Secondary Succession after Slash-and-Burn Cultivation in Papuan Lowland Forest, Indonesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Data Analysis
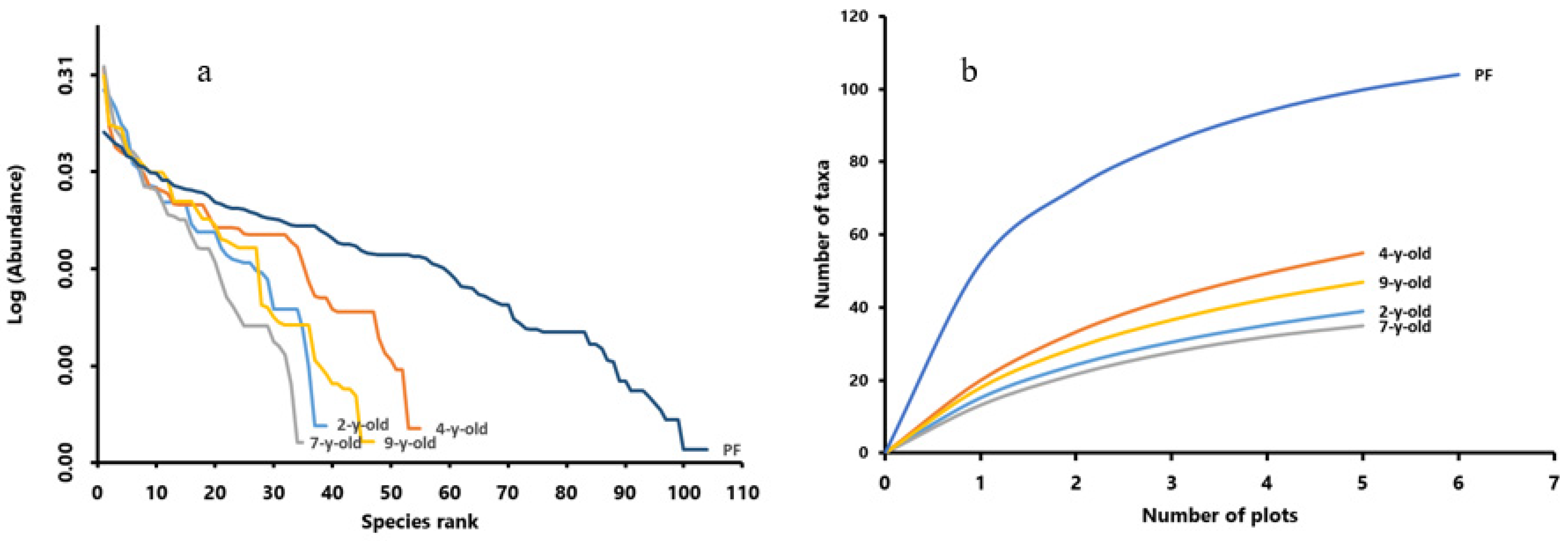
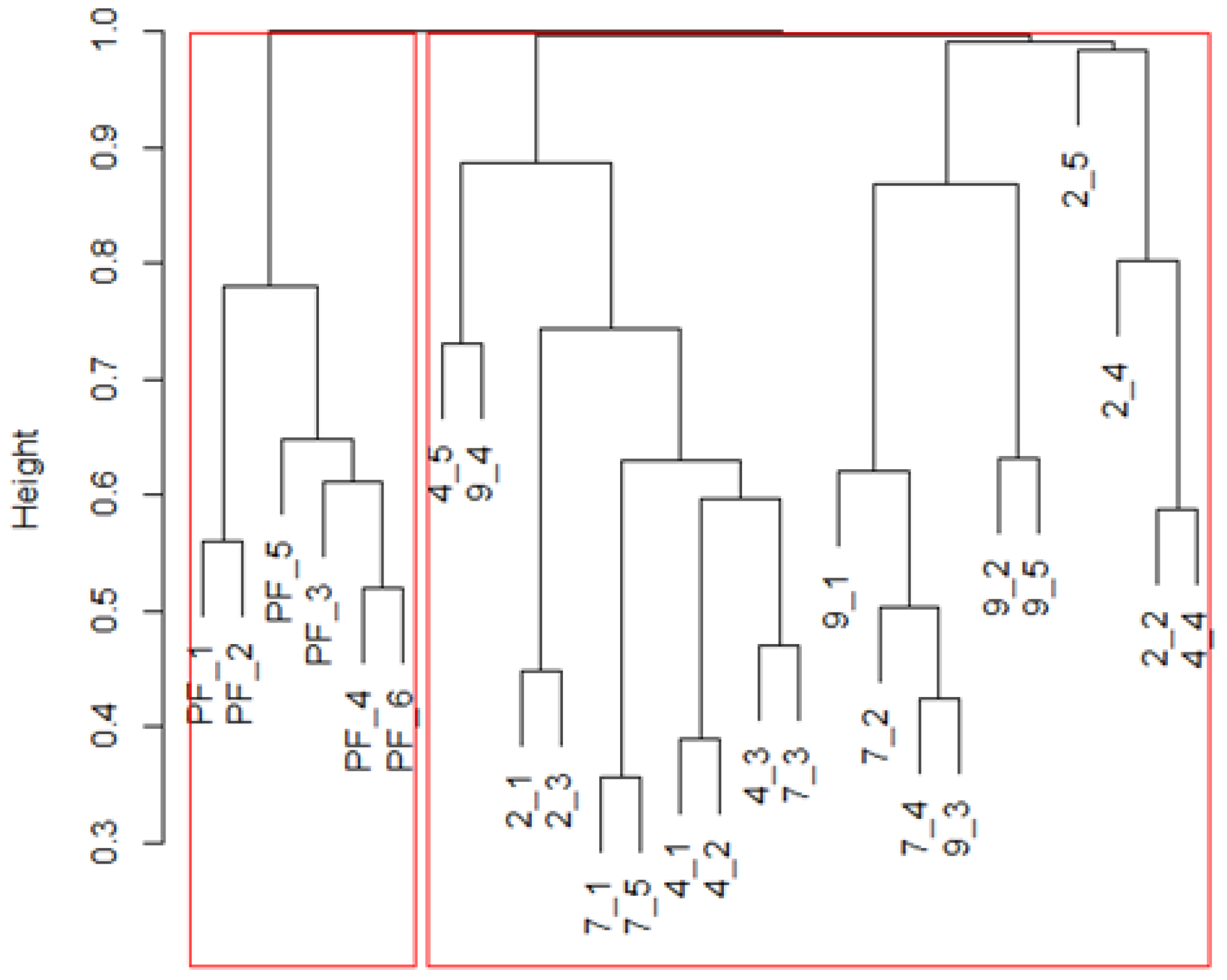
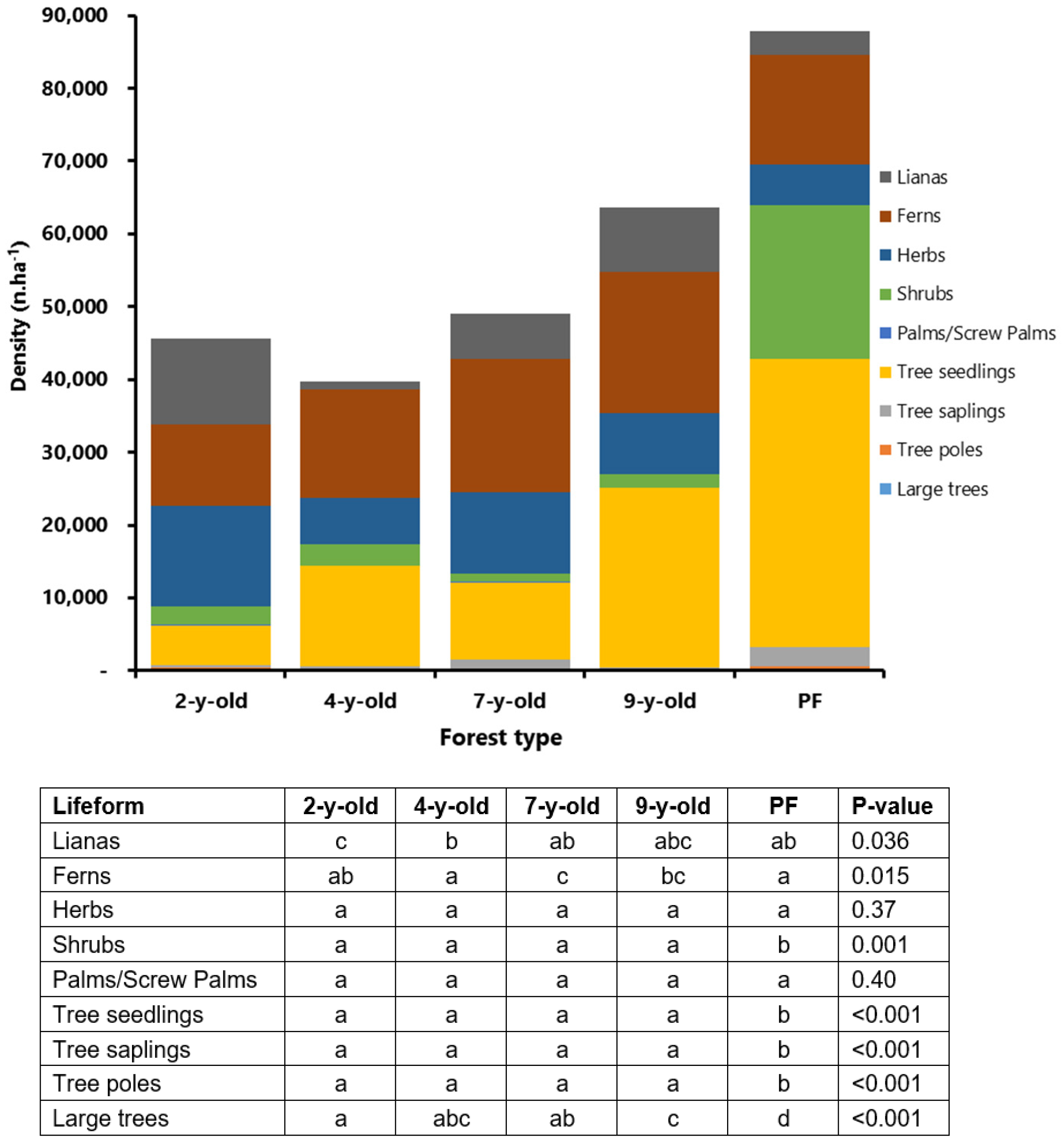
3. Results
Species Richness of Vegetation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Forest Type | ||||
|---|---|---|---|---|---|
| 2-Year-Old | 4-Year-Old | 7-Year-Old | 9-Year-Old | PF | |
| Actinodaphne nitida Teschner | √ | ||||
| Ageratum conyzoides (L.) L. | √ | ||||
| Aglaia spectabilis (Miq.) S.S.Jain and S.Bennet | √ | ||||
| Aglaia brassii Merr. and L.M.Perry | √ | √ | |||
| Alpinia galanga (L.) Willd. | √ | √ | |||
| Alpinia sp. | √ | ||||
| Alstonia scholaris (L.) R.Br. | √ | √ | √ | √ | √ |
| Ananas comosus (L.) Merr. | √ | ||||
| Anisoptera thurifera (Blanco) Blume | √ | ||||
| Antiaris toxicaria (J.F.Gmel.) Lesch. | √ | ||||
| Archidendron pachycarpum (Warb.) Dewit | √ | ||||
| Archidendron parviflorum Pulle | √ | √ | √ | ||
| Archidendron sp. | √ | ||||
| Areca catechu L. | √ | √ | |||
| Artocarpus altilis (Parkinson ex F.A.Zorn) Fosberg | √ | √ | √ | √ | |
| Artocarpus integer (Thunb.) Merr. | √ | ||||
| Baccaurea sp. | √ | ||||
| Bidens pilosa L. | √ | ||||
| Blechnum patersonii (R.Br.) Mett. | √ | ||||
| Bubbia sp. | √ | ||||
| Buchanania arborescens (Blume) Blume | √ | ||||
| Calophyllum inophyllum L. | √ | ||||
| Campnosperma coriaceum (Jack) Hallier f. | √ | ||||
| Cananga odorata (Lam.) Hook.f. and Thomson | √ | √ | √ | √ | |
| Canarium hirsutum Willd. | √ | ||||
| Celtis latifolia (Blume) Planch. | √ | ||||
| Ceodes umbellifera J.R.Forst. and G.Forst. | √ | ||||
| Cerbera floribunda K.Schum. | √ | ||||
| Chionanthus aff. macrocarpus Blume | √ | ||||
| Chionanthus sp. | √ | ||||
| Chisocheton ceramicus (Miq.) C.DC. | √ | ||||
| Cocos nucifera L. | √ | √ | |||
| Coffea sp. | √ | ||||
| Cryptocarya massoy (Oken) Kosterm. | √ | ||||
| Curcuma zanthorrhiza Roxb. | √ | ||||
| Cynometra browneoides (Harms) Rados. | √ | √ | |||
| Diospyros discolor Willd. | √ | √ | |||
| Dracontomelon dao (Blanco) Merr. and Rolfe | √ | √ | √ | √ | |
| Durio zibethinus L. | √ | √ | |||
| Dysoxylum mollissimum Blume | √ | √ | √ | √ | √ |
| Dysoxylum parasiticum (Osbeck) Kosterm. | √ | √ | |||
| Elaeocarpus angustifolius Blume | √ | ||||
| Endospermum moluccanum (Teijsm. and Binn.) Kurz | √ | √ | √ | ||
| Falcataria falcata (L.) Greuter and R.Rankin | √ | ||||
| Ficus aff. annulata Blume | √ | ||||
| Ficus benjamina L. | √ | ||||
| Ficus drupacea Thunb. | √ | ||||
| Ficus macrothyrsa Corner | √ | √ | √ | √ | |
| Ficus racemifera Roxb. | √ | ||||
| Ficus septica Burm.f. | √ | √ | √ | ||
| Ficus variegata Blume | √ | √ | √ | ||
| Ficus sp. 1 | √ | √ | √ | √ | |
| Ficus sp. 2 | √ | √ | |||
| Ficus sp. 3 | √ | √ | |||
| Ficus sp. 4 | √ | ||||
| Ficus sp. 5 | √ | ||||
| Garcinia sp. | √ | ||||
| Gmelina sp. | √ | ||||
| Gnetum gnemon L. | √ | √ | √ | √ | |
| Gonocaryum littorale (Blume) Sleumer | √ | ||||
| Grass A | √ | √ | √ | ||
| Gymnacranthera farquhariana (Wall ex. Hook.f. and Thomson) Warb. | √ | √ | |||
| Harpullia sp. | √ | ||||
| Hibiscus tiliaceus L. | √ | ||||
| Hibiscus sp. | √ | √ | |||
| Homalium foetidum (Roxb.) Benth. | √ | ||||
| Hopea sp. | √ | ||||
| Horsfieldia irya (Gaertn.) Warb. | √ | √ | √ | ||
| Hylodesmum repandum (Vahl) H.Ohashi and R.R.Mill | √ | √ | |||
| Imperata cylindrica (L.) Raeusch. | √ | √ | √ | √ | |
| Intsia bijuga (Colebr.) Kuntze | √ | √ | √ | ||
| Koordersiodendron pinnatum (Blanco) Merr. | √ | √ | √ | √ | |
| Lauraceae sp. | √ | ||||
| Leea aculeata Blume ex Spreng. | √ | √ | |||
| Lepiniopsis ternatensis Valeton | √ | √ | √ | ||
| Leucaena leucocephala (Lam.) de Wit | √ | ||||
| Liana A | √ | √ | |||
| Liana B | √ | ||||
| Liana C | √ | ||||
| Liana D | √ | ||||
| Litsea ledermannii Teschner | √ | ||||
| Litsea timoriana Span. | √ | √ | √ | ||
| Litsea tuberculata (Blume) Boerl. | √ | ||||
| Litsea sp. | √ | ||||
| Lunasia amara Blanco | √ | √ | |||
| Maasia glauca (Hassk.) Mols, Kessler and Rogstad | √ | ||||
| Macaranga sp. 1 | √ | √ | √ | √ | |
| Macaranga sp. 2 | √ | √ | √ | √ | √ |
| Mallotus floribundus (Blume) Müll.Arg. | √ | ||||
| Mangifera indica L. | √ | ||||
| Maniltoa sp. | √ | ||||
| Mastixiodendron pachyclados (K.Schum.) Melch. | √ | √ | √ | √ | |
| Medusanthera laxiflora (Miers) R.A.Howard | √ | √ | |||
| Melicope elleryana (F.Muell.) T.G.Hartley | √ | √ | |||
| Merremia sp. | √ | ||||
| Mimusopselengi L. | √ | ||||
| Monoon polycarpum (Burck) B.Xue and R.M.K.Saunders | √ | ||||
| Monstera sp. | √ | √ | √ | √ | |
| Mucuna novo-guineensis Scheff. | √ | √ | |||
| Musa sp. | √ | √ | |||
| Myristica fatua Houtt. | √ | ||||
| Myristica aff. gigantea King | √ | ||||
| Myristica sp. | √ | ||||
| Neolamarckia cadamba (Roxb.) Bosser | √ | √ | |||
| Neonauclea acuminata Ridsdale | √ | ||||
| Neonauclea sp. | √ | ||||
| Nephelium lappaceum L. | √ | √ | |||
| Nephrolepis sp. | √ | √ | √ | √ | √ |
| Ochrosia sp. | √ | ||||
| Octomeles sumatrana Miq. | √ | √ | √ | √ | |
| Orchidaceae sp. | √ | ||||
| Ormosia calavensis Azaola | √ | ||||
| Ormosia sp. | √ | ||||
| Osmoxylon aff. globulare Philipson | √ | √ | |||
| Palaquium amboinense Burck | √ | √ | |||
| Palaquium sp. | √ | √ | √ | ||
| Pandanus tectorius Parkinson ex Du Roi | √ | ||||
| Pimelodendron amboinicum Hassk. | √ | √ | |||
| Piper aduncum L. | √ | √ | √ | √ | √ |
| Piper sp. | √ | ||||
| Pipturus argenteus (G.Forst.) Wedd. | √ | ||||
| Polyalthia sp. 1 | √ | ||||
| Polyalthia sp. 2 | √ | ||||
| Pometia pinnata J.R.Forst. and G.Forst. | √ | √ | √ | ||
| Premna corymbosa Rottler | √ | √ | √ | √ | |
| Prunus arborea (Blume) Kalkman | √ | √ | |||
| Pterygota horsfieldii (R.Br.) Kosterm. | √ | √ | |||
| Pterygota sp. | √ | ||||
| Rhus taitensis Guill. | √ | √ | |||
| Rhus sp. | √ | ||||
| Sapindaceae sp. | √ | ||||
| Semecarpus papuanus Lauterb. | √ | ||||
| Spathiostemon javensis Blume | √ | √ | √ | ||
| Spondias dulcis Parkinson | √ | √ | √ | ||
| Stachytarpheta jamaicensis (L.) Vahl | √ | ||||
| Sterculia aff. elongata Ridl. | √ | √ | |||
| Sterculia macrophylla Vent. | √ | √ | √ | ||
| Sterculia parkinsonii F.Muell. | √ | ||||
| Sterculia urceolata Sm. | √ | √ | √ | √ | |
| Syzygium sp. | √ | √ | |||
| Tectaria aff. zollingeri (Kurz) Holttum | √ | √ | |||
| Teijsmanniodendron bogoriense Koord. | √ | ||||
| Thelypteridiaceae sp. | √ | √ | √ | √ | |
| Timonius timon (Spreng.) Merr. | √ | ||||
| Timonius sp. | √ | ||||
| Tree A | √ | ||||
| Tree B | √ | √ | √ | ||
| Uncaria sp. 1 | √ | ||||
| Uncaria sp. 2 | √ | ||||
| Vigna trilobata L. (Verdc.) | √ | ||||
| Vitex sp. | √ | √ | √ | ||
| Ziziphus sp. | √ | ||||
References
- Cámara-Leret, R.; Frodin, D.G.; Adema, F.; Anderson, C.; Appelhans, M.S.; Argent, G.; Guerrero, S.A.; Ashton, P.S.; Baker, W.J.; Barfod, A.S.; et al. New Guinea has the world’s richest island flora. Nature 2020, 584, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Murdjoko, A.; Ungirwalu, A.; Mardiyadi, Z.; Tokede, M.J.; Djitmau, D.A.; Benu, N.M.H. Floristic composition of Buah Hitam habitats in lowland tropical mixed forest of West Papua, Indonesia. Florest. Ambient. 2021, 28, e20210042. [Google Scholar] [CrossRef]
- Ungirwalu, A.; Awang, S.A.; Murdjoko, A. Model aplikasi agroforestri tumbuhan Buah Hitam (Haplolobus monticola Husson) berbasis pengetahuan lokal etnis Wandamen-Papua: Prospek pengembangan perhutanan sosial di Papua. In Prosiding Seminar Nasional Silvikultur II: Pembaruan Silvikultur untuk Mendukung Pemulihan Fungsi Hutan menuju Ekonomi Hijau, Universitas Gadjah Mada, Yogyakarta, 28 Agustus 2014; Prehaten, D., Syahbudin, A., Andiyani, R.D., Eds.; Fakultas Kehutanan, Universitas Gadjah Mada: Yogyakarta, Indonesia, 2014; pp. 268–274. [Google Scholar]
- Vallet, A.; Locatelli, B.; Levrel, H.; Brenes Pérez, C.; Imbach, P.; Estrada Carmona, N.; Manlay, R.; Oszwald, J. Dynamics of ecosystem services during forest transitions in Reventazón, Costa Rica. PLoS ONE 2016, 11, e0158615. [Google Scholar] [CrossRef] [PubMed]
- Wakhidah, A.Z.; Chikmawati, T.; Purwanto, Y. Homegarden ethnobotany of two Saibatin villages in Lampung, Indonesia: Species diversity, uses, and values. For. Soc. 2020, 4, 338–357. [Google Scholar] [CrossRef]
- Gaveau, D.L.A.; Santos, L.; Locatelli, B.; Salim, M.A.; Husnayaen, H.; Meijaard, E.; Heatubun, C.D.; Sheil, D. Forest loss in Indonesian New Guinea (2001–2019): Trends, drivers and outlook. Biol. Conserv. 2021, 261, 109225. [Google Scholar] [CrossRef]
- Manner, H.I. Ecological succession in new and old swiddens of montane Papua New Guinea. Hum. Ecol. 1981, 9, 359–377. [Google Scholar] [CrossRef]
- Ungirwalu, A.; Awang, S.A.; Suryanto, P.; Maryudi, A. The ethno-techno-conservation approach in the utilization of Black Fruit (Haplolobus sp.) by the Wandamen ethnic of Papua, Indonesia. Biodiversitas 2017, 18, 1336–1343. [Google Scholar] [CrossRef]
- Heinimann, A.; Mertz, O.; Frolking, S.; Christensen, A.E.; Hurni, K.; Sedano, F.; Chini, L.P.; Sahajpal, R.; Hansen, M.; Hurtt, G. A global view of shifting cultivation: Recent, current, and future extent. PLoS ONE 2017, 12, e0184479. [Google Scholar] [CrossRef]
- Mukul, S.A.; Herbohn, J. The impacts of shifting cultivation on secondary forests dynamics in tropics: A synthesis of the key findings and spatio temporal distribution of research. Environ. Sci. Policy 2016, 55, 167–177. [Google Scholar] [CrossRef]
- Schmidt-Vogt, D.; Leisz, S.J.; Mertz, O.; Heinimann, A.; Thiha, T.; Messerli, P.; Epprecht, M.; Cu, P.V.; Chi, V.K.; Hardiono, M.; et al. An assessment of trends in the extent of swidden in Southeast Asia. Hum. Ecol. 2009, 37, 269–280. [Google Scholar] [CrossRef]
- Angelsen, A. Shifting cultivation and “deforestation”: A study from Indonesia. World Dev. 1995, 23, 1713–1729. [Google Scholar] [CrossRef]
- Brearley, F.Q.; Prajadinata, S.; Kidd, P.S.; Proctor, J.; Suriantata. Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. For. Ecol. Manag. 2004, 195, 385–397. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.; Liu, S.; He, F.; Letcher, S.G. Recovery of woody plant diversity in tropical rain forests in southern China after logging and shifting cultivation. Biol. Conserv. 2012, 145, 225–233. [Google Scholar] [CrossRef]
- Fujiki, S.; Nishio, S.; Okada, K.-I.; Nais, J.; Kitayama, K. Plant communities and ecosystem processes in a succession-altitude matrix after shifting cultivation in the tropical montane forest zone of northern Borneo. J. Trop. Ecol. 2017, 33, 33–49. [Google Scholar] [CrossRef]
- Villa, P.M.; Martins, S.V.; Nolasco de Oliveira Neto, S.; Rodrigues, A.C.; Martorano, L.G.; Delgado Monsanto, L.; Cancio, N.M.; Gastauer, M. Intensification of shifting cultivation reduces forest resilience in the northern Amazon. For. Ecol. Manag. 2018, 430, 312–320. [Google Scholar] [CrossRef]
- Ribeiro Filho, A.A.; Adams, C.; Murrieta, R.S.S. The impacts of shifting cultivation on tropical forest soil: A review. Bol. Mus. Para. Emílio Goeldi Cienc. Hum. Belém 2013, 8, 693–727. [Google Scholar] [CrossRef]
- Kukla, J.; Whitfeld, T.J.S.; Cajthaml, T.; Baldrian, P.; Veselá-Šimáčková, H.; Novotný, V.; Frouz, J. The effect of traditional slash-and-burn agriculture on soil organic matter, nutrient content, and microbiota in tropical ecosystems of Papua New Guinea. Land. Degrad. Dev. 2019, 30, 166–177. [Google Scholar] [CrossRef]
- Polak, M. The botanical diversity in the Ayawasi area, Irian Jaya, Indonesia. Biodivers. Conserv. 2000, 9, 1345–1375. [Google Scholar] [CrossRef]
- Sheil, D.; Boissière, M.; van Heist, M.; Rachman, I.; Basuki, I.; Wan, M.; Watopa, Y. The floodplain forests of the Mamberamo Basin, Papua, Indonesia (western New Guinea): Vegetation, soils, and local use. Forests 2021, 12, 1790. [Google Scholar] [CrossRef]
- van Heist, M.; Sheil, D.; Rachman, I.; Gusbager, P.; Raweyai, C.; Yoteni, H. The forests and related vegetation of Kwerba, on the Foja foothills, Mamberamo, Papua (Indonesian New Guinea). Blumea 2010, 55, 153–161. [Google Scholar] [CrossRef][Green Version]
- Robiansyah, I. Diversity and biomass of tree species in Tambrauw, West Papua, Indonesia. Biodiversitas 2018, 19, 377–386. [Google Scholar] [CrossRef]
- Tawer, P.; Maturbongs, R.; Murdjoko, A.; Jitmau, M.; Djitmau, D.; Siburian, R.; Ungirwalu, A.; Wanma, A.; Mardiyadi, Z.; Wanma, J.; et al. Vegetation dynamic post-disturbance in tropical rain forest of Bird’s Head Peninsula of West Papua, Indonesia. Ann. Silvic. Res. 2021, 46, 48–58. [Google Scholar]
- Badan Pusat Statistik Kabupaten Manokwari. Kabupaten Manokwari Dalam Angka 2021; Badan Pusat Statistik Kabupaten Manokwari: Manokwari, Indonesia, 2021; p. 13.
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘vegan’ Community ecology package. Version 2.5–6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 3 May 2021).
- Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 11 October 2021).
- Sillitoe, P.; Shiel, R.S. Soil fertility under shifting and semi-continuous cultivation in the Southern Highlands of Papua New Guinea. Soil Use Manag. 1999, 15, 49–55. [Google Scholar] [CrossRef]
- Whitfield, T.J.S.; Lasky, J.R.; Damas, K.; Sosanika, G.; Molem, K.; Montgomery, R.A. Species richness, forest structure, and functional diversity during succession in the New Guinea lowlands. Biotropica 2014, 46, 538–548. [Google Scholar] [CrossRef]
- Hattori, D.; Kenzo, T.; Shirahama, T.; Harada, Y.; Kendawang, J.J.; Ninomiya, I.; Sakurai, K. Degradation of soil nutrients and slow recovery of biomass following shifting cultivation in the heath forests of Sarawak, Malaysia. For. Ecol. Manag. 2019, 432, 467–477. [Google Scholar] [CrossRef]
- Klanderud, K.; Mbolatiana, H.Z.H.; Vololomboahangy, M.N.; Radimbison, M.A.; Roger, E.; Totland, Ø.; Rajeriarison, C. Recovery of plant species richness and composition after slash-and-burn agriculture in a tropical rainforest in Madagascar. Biodivers. Conserv. 2010, 19, 187–204. [Google Scholar] [CrossRef]
- Pereira Cabral Gomes, E.; Sugiyama, M.; Fernandes de Oliveira Junior, C.J.; Medeiros Prado, H.; Ribeiro Filho, A.A.; Adams, C. Post-agricultural succession in the fallow swiddens of southeastern Brazil. For. Ecol. Manag. 2020, 475, 118398. [Google Scholar] [CrossRef]
- Siahaya, M.E.; Hutauruk, T.R.; Aponno, H.S.E.S.; Hatulesila, J.W.; Mardhanie, A.B. Traditional ecological knowledge on shifting cultivation and forest management in east Borneo, Indonesia. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 14–23. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef] [PubMed]
- Tongkoom, K.; Marohn, C.; Piepho, H.P.; Cadisch, G. Ecosystem recovery indicators as decision criteria on potential reduction of fallow periods in swidden systems of northern Thailand. Ecol. Indic. 2018, 95, 554–567. [Google Scholar] [CrossRef]
- Villa, P.M.; Martins, S.V.; Nolasco de Oliveira Neto, S.; Rodrigues, A.C.; Safar, N.V.H.; Delgado Monsanto, L.; Cancio, N.M.; Ali, A. Woody species diversity as an indicator of the forest recovery after shifting cultivation disturbance in the northern Amazon. Ecol. Indic. 2018, 95, 687–694. [Google Scholar] [CrossRef]
- Lu, X.; Zang, R.; Ding, Y.; Letcher, S.G.; Long, W.; Huang, Y. Variations and trade-offs in functional traits of tree seedlings during secondary succession in a tropical lowland rain forest. Biotropica 2014, 46, 404–414. [Google Scholar] [CrossRef]
- Hawes, J.E.; Vieira, I.C.G.; Magnago, L.F.S.; Berenguer, E.; Ferreira, J.; Aragão, L.E.O.C.; Cardoso, A.; Lees, A.C.; Lennox, G.D.; Tobias, J.A.; et al. A large-scale assessment of plant dispersal mode and seed traits across human-modified Amazonian forests. J. Ecol. 2020, 108, 1373–1385. [Google Scholar] [CrossRef]
- Murdjoko, A.; Jitmau, M.M.; Djitmau, D.A.; Siburian, R.H.S.; Ungirwalu, A.; Wanma, A.O.; Mardiyadi, Z.; Rumatora, A.; Mofu, W.Y.; Sineri, A.S.; et al. Heterospecific and conspecific associations of trees in lowland tropical forest of New Guinea. Biodiversitas 2020, 21, 4405–4418. [Google Scholar] [CrossRef]
- Chong, K.Y.; Corlett, R.T.; Nuñez, M.A.; Chiu, J.H.; Courchamp, F.; Dawson, W.; Kuebbing, S.; Liebhold, A.M.; Padmanaba, M.; Souza, L.; et al. Are terrestrial biological invasions different in the tropics? Annu. Rev. Ecol. Evol. Syst. 2020, 52, 291–314. [Google Scholar] [CrossRef]
- Hartemink, A.E. The invasive shrub Piper aduncum in Papua New Guinea: A review. J. Trop. For. Sci. 2010, 22, 202–213. [Google Scholar]
- Kuswandi, R.; Murdjoko, A. Population structures of four tree species in logged-over tropical forest in south Papua, Indonesia: An integral projection model approach. Indones. J. For. Res. 2015, 2, 93–101. [Google Scholar]
- Murdjoko, A. Recuperation of non-commercial trees in logged forest in southern Papua, Indonesia. J. Manaj. Hutan Trop. 2013, 19, 94–102. [Google Scholar]
- Murdjoko, A.; Marsono, D.; Sadono, R.; Hadisusanto, S. Population dynamics of Pometia for the period of post-selective logging in tropical rainforest, southern Papua, Indonesia. Biosaintifika 2016, 8, 321–330. [Google Scholar] [CrossRef]
- McNamara, S.; Erskine, P.D.; Lamb, D.; Chantalangsy, L.; Boyle, S. Primary tree species diversity in secondary fallow forests of Laos. For. Ecol. Manag. 2012, 281, 93–99. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Junqueira, A.B.; Crouzeilles, R.; Peña-Claros, M.; Mesquita, R.C.G.; Bongers, F. The role of land-use history in driving successional pathways and its implications for the restoration of tropical forests. Biol. Rev. 2021, 96, 1114–1134. [Google Scholar] [CrossRef]
- Cuni Sanchez, A.; Lindsell, J.A. The role of remnant trees in carbon sequestration, vegetation structure and tree diversity of early succession regrowing fallows in eastern Sierra Leone. Afr. J. Ecol. 2017, 55, 188–197. [Google Scholar] [CrossRef]
- Sandor, M.E.; Chazdon, R.L. Remnant trees affect species composition but not structure of tropical second-growth forest. PLoS ONE 2014, 9, e83284. [Google Scholar]
- Lepš, J.; Novotný, V.; Čížek, L.; Molem, K.; Isua, B.; Boen, W.; Kutil, R.; Auga, J.; Kasbal, M.; Manumbor, M.; et al. Successful invasion of the Neotropical species Piper aduncum in rain forests in Papua New Guinea. Appl. Veg. Sci. 2002, 5, 255–262. [Google Scholar] [CrossRef]
- Labrière, N.; Locatelli, B.; Laumonier, Y.; Freycon, V.; Bernoux, M. Soil erosion in the humid tropics: A systematic quantitative review. Agric. Ecosyst. Environ. 2015, 203, 127–139. [Google Scholar] [CrossRef]
- Li, P.; Feng, Z.; Jiang, L.; Liao, C.; Zhang, J. A review of swidden agriculture in Southeast Asia. Remote Sens. 2014, 6, 1654–1683. [Google Scholar] [CrossRef]
- Trethowan, L.A.; Eiserhardt, W.L.; Girmansyah, D.; Kintamani, E.; Utteridge, T.M.A.; Brearley, F.Q. Floristics of forests across low nutrient soils in Sulawesi, Indonesia. Biotropica 2020, 52, 1309–1318. [Google Scholar] [CrossRef]
- Utteridge, T.M.A.; Jennings, L.V.S. Trees of New Guinea; Kew Publishing: Richmond, UK, 2021. [Google Scholar]
- Rochmyaningsih, D. Massive road project threatens New Guinea’s biodiversity. Science 2021, 374, 246–247. [Google Scholar] [CrossRef]
- Cámara–Leret, R.; Dennehy, Z. Indigenous knowledge of New Guinea’s useful plants: A review. Econ. Bot. 2019, 73, 405–415. [Google Scholar] [CrossRef]
- Ungirwalu, A.; Awang, S.A.; Runtuboi, Y.Y.; Peday, M.Y.; Marwa, J.; Maitar, B.; Murdjoko, A.; Fatem, S.M. Customary forests in West Papua: Contestation of desires or needs? For. Soc. 2021, 5, 365–375. [Google Scholar] [CrossRef]
- Brearley, F.Q.; Adinugroho, W.C.; Cámara-Leret, R.; Krisnawati, H.; Ledo, A.; Qie, L.; Smith, T.E.L.; Aini, F.; Garnier, F.; Lestari, N.S.; et al. Opportunities and challenges for an Indonesian forest monitoring network. Ann. For. Sci. 2019, 76, 54. [Google Scholar] [CrossRef]


| Variable | Forest Type | ||||
|---|---|---|---|---|---|
| 2-Year-Old | 4-Year-Old | 7-Year-Old | 9-Year-Old | PF | |
| SOM (%) | 18.40 ± 4.82 | 8.05 ± 3.15 | 9.38 ± 2.77 | 3.35 ± 0.36 | 10.00 ± 0.81 |
| b | ab | ab | a | b | |
| Total N (%) | 0.47 ± 0.05 | 0.41 ± 0.07 | 0.54 ± 0.05 | 0.32 ± 0.03 | 0.84 ± 0.02 |
| ab | ab | b | a | c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdjoko, A.; Brearley, F.Q.; Ungirwalu, A.; Djitmau, D.A.; Benu, N.M.H. Secondary Succession after Slash-and-Burn Cultivation in Papuan Lowland Forest, Indonesia. Forests 2022, 13, 434. https://doi.org/10.3390/f13030434
Murdjoko A, Brearley FQ, Ungirwalu A, Djitmau DA, Benu NMH. Secondary Succession after Slash-and-Burn Cultivation in Papuan Lowland Forest, Indonesia. Forests. 2022; 13(3):434. https://doi.org/10.3390/f13030434
Chicago/Turabian StyleMurdjoko, Agustinus, Francis Q. Brearley, Antoni Ungirwalu, Dony A. Djitmau, and Nithanel M. H. Benu. 2022. "Secondary Succession after Slash-and-Burn Cultivation in Papuan Lowland Forest, Indonesia" Forests 13, no. 3: 434. https://doi.org/10.3390/f13030434
APA StyleMurdjoko, A., Brearley, F. Q., Ungirwalu, A., Djitmau, D. A., & Benu, N. M. H. (2022). Secondary Succession after Slash-and-Burn Cultivation in Papuan Lowland Forest, Indonesia. Forests, 13(3), 434. https://doi.org/10.3390/f13030434








