commercial spruce stands over 50 years old in the Borecka Forest, northeastern Poland, that have previously been infested by H. parviporum. We determined the fungal biota inhabiting the wood of trees and stumps in selected stands growing on old forest sites and on post-agricultural sites. We hypothesized that: (i) infection with H. parviporum can be detected early by tomographic examination of the logs, and (ii) stumps of felled and dead trees with internal rot are, on post-agricultural soil, a more adequate food source for various fungi of different trophic groups than stumps on old forest soil.
2. Materials and Methods
2.1. Research Plots
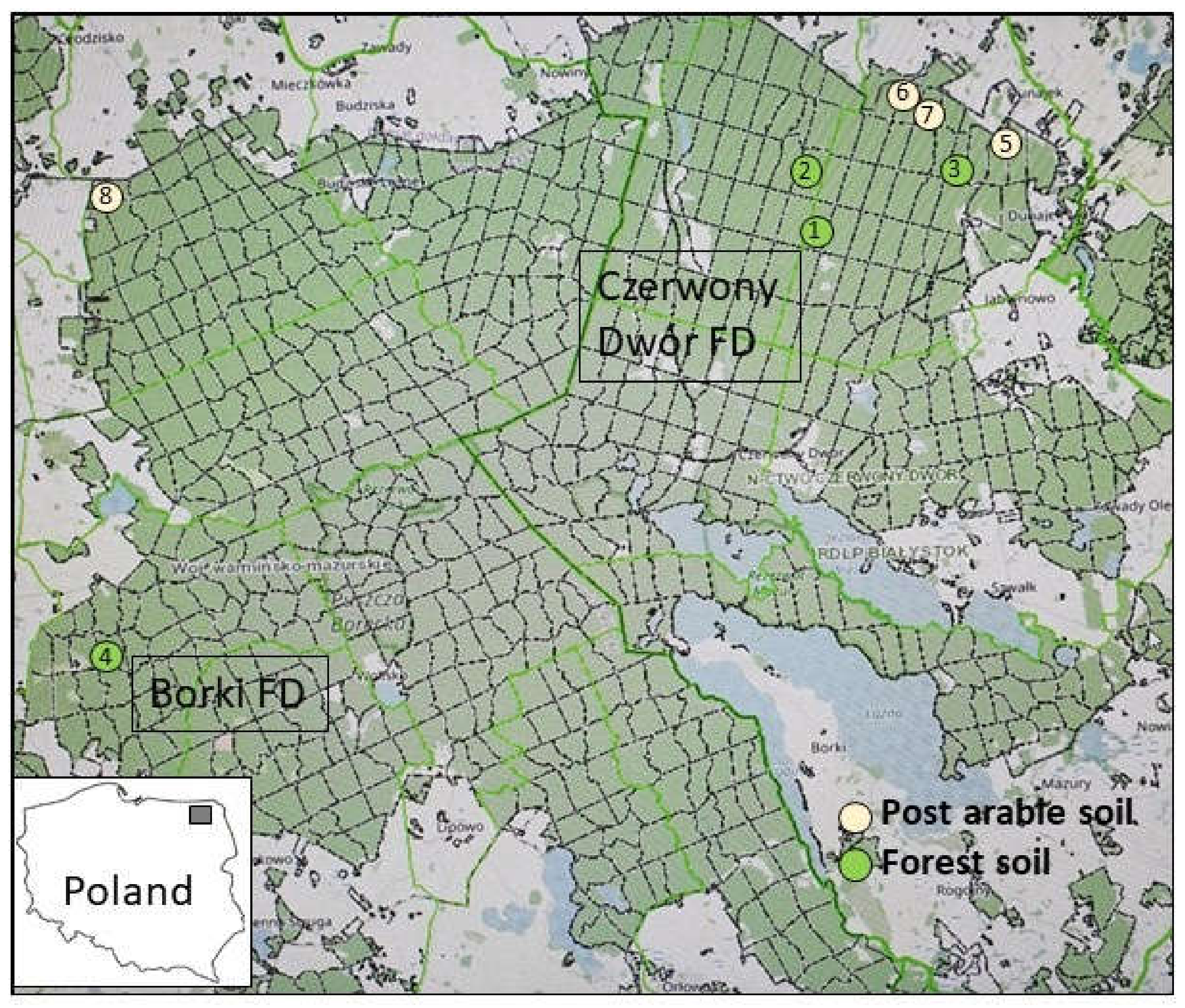
The study was performed in summer 2017 (PICUS examination) and autumn 2021 (stumps examination) in spruce stands (over 50 years old) in northeastern Poland (Figure 1) in the Borki and Czerwony Dwór Forest Districts (FDs), which belong to the Białystok Regional Directorate. Both FDs are mainly located in the Borecka Forest, a large forest complex (230 km2) in the lake area of the 842.86 Ełk Lake District (Masurian Lakeland) [39]; part of Borecka Forest is a dedicated nature reserve. According to the annual reports of the State Forests [40], both forest areas show significant damage by fungal pathogens, mainly Heterobasidion spp. and Armillaria ostoyae. Based on personal communication with foresters and an inventory, eight threatened stands were selected, four on former agricultural land and four old forest stands (Figure 1, Table 1). The old forest stands were selected randomly, whereas the stands on former agricultural sites were located on the edge of Borecka Forest. The numbers of the compartments were obtained from the Forest Data Bank [41].
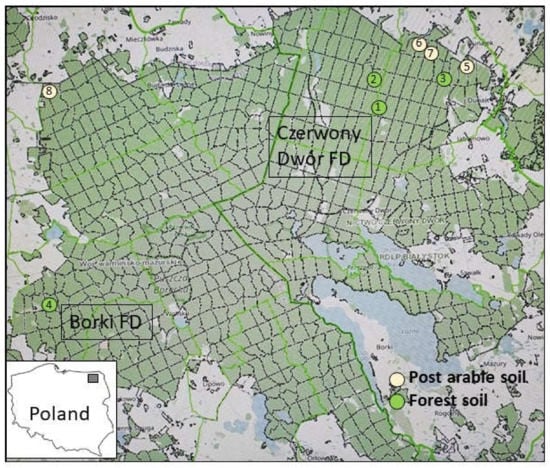
Figure 1.
Localization of the eight evaluated plots in Czerwony Dwór and Borki Forest Districts in Poland (Map source: [41]).

Table 1.
Description of the assessed compartments in the two forest types.
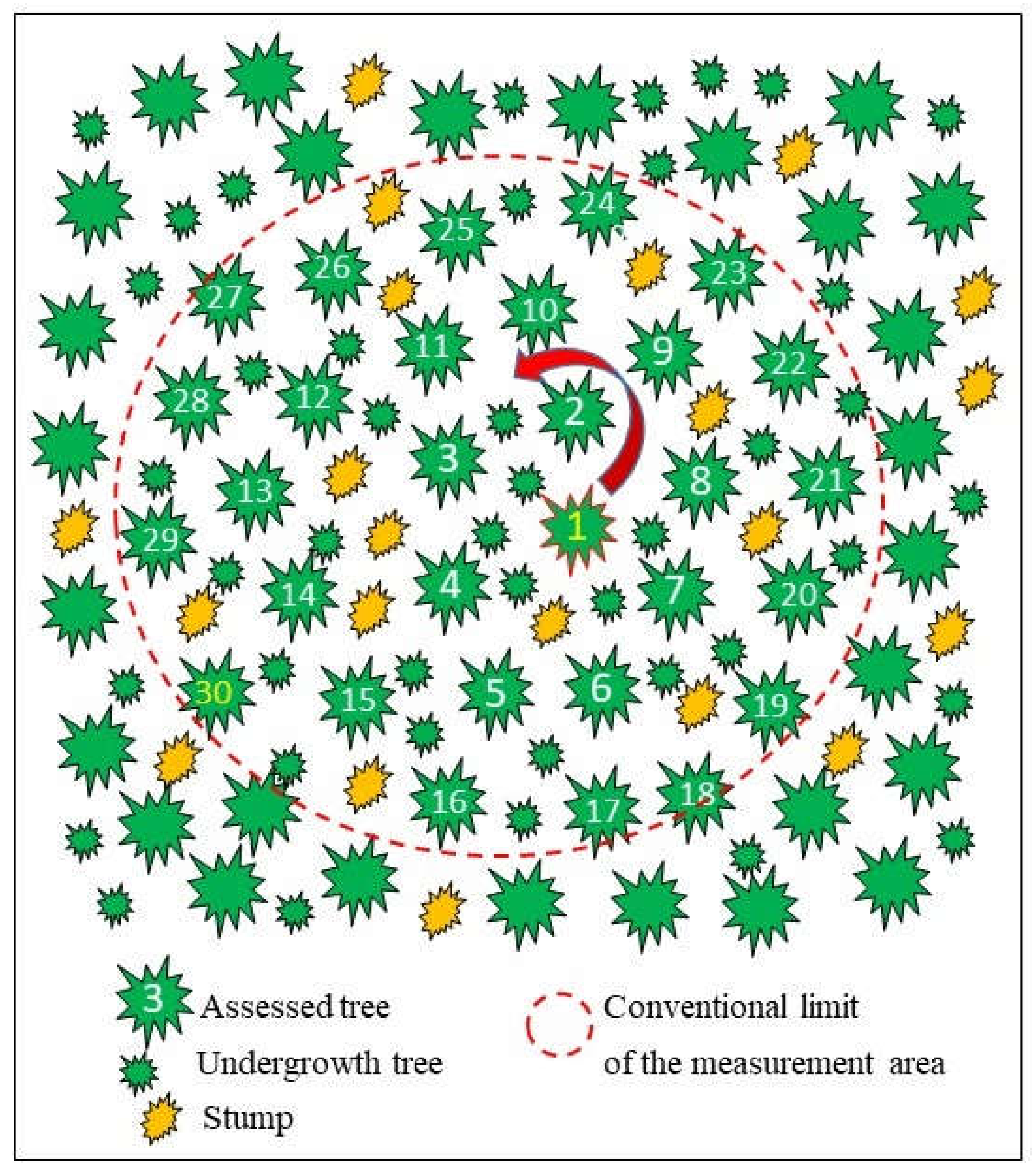
In each of these stands, spruce stumps with symptoms of internal decay typical of H. parviporum or with basidiomata were searched. One randomly selected stump served as the reference for further evaluation, and the tree closest to it was selected as the plot center (No. 1 in Figure 2). Subsequently, 30 consecutive trees were selected in a spiral and numbered (1–30). The trees showed no signs of crown thinning or dieback. The undergrowth and the small trees in the lower floors of the plot were omitted. The diameter of this area was measured and represented the survey plot (Figure 2). Within each plot, all spruce stumps and all trees were counted and examined for the presence of sporocarps (Table 1).
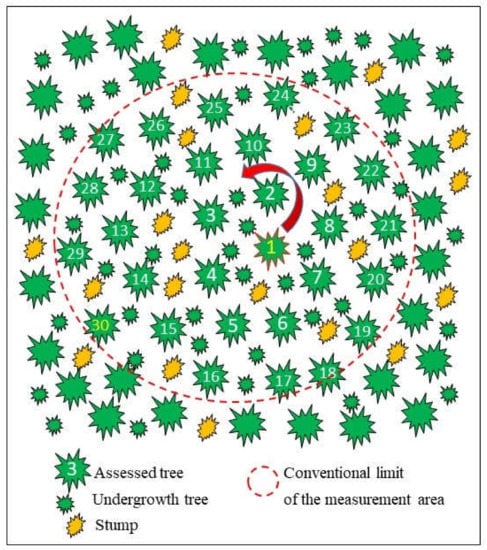
Figure 2.
Diagram showing the selection and location of 30 trees and stumps within the conventional survey plot.
2.2. Computed Tomographic Analysis
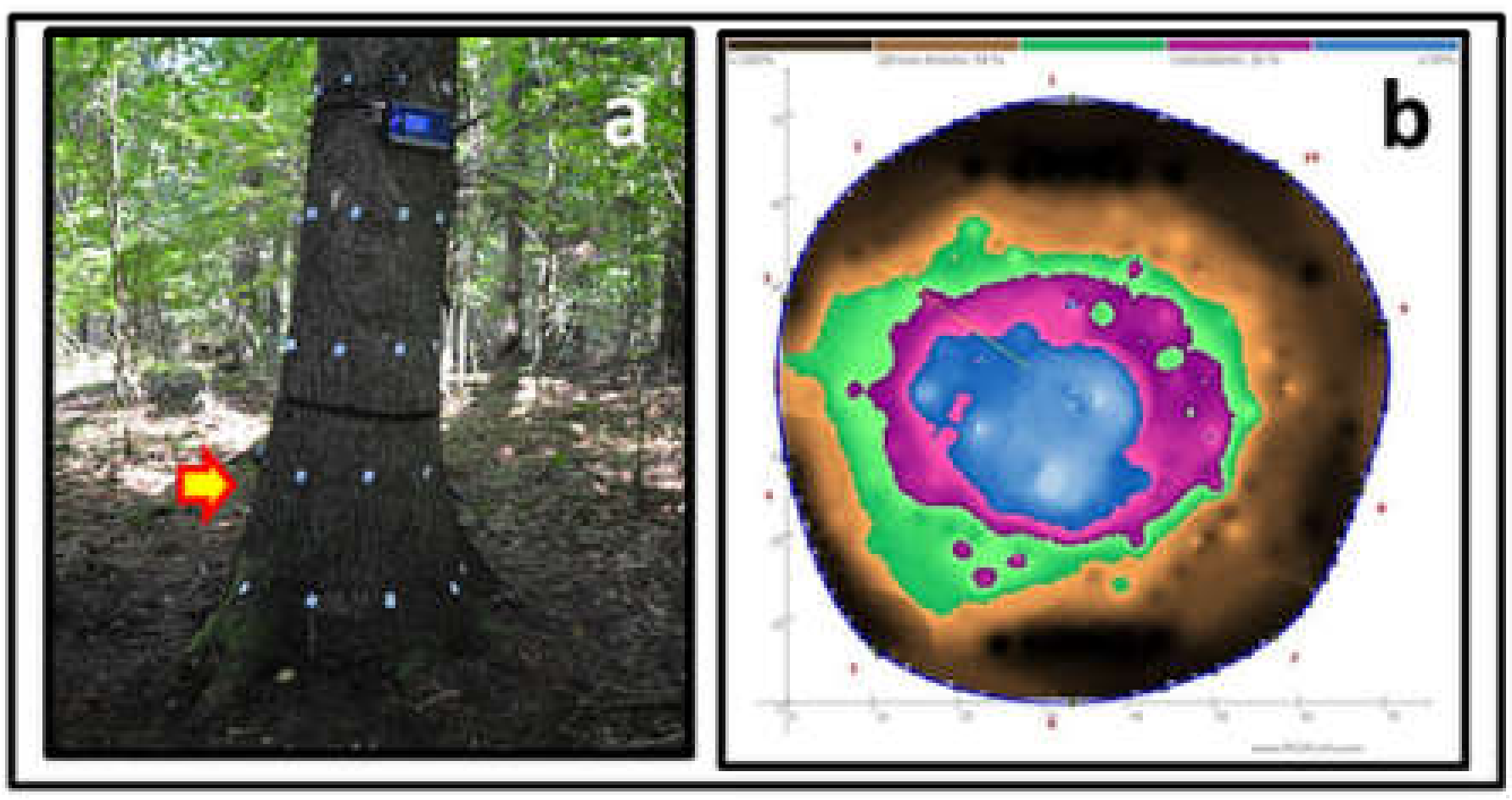
All 240 trees (30 trees in each of the 8 plots) were examined with PICUS Sonic® to determine the presence or absence of internal rot (Figure 3).

Figure 3.
One of the trees cut down for DNA analysis of fungi inside the spruce trunk. The crown of the tree showed no symptoms of defoliation or discoloration, whereas evaluation via PICUS Sonic Tomography showed the presence of stem internal rot. Photo by M. Damszel.
Tomographic assessment of wood decay inside the tree trunk [42,43] was performed at a height of 0.1 and 0.6 m above the ground (Figure 4), using the Sonic Tomograph—PICUS 3 Q74 EXP and the PICUS GMS Calliper 3 measuring device [26].
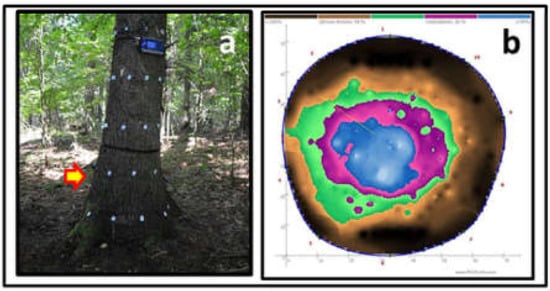
Figure 4.
Measurement with PICUS Sonic (a), and tomogram of the trunk presented in Figure 3 at a height of 0.6 m (b). Photo by W. Kowalczuk.
2.3. Assessment of Sporocarps
The collected specimens, growing in the root necks of all standing trees and on tree stumps within each plot, were identified using standard mycotaxonomic methods [44]. Species were identified using keys [45,46,47,48,49], and dried specimens were stored in the fungarium of the Forest Protection Department of the Warsaw University of Life Sciences (SGGW). The nomenclature of fungi followed the Index Fungorum database [50]. Threat categories were assigned according to the “Red List of Macrofungi in Poland” [51]; endangered and rare fungal species were listed according to Kujawa et al. (2021) [52].
2.4. Preparation of Pure Cultures and Molecular Analyses
Two trees showing signs of decay previously indicated by PICUS were randomly selected from the measurement plots (one from forest plot 49b and the other from arable site plot 6a). The trees were felled in October 2020. After debarking the trunk, five replicates of wood samples were cut at a height of 0.1 and 0.6 m, placed in sterile glass tubes, and stored in the refrigerator prior to further analyses. In the laboratory, the samples were surface-sterilized, cut into small inocula, and placed on 2% malt extract agar (MEA) supplemented with 50 mg/L streptomycin. Cultures were incubated at 22 °C for 7 days and sub-cultured as necessary. The resulting colonies were transferred to plates containing 2% MEA to obtain pure cultures for DNA analyses.
Fungal genomic DNA from mycelium, rhizomorphs, and sporocarps was extracted according to Kubiak et al. (2016) [53]. For the identification of fungal isolates obtained from decaying wood, the ITS rDNA region was amplified and sequenced using the primers ITS1F and ITS4 [54,55]. Identification of Heterobasidion specimens was performed by species-specific PCR with the primer pairs MJ-F/MJ-R (H. annosum) and KJ-F/KJ-R (H. parviporum) [56]. Identification of Armillaria species was performed based on sequencing of a portion of the translation elongation factor 1 alpha (tef 1-alpha, amplified according to Szewczyk et al. (2015) [57]. The amplified regions had a size of 100 bp (H. annosum), 350 bp (H. parviporum), and 600–650 bp (tef 1-alpha). Both strands of PCR products of the ITS region and the tef 1-alpha gene were sequenced using a 3730XL DNA analyzer (Applied Biosystems, Waltham, MA, USA) at Genomed (Warsaw, Poland). Nucleotide sequences were read and edited using FinchTV v. 1.4.0 (Geospiza Inc., Seattle, WA, USA) and aligned with sequences publicly available in GenBank (http://www.ncbi.nlm.nih.gov; 12 January 2022), using the BLASTn algorithm to confirm the taxonomy of the fungi studied.
2.5. Statistical Analyses
To determine the species diversity in the studied groups, several indices were calculated: Chao1, Ace, Shannon, Simpson, and Fisher. Alpha biodiversity was analyzed using the phyloseq package R [58]. Statistical analysis of the selected indices was performed using the R function pairwise.wilcox.test as Wilcoxon sum in pairs. Chi-square statistical analysis was performed for the frequencies presented in Table 2 and Table 3. Statistical analysis was performed using the Pearson chi-square test, and differences were considered statistically significant at p < 0.1. Correlation analysis for some of the tested results was performed using the ggpubr library of the R package. The method chosen was the non-parametric Spearman correlation. The alpha biodiversity of community samples describing individual taxa within a defined area or a collective list of species present in a given area was assessed. Both ACE and Chao1 are richness estimators, whereas Shannon and Simpson are diversity indices. The Fisher index was theoretically adjusted for sampling bias.

Table 2.
Frequency (%) of fungal taxa on Norway spruce stumps within plots on old forest sites (1–Statistical analysis was performed using the Pearson chi-square test, and differences were considered statistically significant at p < 0.1. Correlation analysis for some of the tested results was performed using the ggpubr library of the R package. The method chosen was the non-parametric Spearman correlation. The alpha biodiversity of community samples describing individual taxa within a defined area or a collective list of species present in a given area was assessed. Both ACE and Chao1 are richness estimators, whereas Shannon and Simpson are diversity indices. The Fisher index was theoretically adjusted for sampling bias4) and post-agricultural sites (5–8) (trophic categories: E–ectomycorrhizal fungi, M—mycoparasites, P—parasites, S—saprotrophs.

Table 3.
Frequency (%) of fungal taxa on Norway spruce trees within plots on old forest (1–4) and post-agricultural soils (5–8) (trophic categories: P—parasites and S—saprotrophs); * W—in wood inside trunk (PCR-confirmed).
3. Results
Computed tomographic analysis of the stems showed the presence of internal rot in 13.3–73.3% of the trees studied (Table 1). On average, the percentage of trees with internal damage was 43.3% on forest sites and 39.2% on former agricultural sites. For both soil types, the differences in the proportions of trees in which H. parviporum was detected by PICUS were not significant (pchi-sq. > 0.05).
The average percentage of stumps with decay symptoms (hollow) and/or basidiomata of both pathogens was 11.3 and 16.3%, respectively (Table 1). In both categories, the proportion of stumps with rot increased with stand age. It is noteworthy that the crowns of all trees with internal wood decay found by tomography showed no signs of thinning or discoloration of the needles.
In the eight studied plots representing the economic spruce stands of Borecka Forest, a total of 79 fungal taxa were found. In total, 417 records (395 on stumps and 22 on trees), including from the inner parts of the felled trunks, were obtained (Table 2 and Table 3). The occurrence of fungi inhabiting the wood of spruce growing on former agricultural sites (57 taxa) was higher than that of spruce growing on forest sites (45 taxa), with 224 versus 193 records.
The taxa found belonged to Ascomycota (8 species) and Mucoromycota (1), but mainly to Basidiomycota (70 taxa), with the representative orders Agaricales (30) and Polyporales (13) predominating. The sporocarps belonged to a total of 18 orders and 45 families at least. Among the identified taxa (59 saprotrophs, 14 parasites), four species (Helvella macropus, Paxillus involutus, Tomentella bryophila, T. radiosa) are classified as ectomycorrhizal fungi, and two species (Hypocrea pulvinata, Tremella encephala) are mycoparasites (Table 2 and Table 3).
In the old forest sites, 45 species were identified, of which 21 were only found in these sites (e.g., Amphinema byssoides, Armillaria borealis, Gloeophyllum odoratum, Phlebiopsis gigantea, Pholiota squarrosa, Pluteus pouzarianus, Pseudohydnum gelatinosum, or Sistotrema brinkmannii), whereas 57 species were recorded in the post-agricultural sites, of which 34 taxa only occurred in these sites (e.g., Bjerkandera adusta, Kuehneromyces mutabilis, Mycena galericulata, Pholiota flammans, Pleurotus dryinus, P. ostreatus, P. pulmonarius, Trichomolopsis rutilans, Xylodon flaviporus). Overall, 24 species occurred on both site types (e.g., Amaropostia stiptica, Armillaria cepistipes, Cyanosporus caesius, Heterobasidion parviporum, Hypholoma capnoides, H. fasciculare, Onnia tomentosa, Postia ptychogaster, Stereum sanguinolentum). Only three species were found on the wood of spruce trees growing on both soil types: Amaropostia stiptica, Heterobasidion parviporum, and Onnia tomentosa. For the post-agricultural soils, 15 species were recorded, whereas in the forest soils, we found 6 species (Table 2 and Table 3).
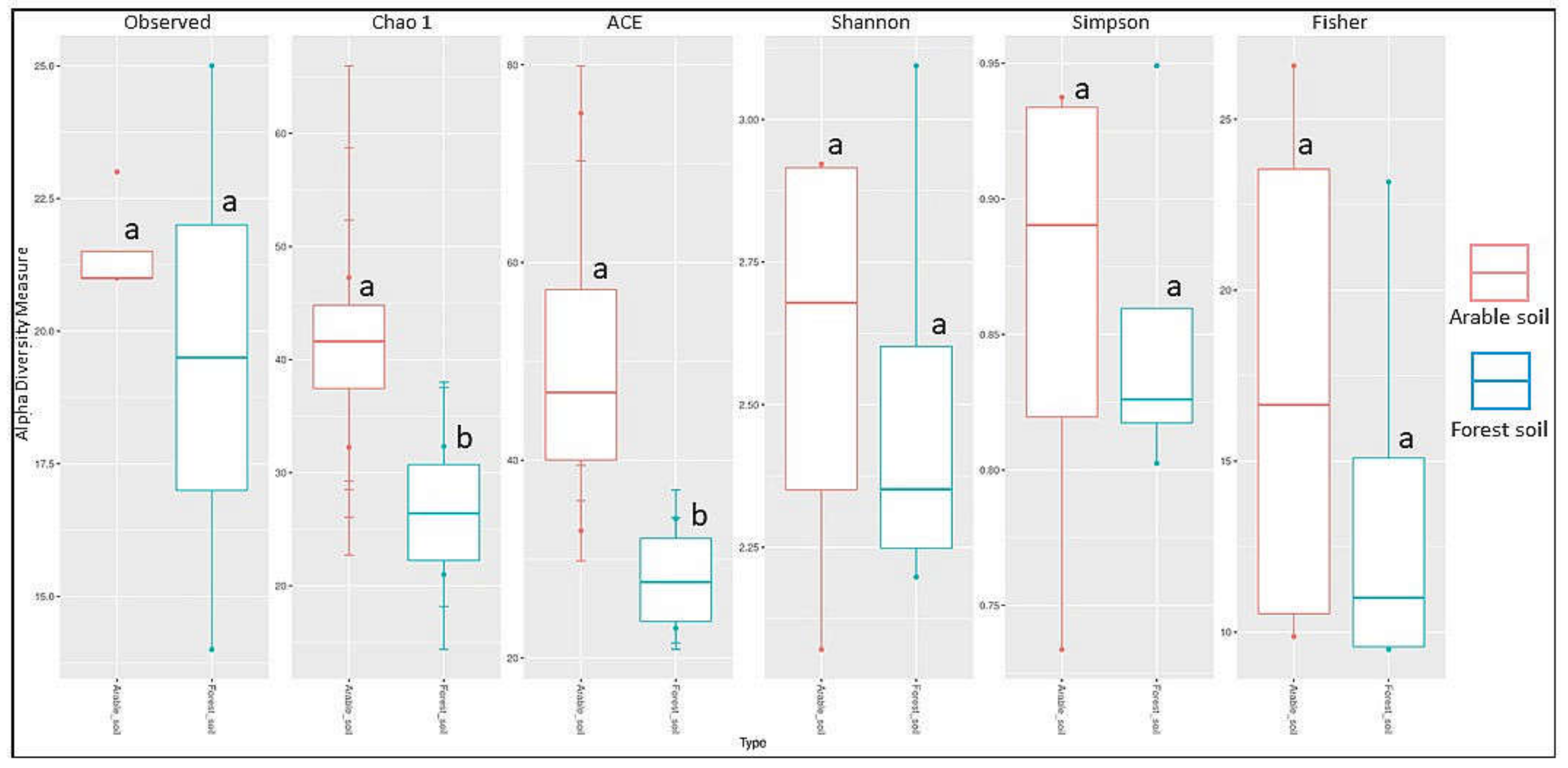
Significant differences in the Chao1 diversity index indicate (Table 4) that the origin of the soil—farm or forest soil—influences the alpha diversity of the fungal communities in the studied forests. The values of the Shannon and Simpson indices showed that the two communities were similar in terms of species number, with p = 0.88. The probability for the Chao1 index, which counts singletones and doubletones, was at the 0.05 limit, indicating a difference between the groups in the low-identified species (where we had one or two of the stumps). Regarding the ACE indicator, which is a non-parametric method for estimating the total number of species based on the coverage (size) of the sample, a probability at the 0.05 limit means that between groups of samples, the distribution of coverage (size) varies (Figure 5). Fungi belonging to four trophic groups were found on the studied tree stumps: saprotrophs (55 taxa), parasites (8), ectomycorrhizal (4), and mycoparasites (2) based on 395 records (185 in forest soil, 210 in arable soil). On the examined trees, 8 saprotrophs and 10 parasites were found based on 22 records (8 in forest soil, 14 in arable soil) (Table 2 and Table 3).

Table 4.
Values of some ecological indices describing the fungal communities in the forest (s1–s4) and arable (s5–s8) soils.
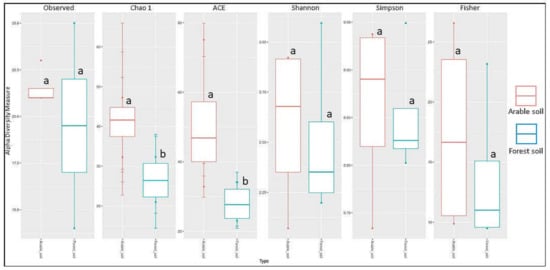
Figure 5.
Alpha diversity in arable soil (red) and forest soil (blue) taxa groups based on the number of observed species, Chao1, ACE, Shannon, Simpson, and Fisher index; a, b indicate significant differences between groups.
In the saprotroph group, three fungal species of the genus Hypholoma were found, among others: H. capnoides and H. fasciculare occurred in six plots and H. lateritia in one plot (Table 2 and Table 3). The shares of stumps with basidiomata of H. capnoides were similar for post-agricultural soil (11 stumps) and forest soil (10 stumps). Hypholoma fasciculare basidiomata were found more frequently on stumps in post-agricultural soil (10 stumps) compared to stumps in forest soil (6 stumps) (Table 2).
The presence of root pathogens (H. parviporum and two species of Armillaria) in stumps and trees on all plots is striking, although Armillaria spp. were detected more frequently (122 stumps and trees versus 25 stumps and trees). Although the presence of A. borealis was confirmed via mycelium under bark and basidiomata with PCR (NCBI sequences OL634955 and −56), only in spruce stumps in one plot (No. 2) on forest soil, A. cepistipes was detected in most plots (Table 2 and Table 3), both in forest (NCBI sequences OL652577, −80) and agricultural soils (NCBI sequences OL652578, −79, −81, −82, −83).
There was no significant correlation between the presence of a particular taxa in arable soil or forest soil, while the highest correlation was found for Armillaria spp. (R = 0.8, p = 0.33), all fungi R = −0.77, p = 0.23) and H. parviporum + Armillaria spp. stumps (R = −0.63, p = 0.37). Weak correlations were detected for Fungi on stumps vs. All stumps (R = 0.58, p = 0.13), and PICUS+ vs. All stumps (R = −0.49, p = 0.21). The analyzes performed also showed a strong negative correlation (p < 0.1) between trees with rot confirmed tomographically (PICUS+) (n) and the number of trees in the study plot (R = −0.96, p = 0.00011), and a positive correlation between trees with rot (PICUS+) (n) and stand age (Table 5).

Table 5.
Correlation coefficient and Spearman p-values for the compared variables (counts).
Basidiomata were found more frequently on stumps than in the root necks of standing trees. In wood samples taken from different sections of two felled trees that showed symptoms of wood decay on tomographic examination, genetic analysis confirmed the presence of the DNA of the pathogens with 100% identity: H. parviporum (NCBI sequence OL691107-OL691110), A. cepistipes (see above), both at 0.1 and 0.6 m, as well as Sistotrema brinkmannii (NCBI sequence OL691111) and Resinicium bicolor (NCBI sequence OL691112). On the other hand, the presence of H. parviporum was not confirmed at a height of 6.0 m, despite visible symptoms of wood decay (Figure 6). The presence of both pathogens, whose sequences were submitted to GenBank, was confirmed from both perimeters of the surface.

Figure 6.
Symptoms of heartwood rot caused by H. parviporum on successive 1-m sections of a spruce trunk. Photo by M. Damszel.
The six species collected were red-listed fungi. Two of them (Onnia tomentosa, Pleurotus pulmonarius) are listed in the threatened category and described as vulnerable (V), whereas four species (Entoloma byssisedum, Galerina triscopa, Physisporinus vitreus, and Postia ptychogaster) are rare (R). Of the identified species, 20 (Amphinema byssoides, Armillaria borealis, A. cepistipes, Clitopilus hobsonii, Entoloma byssisedum, Galerina triscopa, Hyphoderma roseocremeum, Hyphodontia arguta, H. pallidula, Onnia tomentosa, Physisporinus vitreus, Pleurotus pulmonarius, Pluteus pouzarianus, Postia ptychogaster, P. tephroleuca, Ramaria apiculata, Sistotrema brinkmannii, Trechispora hymenocystis, T. nivea, and T. mollusca) are included in the “Register of Protected and Endangered Fungal Species in Poland (GREJ)”.
4. Discussion
In this study, the presence of fungi was identified by classical methods. Root pathogens were detected using genetic methods, both on 821 trees, including 240 PICUS-trees, and on 339 stumps in both site types (four stands on old forest soil and four stands on post-agricultural soil). Based on our results, the wood of both standing spruce with living crowns and stumps is commonly colonized by fungi belonging to different trophic groups, with different interactions among them [59,60,61]. The difference in the occurrence of different taxa (values of Chao1 and ACE) may be caused by differences in the structure of spruce wood due to different soil fertility levels (arable was made fertile in the past) [62,63].
A similar number of wood-inhabiting species (58), compared to our study (57), in spruce stands on former agricultural land was found in a 3-year study in Slovakia in spruce stands aged 21–51 years [64]. It is worth noting that in the cited studies, typical spruce pathogens, such as Armillaria spp. and Heterobasidion spp., only sporadically occurred, in contrast to our results. We detected numerous species of pathogens (Heterobasidion parviporum and Armillaria spp.) as well as saprotrophs and symbiotrophs. Kubart et al. (2016) [65] point out that the presence of stump fungi is common in spruce stands in different regions of Sweden, whereas saprotrophic fungi, especially Resinicium bicolor, Fomitopsis pinicola, and Hyphodontia spp., which were also found in our studies, are present in large numbers.
It is interesting to note that the occurrence of fungi on spruce stumps and trees growing on an old arable soil was more numerous and diverse than on forest soils. This is probably due to the greater fertility of arable soils, which favors the formation of a wider annual increment [66,67], as well as to different fungal communities present in the environment of both types of stands in the past and today [68,69].
The analysis showed the strong negative correlation between the number of trees with tomographically confirmed decay and all stumps on the plots and positive correlation with stand age, which can be attributed to the systematic removal of dead trees by the forest administration and thus the increasing number of stumps. The weak correlation between all stumps and the number of inhabiting taxa is understandable. It is surprising that communities frequency within a dense forest complex, i.e., a Borecka forest, differ considerably depending on the type of soil (forest or arable) on which the stands grow. For similar trends in colonization of stumps and logs in a habitat, see Kubartova et al. (2012) [70].
Among the identified fungi, several taxa are effective competitors of food root pathogens (including Hypholoma, Pleurotus, and Phlebiopsis) and have therefore been studied and used in the biological protection against these pathogens [71,72,73,74,75]. The relatively high proportion of Hypholoma species suggests that fungi of this genus are widespread and important organisms that can limit the population of root pathogens in spruce stands [76,77].
Interestingly, several fungi described by Wojewoda and Ławrynowicz (2006) [51] as threatened/vulnerable (Onnia tomentosa, Pleurotus pulmonarius) or rare (Entoloma byssisedum, Galerina triscopa, Physisporinus vitreus, Postia ptychogaster) were identified in this study. In Poland, O. tomentosa is known from about a dozen sites, mainly from large, well-preserved forest complexes in the mountains (in the south of the country) and in the north [78,79]; this species has not been reported from the Borecka Forest. According to Ryvarden et al. (2017) [49], terrestrial basidioma often develop in large numbers from roots in old spruce stands. In our study, basidiocarps were found in both old (111 years old, forest plot 4) and a much younger, 74-year-old tree stand (arable plot 7). P. pulmonarius is known from numerous sites in Poland [79] and does not seem to be a threat to any species. It grows on various deciduous trees and in North America also on conifers, e.g., on Abies [80,81]. In Poland, P. pulmonarius has rarely been reported on conifers, e.g., Picea abies [82,83], and recently, it has been found on the trunk of a fallen Pinus sylvestris in a wind-damage area in Kampinos National Park [84]. The occurrence of numerous rare fungal species in spruce stands, both in managed and old forests, has been reported previously [85,86,87,88,89].
Gori et al. (2013) [90] found that in the Alps, spruce infection and wood rot can last for up to 80 years, with variable growth decline depending on climatic altitudes and the effects of drought [91]. The trees tested showed extensive heartwood decay, suggesting that infection began in the younger age classes. In the Borecka Forest, the proportion of H. parviporum-positive trees was 13.3–70.3%, although the trees were older than 50 years. The large number of undergrowth trees in the studied plots indicates a high density of root systems and an easy penetration of the mycelium of both root pathogens (H. parviporum and Armillaria spp.) into the trees due to numerous secondary infections through the roots [37,92,93,94]. In Norway, significant threats are caused by both Heterobasidion fungi, with a high proportion of H. parviporum, 98.5% [95]. The authors found that 68.2% of the trees were infected in 44-year-old regenerated spruce stands.
The Armillaria species found here (A. cepistipes and A. borealis) are mainly described as weak pathogens of deciduous trees and as opportunistic pathogens or saprotrophs of conifers [96,97,98,99]. In the current study, A. cepistipes was identified by genetic analyses of DNA from underneath bark, rhizomorphs, and basidiocarps collected in all stands studied, but surprisingly, more frequently in stands established on post-agricultural soils than on forest soils, which is described by other authors [100,101,102].
In a previous study, both H. parviporum and A. cepistipes have also been re-isolated from within the wood after felling standing trees whose crowns showed no symptoms of disease [103]. This confirms the thesis that without computed tomographic assessment of the interior of the wood, trees infected with the pathogen can grow unproductively for decades as commodities in managed spruce stands [19,37,104,105]. Another question is their ecological role in the ecosystem, where the pathogen and the resulting decomposition of wood during forest succession can provide suitable opportunities for the development of other organisms such as other fungi, birds, bats, insects, and squirrels [106,107,108,109,110].
Rigerte et al. (2019) [34] found 11 taxa described only as fungal root endophytes in spruce, none of which were identified in the present study. Numerous saprotrophs and symbiotrophs found on stumps and trees indicate that trees formerly infested by root pathogens are still attractive as food sources and are not discriminated against by the enzymes and metabolites of the pathogens [111].
5. General Conclusions
Our results show that trees older than 50 years, regardless of the type of soil on which the spruce stands grow (forest, post-agricultural), are subjected to advanced internal wood rot. This means that the infection of the roots took place many years earlier and that the development of the pathogen, detected by tomography inside the trunk, takes at least a dozen years. Consequently, in some valuable spruce stands, the degree of infestation of spruce with root pathogens should be determined by computed tomography at the age of 30–40 years.
Trees or stem sections showing advanced heartwood decay could be termed “dead-wood”, and some of them should be left in the stand from an ecological point of view to contribute to an increased biodiversity.
Because of the significant proportion of fungi of the genus Armillaria in the studied stand, which is generally underestimated in risk assessments, monitoring methods for assessing the presence of these pathogens in spruce stands need to be revised, including the severity of rhizomorph occurrence in the soil.
Author Contributions
A.S., M.D., K.S. and Z.S. substantially contributed to the conceptualization, resources, writing of the original draft, review and editing of the text; W.K., A.S. and M.D. performed the field experiments; K.S. and A.S. analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Warmia and Mazury University in Olsztyn, Poland (Project No. 30.610.019-110).
Data Availability Statement
Sequences OL634955-56; OL652577-83; OL691107-OL691112 are available at http://www.ncbi.nlm.nih.gov(accessed on 12 December 2021).
Acknowledgments
The authors thank Agata Młodzińska, Bioidea Poland, for her constructive bio-informatic additions, the Czerwony Bór and Borki Forest Districts administration for help in the field works and the anonymous reviewers for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woodward, S.; Stenlid, J.; Karjalainen, R.; Hüttermann, A. Heterobasidion Annosum. Biology, Ecology, Impact and Control; CAB International: Wallingford, UK, 1998. [Google Scholar]
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref s.l. Mol. Plant Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef]
- Żółciak, A.; Bohacz, J. Ligninolytic activity of Heterobasidion parviporum isolates in cultivation on Norway spruce wood. Sylwan 2016, 160, 1027–1035. [Google Scholar] [CrossRef]
- Burdsall, H.H., Jr.; Volk, T.J. Armillaria solidipes, an older name for the fungus called Armillaria ostoyae. North. Am. Fungi 2008, 3, 261–267. [Google Scholar] [CrossRef]
- Oliva, J.; Bernat, M.; Stenlid, J. Heartwood stump colonisation by Heterobasidion parviporum and H. annosum s.s. in Norway spruce (Picea abies) stands. For. Ecol. Manag. 2013, 295, 1–10. [Google Scholar] [CrossRef]
- Sierota, Z.; Grodzki, W.; Szczepkowski, A. Abiotic and Biotic Disturbances Affecting Forest Health in Poland over the Past 30 Years: Impacts of Climate and Forest Management Forests. Forests 2019, 10, 75. [Google Scholar] [CrossRef]
- Boddy, L. Fungal community ecology and wood decomposition processes in angiosperms: From standing tree to complete decay of coarse woody debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar]
- Chapin III, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar]
- Hammel, K.E.; Kapich, A.N.; Jensen, K.A., Jr.; Ryan, Z.C. Reactive oxygen species as agents of wood decay by fungi. Enzym. Microb. Technol. 2002, 30, 445–453. [Google Scholar] [CrossRef]
- Hietala, A.M.; Dörsch, P.; Kvaalen, H.; Solheim, H. Carbon dioxide and methane formation in Norway spruce stems infected by white-rot fungi. Forests 2015, 6, 3304–3325. [Google Scholar] [CrossRef]
- Sierota, Z.; Żółciak, A.; Małecka, M.; Sikora, K.; Damszel, M. An approach to calculate CO2 release through Norway spruce wood decay by Heterobasidion parviporum. Dendrobiology 2018, 79, 91–96. [Google Scholar] [CrossRef]
- Swift, M.J. The roles of fungi and animals in the immobilisation and release of nutrient elements from decomposing branch-wood. In Soil Organisms as Components of Ecosystems; Lohm, U., Persson, T., Eds.; Swedish Natural Science Research Council: Stockholm, Sweden, 1977; Volume 25, pp. 193–202. [Google Scholar]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Hendrickson, O. Abundance and activity of N2-fixing bacteria in decaying wood. Can. J. For. Res. 1991, 21, 1299–1304. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Pulliam, W.M.; Lodge, D.J.; Quinones-Orfila, V.; Fetcher, N.; Guzman-Grajales, S.; Parrotta, J.A.; Asbury, C.E.; Walker, L.R.; Waide, R.B. Nitrogen immobilization by decomposing woody debris and the recovery of tropical wet forest from hurricane damage. Oikos 1995, 72, 314–322. [Google Scholar] [CrossRef]
- Metzler, B. Quantitative assessment of fungal colonization in Norway spruce after green pruning. Eur. J. For. Path. 1997, 27, 1–11. [Google Scholar] [CrossRef]
- Eriksson, K.-E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer-Verlag: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Nagy, N.E.; Ballance, S.; Kvaalen, H.; Fossdal, C.G.; Solheim, H.; Hietala, A.M. Xylem defense wood of Norway spruce compromised by the pathogenic white-rot fungus Heterobasidion parviporum shows a prolonged period of selective decay. Planta 2012, 236, 1125–1133. [Google Scholar] [CrossRef]
- Klavina, D.; Bruna, L.; Zaluma, A.; Burnevica, N.; Polmanis, K.; Gaitnieks, T.; Piri, T. Infection and Spread of Root Rot Caused by Heterobasidion parviporum in Picea abies Stands after Thinning: Case Studies on Former Pasture and Meadow Lands. Environ. Sci. Proc. 2021, 3, 5230. [Google Scholar] [CrossRef]
- Shortle, W.C.; Smith, K.T. Electrical properties and rate of decay in spruce and fir wood. Phytopathology 1987, 77, 811–814. [Google Scholar] [CrossRef]
- Mattheck, C.G.; Bethge, K.A. Detection of decay in trees with the Metriguard Stress Wave Timer. J. Arbor. 1993, 19, 374–378. [Google Scholar]
- Sierota, Z.; Wrzosek, M.; Małecka, M.; Zółciak, A. Decay indices for evaluating wood decomposition activity. Biocontrol Sci. Technol. 2016, 26, 163–173. [Google Scholar] [CrossRef]
- Nicolotti, G.; Socco, L.V.; Martinis, R.; Godio, A.; Sambuelli, L. Application and comparison of three tomographic techniques for detection of decay in trees. J. Arboric. 2003, 29, 66–78. [Google Scholar]
- Chomicz, E. Bezinwazyjne metody wykrywania defektów wewnątrz pni drzew stojących (Tomograf PiCUS Sonic and PiCUS Treetronic). Leś. Pr. Bad. 2007, 3, 117–122. [Google Scholar]
- Szczepkowski, A.; Kowalczuk, W. Nowe stanowisko Perenniporia fraxinea (Polyporaceae, Basidiomycota) w Polsce i tomograficzna analiza drewna porażonego drzewa. Przegląd Przyr. 2018, 29, 116–121. [Google Scholar]
- Nilsson, K.; Bjurman, J. Chitin as an indicator of the biomass of two wood-decay fungi in relation to temperature, incubation time, and media composition. Can. J. Microbiol. 1998, 44, 575–581. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Finlay, R.D. Activities of chitinolytic enzymes during primary and secondary colonization of wood by basidiomycetous fungi. New Phytol. 2006, 169, 389–397. [Google Scholar] [CrossRef]
- Piri, T.; Korhonen, K. Spatial distribution and persistence of Heterobasidion parviporum genets on a Norway spruce site. For. Pathol. 2007, 37, 1–8. [Google Scholar] [CrossRef]
- Piri, T.; Hamberg, L. Persistence and infectivity of Heterobasidion parviporum in Norway spruce root residuals following stump harvesting. For. Ecol. Manag. 2015, 353, 49–58. [Google Scholar] [CrossRef]
- Oliva, J.; Rommel, S.; Fossdal, C.G.; Hietala, A.M.; Nemesio-Gorriz, M.; Solheim, H.; Elfstrand, M. Transcriptional responses of Norway spruce (Picea abies) inner sapwood against Heterobasidion parviporum. Tree Physiol. 2015, 35, 1007–1015. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, H.; Vainio, E.J.; Raffaello, T.; Kovalchuk, A.; Morin, E.; Duplessis, S.; Asiegbu, F.O. Intraspecific comparative genomics of isolates of the Norway spruce pathogen (Heterobasidion parviporum) and identification of its potential virulence factors. BMC Genom. 2018, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Sipari, N.; Asiegbu, F.O. Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol. Control 2016, 99, 53–63. [Google Scholar] [CrossRef]
- Rigerte, L.; Blumenstein, K.; Terhonen, E. New R-Based Methodology to Optimize the Identification of Root Endophytes against Heterobasidion parviporum. Microorganisms 2019, 7, 102. [Google Scholar] [CrossRef]
- Awan, H.U.M.; Asiegbu, F.O. Interspecific interactions within fungal communities associated with wood decay and forest trees. In Forest Microbiology: Tree Microbiome: Phyllosphere, Endosphere and Rhizosphere; Asiegbu, F.O., Kovalchuk, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 75–108. [Google Scholar] [CrossRef]
- Gonthier, P.; Garbelotto, M.; Nicolotti, G. Swiss stone pine trees and spruce stumps represent an important habitat for Heterobasidion spp. in subalpine forests. For. Pathol. 2003, 33, 191–203. [Google Scholar] [CrossRef]
- Piri, T.; Valkonen, S. Incidence and spread of Heterobasidion root rot in uneven-aged Norway spruce stands. Can. J. For. Res. 2013, 43, 872–877. [Google Scholar] [CrossRef]
- Honkaniemi, J.; Ojansuu, R.; Piri, T.; Kasanen, R.A.O.; Lehtonen, M.; Salminen, H.; Kalliokoski, T.; Mäkinen, H. Hmodel, a Heterobasidion annosum model for even-aged Norway spruce stands. Can. J. For. Res. 2014, 44, 796–809. [Google Scholar] [CrossRef]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Dobrowolski, R.; Grzegorczyk, I.; Jodłowski, M.; et al. Physico-geographical mesoregions of Poland: Verifcation and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Szmidla, H. Warunki Pogodowe w 2020. In Krótkoterminowa Prognoza Występowania Ważniejszych Szkodników i Chorób Infekcyjnych Drzew Leśnych w Polsce w 2021 roku; Jabłoński, T., Ed.; Instytut Badawczy Leśnictwa: Sękocin Stary, Poland, 2021; pp. 13–18. [Google Scholar]
- Bank Danych o Lasach. Available online: https://www.bdl.lasy.gov.pl/ (accessed on 11 November 2021).
- Gilbert, E.A.; Smiley, E.T. Picus Sonic Tomography for the quantification of decay in white oak (Quercus alba) and hickory (Carya spp.). J. Arboric. 2004, 30, 277–281. [Google Scholar]
- Deflorio, G.; Fink, S.; Schwarze, F.W.M.R. Detection of incipient decay in tree stems with sonic tomography after wounding and fungal inoculation. Wood Sci. Technol. 2008, 42, 117–132. [Google Scholar] [CrossRef]
- Clemençon, H. Methods for Working with Macrofungi. Laboratory Cultivation and Preparation of Larger Fungi for Light Microscopy; IHW-Verlag: Eching, Germany, 2009. [Google Scholar]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland, Ascomycetes; Verlag Mykologia: Luzern, Switzerland, 1984; Volume 1. [Google Scholar]
- Bernicchia, A. Polyporaceae s.l. Fungi Europaei 10; Edizioni Canduso: Alassio, Italy, 2005. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Corticiaceae s.l. Fungi Europaei 12; Edizioni Candusso: Alassio, Italy, 2010. [Google Scholar]
- Knudsen, H.; Vesterholt, J. Funga Nordica. Agaricoid, Boletoid, Clavarioid, Cyphelloid and Gastroid Genera, 2nd ed.; Nordsvamp: Copenhagen, Denmark, 2012. [Google Scholar]
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe, 2nd ed.; Fungiflora AS: Oslo, Norway, 2017. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org/authorsoffungalnames.htm (accessed on 11 November 2021).
- Wojewoda, W.; Ławrynowicz, M. Red list of the macrofungi in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szeląg, Z., Eds.; Polish Academy of Sciences: Kraków, Poland, 2006; pp. 53–70. [Google Scholar]
- Kujawa, A.; Gierczyk, B.; Ślusarczyk, T. GREJ - Rejestr gatunków grzybów chronionych i zagrożonych [Register of protected and en-dangered fungi species in Poland]. In Atlas grzybów Polski [Fungi of Poland]; Snowarski, M. Ed. Available online: http://www.grzyby.pl/rejestr-grzybow-chronionych-i-zagrozonych.htm (accessed on 11 November 2021).
- Kubiak, K.; Damszel, M.; Sikora, K.; Przemieniecki, S.; Małecka, M.; Sierota, Z. Colonization of fungi and bacteria in stumps and roots of Scots pine after thinning and treatment with Rotstop. J. Phytopath. 2016, 165, 143–156. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Burns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hantula, J.; Vainio, E. Specific primers for the differentiation of Heterobasidion annosum (s.str.) and H. parviporum infected stumps in northern Europe. Silva Fenn. 2003, 37, 181–187. [Google Scholar] [CrossRef]
- Szewczyk, W.; Kwaśna, J.; Behnke-Borowczyk, J. Populations of Armillaria species in pine plantations in west-central Poland. Dendrobiology 2015, 74, 95–108. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 July 2019).
- Boddy, L. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol. Ecol. 2000, 31, 185–194. [Google Scholar] [CrossRef]
- Jönsson, M.T.; Edman, M.; Jonsson, B.G. Colonization and extinction patterns of wood-decaying fungi in a boreal old-growth Picea abies forest. J. Ecol. 2008, 96, 1065–1075. [Google Scholar] [CrossRef]
- Boddy, L.; Hiscox, J. Fungal ecology, principles and mechanisms of colonisation and competition by saprotrophic fungi. Microbiol. Spectr. 2016, 4, 4–6. [Google Scholar] [CrossRef]
- Bujoczek, L.; Zięba, S.; Banaś, J. Effect of site conditions and site index for the dominant tree species on the amount of deadwood in managed forests. Sylwan 2016, 160, 320–327. [Google Scholar] [CrossRef]
- Piętka, S.; Sotnik, A.; Damszel, M.; Sierota, Z. Coarse woody debris and wood-colonizing fungi-differences between a reserve stand and a managed forest in the Taborz region of Poland. J. For. Res. 2019, 30, 1081–1091. [Google Scholar] [CrossRef]
- Mihál, I.; Luptáková, E.; Pavlík, M. Wood-inhabiting macromycetes communities in spruce stands on former agricultur-al land. J. For. Sci. 2021, 67, 51–65. [Google Scholar] [CrossRef]
- Kubart, A.; Vasaitis, R.; Stenlid, J.; Dahlberg, A. Fungal communities in Norway spruce stumps along a latitudinal gradient in Sweden. For. Ecol. Manag. 2016, 371, 50–58. [Google Scholar] [CrossRef]
- Tomczak, A.; Jelonek, T. Radial variation in the wood properties of Scots pine (Pinus sylvestris L.) grown on former agricultural soil. For. Res. Pap. 2013, 74, 171–177. [Google Scholar] [CrossRef]
- Cukor, J.; Zeidler, A.; Vacek, Z.; Vacek, S.; Šimůnek, V.; Gallo, J. Comparison of growth and wood quality of Norway spruce and European larch: Effect of previous land use. Eur. J. For. Res. 2020, 139, 459–472. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Balami, S.; Vašutová, M.; Košnar, J.; Karki, R.; Khadka, C.; Tripathi, G.; Cudlín, P. Soil fungal communities in abandoned agricultural land has not yet moved towards the seminatural forest. For. Ecol. Manag. 2012, 491, 119181. [Google Scholar] [CrossRef]
- Kubartova, A.; Ottosson, E.; Dahlberg, A.; Stenlid, J. Patterns of fungal communities among and within decaying logs, revealed by 454 sequencing. Mol. Ecol. 2012, 21, 4514–4532. [Google Scholar] [CrossRef]
- Pratt, J.E.; Niemi, M.; Sierota, Z.H. Comparison of three products based on Phlebiopsis gigantea for the control of Heterobasidion annosum in Europe. Biocontr. Sci. Techn. 2000, 10, 469–479. [Google Scholar] [CrossRef]
- Chapman, B.; Xiao, G. Inoculation of stumps with Hypholoma fasciculare as a possible means to control Armillaria root disease. Can. J. Bot. 2000, 78, 129–134. [Google Scholar] [CrossRef]
- Zhang, J.; Ju, Y.; Zhao, B. Advances in the research of Pleurotus poison to nematodes and the potential use for Pleurotus in controlling pine wilt disease. Acta Agric. Univ. Jiangxien. 2002, 4, 441–444. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-JXND200204004.htm (accessed on 11 November 2021).
- Hoff, J.A.; Klopfenstein, N.B.; McDonald, G.I.; Tonn, J.R.; Kim, M.-S.; Zambino, P.J.; Hessburg, P.F.; Rogers, J.D.; Peever, T.L.; Carris, L.M. Fungal endophytes in woody roots of Douglas-fir (Pseudotsuga menziesii) and ponderosa pine (Pinus ponderosa). For. Pathol. 2004, 34, 255–271. [Google Scholar] [CrossRef]
- Thakur, M. Fungi as a Biological Tool for Sustainable Agriculture. In Agriculturally Important Fungi for Sustainable Agriculture. Fungal Biology; Yadav, A.N., Mishra, S., Kour, D., Yadav, N., Kumar, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 255–273. [Google Scholar] [CrossRef]
- Nicolotti, G.; Gonthier, P.; Varese, G.C. Efectiveness of some biocontrol and chemical treatments against Heterobasidion annosum on Norway spruce stumps. Eur. J. For. Pathol. 1999, 29, 339–346. [Google Scholar] [CrossRef]
- Warwell, M.V.; McDonald, G.I.; Hanna, J.W.; Kim, M.-S.; Lalande, B.M.; Stewart, J.E.; Hudak, A.T.; Klopfenstein, N.B. Armillaria altimontana Is Associated with Healthy Western White Pine (Pinus monticola): Potential in Situ Biological Control of the Armillaria Root Disease Pathogen, A. solidipes. Forests 2019, 10, 294. [Google Scholar] [CrossRef]
- Wojewoda, W. Checklist of Polish larger Basidiomycetes; Polish Academy of Science: Kraków, Poland, 2003. [Google Scholar]
- Kujawa, A. Macroscopic fungi of Poland in mycological literature. Available online: http://www.grzyby.pl/grzyby-makroskopijne-Polski-w-literaturze-mikologicznej.htm (accessed on 11 November 2021).
- Hilber, O. The genus Pleurorus (Fr.) Kummer (2); Private: Kelheum, Germany, 1997. [Google Scholar]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkeley, CA, USA, 2000. [Google Scholar]
- Gierczyk, B.; Kujawa, A.; Szczepkowski, A.; Ślusarczyk, T.; Pachlewski, T.; Chachuła, P.; Domian, G. Macrofungi of the Bieszczady Mountains. Acta Mycol. 2019, 54, 1124. [Google Scholar] [CrossRef]
- Chachuła, P.; Dorda, A.; Fiedor, M.; Rutkowski, R. Grzyby Cieszyna; Urząd Miejski w Cieszynie: Cieszyn, Poland, 2015. [Google Scholar]
- Szczepkowski, A. (Institute of Forest Sciences, Warsaw University of Life Sciences—SGGW, Warsaw, Poland); Gierczyk, B. (Faculty of Chemistry, Adam Mickiewicz University in Poznań, Poznań, Poland); Kujawa, A. (Institute for Agricultural and Forest Environment, Polish Academy of Sciences, Poznań, Poland), Ślusarczyk, T. (Naturalists’ Club, Świebodzin, Poland). P. pulmonarius has been found on the trunk of a fallen Pinus sylvestris in a wind-damage area in Kampinos National Park. Unpublished work, 2019.
- Penttilla, R.; Siitonen, J.; Kuusinen, M. Polypore diversity in managed and old-growth boreal Picea abies forests in southern Finland. Biol. Cons. 2004, 117, 271–283. [Google Scholar] [CrossRef]
- Junninen, K.; Komonen, A. Conservation ecology of boreal polypores: A review. Biol. Conserv. 2011, 144, 11–20. [Google Scholar] [CrossRef]
- Norden, J.; Penttilla, R.; Siitonen, J.; Tomppo, E.; Ovaskainen, O. Specialist species of wood-inhabiting fungi struggle while generalists thrive in fragmented boreal forests. J. Ecol. 2013, 101, 701–712. [Google Scholar] [CrossRef]
- Kunttu, P.; Helo, T.; Kulju, M.; Julkunen, J.; Pennanen, J.; Shiryaev, A.G.; Lehtonen, H.; Kotiranta, H. Aphyllophoroid funga (Basidiomycota) of Finland: Range extensions and records of nationally new and rare species. Acta Mycol. 2019, 54, 1128. [Google Scholar] [CrossRef]
- Komonen, A.; Puumala, I.; Várkonyi, G.; Penttilä, R. Wood-decaying fungi in old-growth boreal forest fragments: Extinctions and colonizations over 20 years. Silva Fenn. 2021, 55, 10491. [Google Scholar] [CrossRef]
- Gori, Y.; Cherubini, P.; Camin, F.; La Porta, N. Fungal root pathogen (Heterobasidion parviporum) increases drought stress in Norway spruce stand at low elevation in the Alps. Eur. J. Forest. Res. 2013, 132, 607–619. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant. Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood. Its Biology and Ecology; John Wiley & Sons: Hoboken, NJ, USA, 1988. [Google Scholar]
- Gunulf, A.; Wang, L.; Englund, J.-E.; Rönnberg, J. Secondary spread of Heterobasidion parviporum from small Norway spruce stumps to adjacent trees. For. Ecol. Manage. 2013, 287, 1–8. [Google Scholar] [CrossRef]
- Gaitnieks, T.; Zaļuma, A.; Kenigsvalde, K.; Kļaviņa, D.; Brauners, I.; Piri, T. Susceptibility of Small-Diameter Norway Spruce Understory Stumps to Heterobasidion Spore Infection. Forests 2019, 10, 521. [Google Scholar] [CrossRef]
- Piri, T.; Korhonen, K.T. The effect of winter thinning on the spread of Heterobasidion parviporum in Norway spruce stands. Can. J. For. Res. 2008, 38, 2589–2595. [Google Scholar] [CrossRef]
- Fox, R.T.V. Armillaria Root Rot: Biology and Control of Honey Fungus; Intercept Press: Andover, Hampshirte, UK, 2000. [Google Scholar]
- Prospero, S.; Holdenrieder, O.; Rigling, D. Comparison of the virulence of Armillaria cepistipes and Armillaria ostoyae on four Norway spruce provenances. For. Pathol. 2004, 34, 1–14. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskas, R.; Larsson, K.-H.; Stenlid, J. Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Heinzelmann, R.; Dutech, C.; Tsykun, T.; Labbé, F.; Soularue, J.-P.; Prospero, S. Latest advances and future perspectives in Armillaria research. Can. J. Pl. Path. 2019, 41, 1–23. [Google Scholar] [CrossRef]
- Mańka, M.; Łakomy, P. Effect of thinning in Scotch pine (Pinus sylvestris L.) stand growing on former arable land, on suppressiveness of soil to Heterobasidion annosum (Fr.) Bref. and Armillaria obscura (Schaeff.) Herink. Phytopath. Polon. 1995, 9, 45–51. [Google Scholar]
- Kubiak, K.; Żółciak, A.; Damszel, M.; Lech, P.; Sierota, Z. Armillaria Pathogenesis under Climate Changes. Forests 2017, 8, 100. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Damszel, M.; Ciesielski, S.; Kubiak, K.; Mastalerz, J.; Sierota, Z.; Gorczyca, A. Bacterial microbiome in Armillaria ostoyae rhizomorphs inhabiting the root zone during progressively dying Scots pine. Appl. Soil Ecol. 2021, 164, 103929. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Mukrimin, M.; Zeng, Z.; Rafaello, T.; Liu, M.; Kasanen, R.A.O.; Sun, H.; Asiegbu, F.O. Mycobiome analysis of asymptomatic and symptomatic Norway spruce trees naturally infected by the conifer pathogens Heterobasidion spp. Environ. Microbiol. Rep. 2018, 10, 532–541. [Google Scholar] [CrossRef]
- Seifert, T. Simulating the extent of decay caused by Heterobasidion annosum s. l. in stems of Norway spruce. For. Ecol. Manag. 2007, 248, 95–106. [Google Scholar] [CrossRef]
- Möykkynen, T.; Pukkala, T. Optimising the management of a Norway spruce stand on a site infected by Heterobasidion coll. Scand. J. For. Res. 2009, 24, 149–159. [Google Scholar] [CrossRef]
- Goheen, D.J.; Otrosina, W.J. Characteristics and consequences of root diseases in forests of Western North America. In User’s Guide to the Western Root Disease Model, version 3.0.; Frankel, S.J., Ed.; USDA: Albany, CA, USA, 1998; pp. 3–8. [Google Scholar]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer Verlag: Berlin, Germany, 2000. [Google Scholar]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Wrzosek, M.; Ruszkiewicz-Michalska, M.; Sikora, K.; Damszel, M.; Sierota, Z. The plasticity of fungal interactions. Mycol. Prog. 2017, 16, 101–108. [Google Scholar] [CrossRef]
- Jaworski, T.; Plewa, R.; Hilszczański, J.; Szczepkowski, A.; Horak, J. Saproxylic moths reveal complex within-group and group-environment patterns. J. Insect Conserv. 2016, 20, 677–690. [Google Scholar] [CrossRef]
- Custer, G.F.; van Diepen, L.T.A.; Stump, W.L. Structural and functional dynamics of soil microbes following spruce beetle infestation. Appl. Environ. Microbiol. 2020, 86, e01984–e02003. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).