Forest Conversion and Soil Depth Can Modify the Contributions of Organic and Inorganic Colloids to the Stability of Soil Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil Sampling
2.3. Measurement of Soil Physicochemical Properties and Enzyme Activities
2.4. Soil Aggregate Distribution and Stability Analysis
2.5. Measurement of Organic and Inorganic Soil Colloids
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties and Enzyme Activities
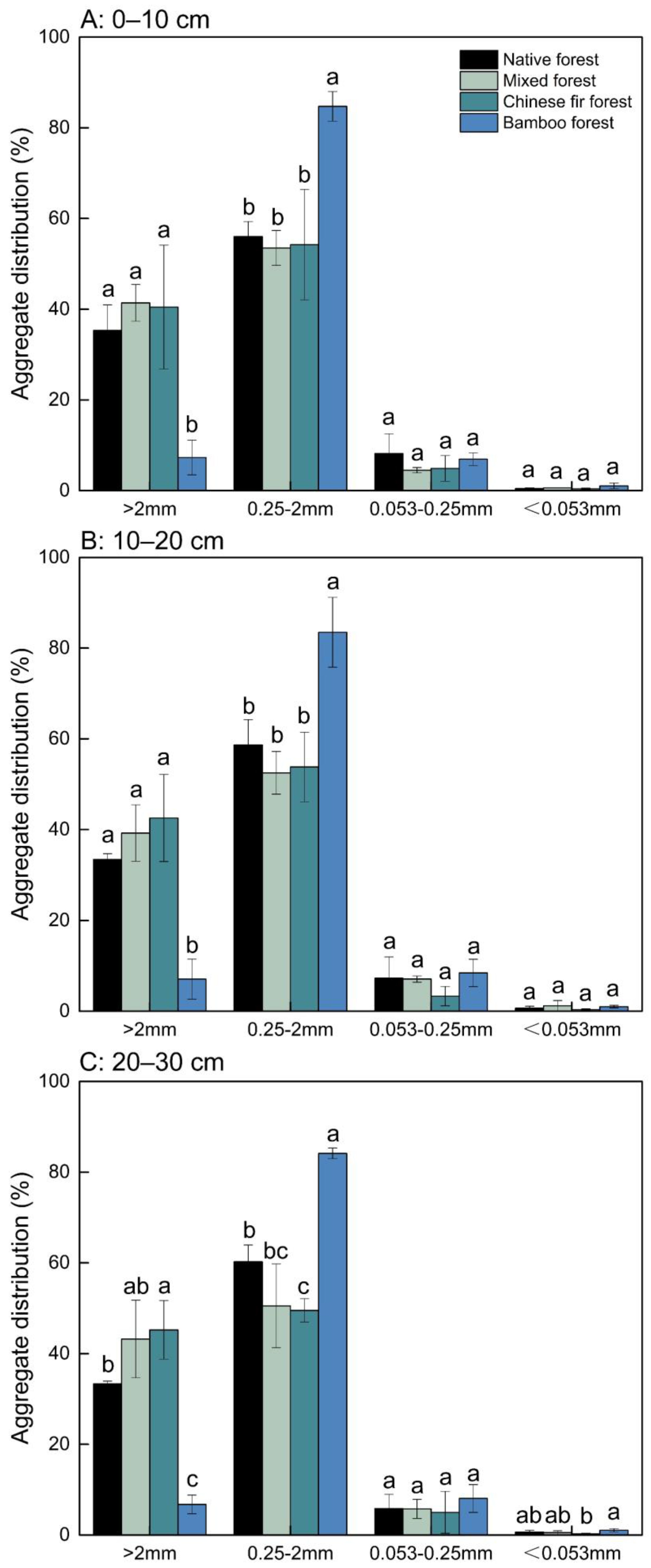
3.2. Soil Aggregate Distribution and Stability
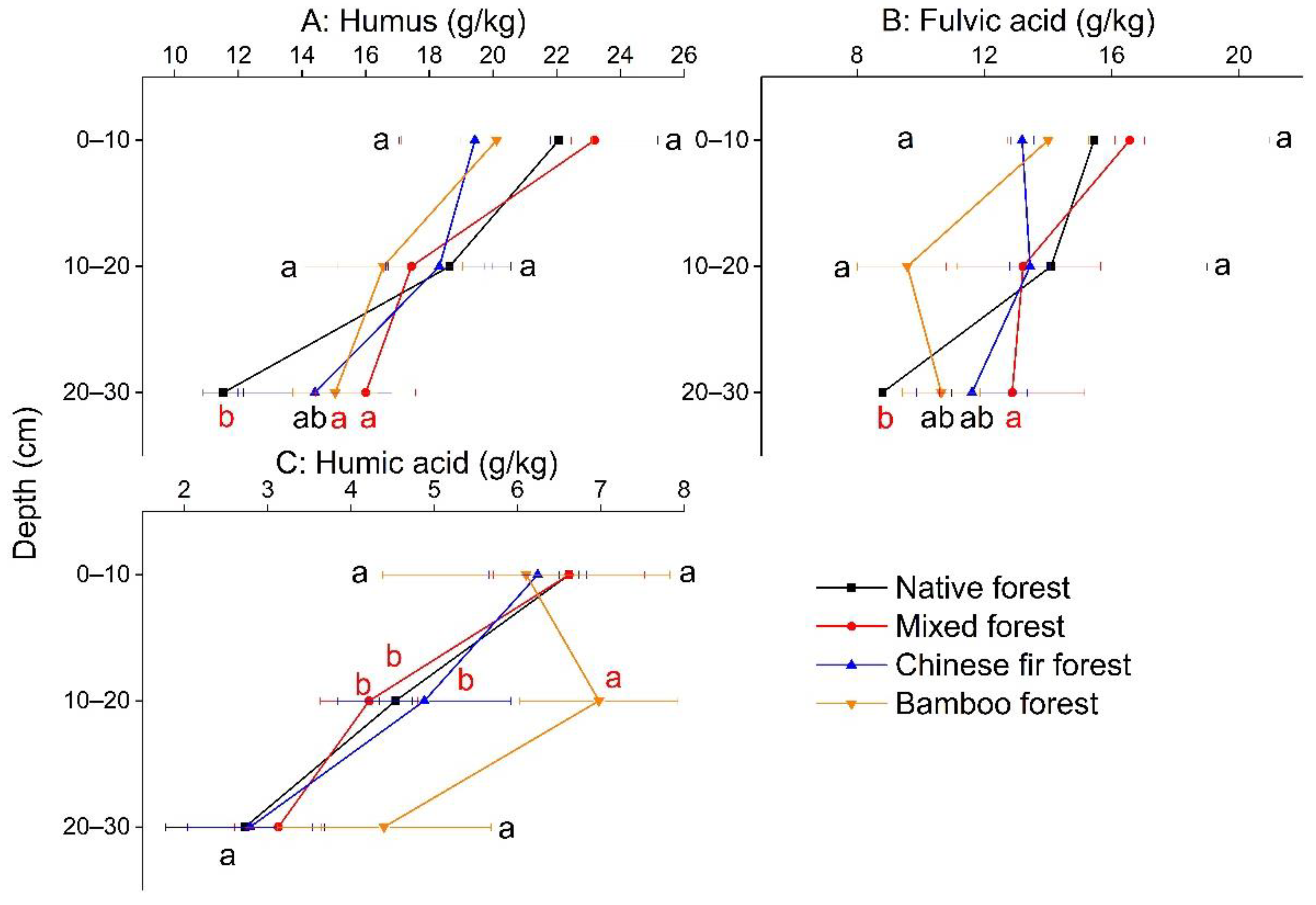
3.3. Soil Organic Colloid
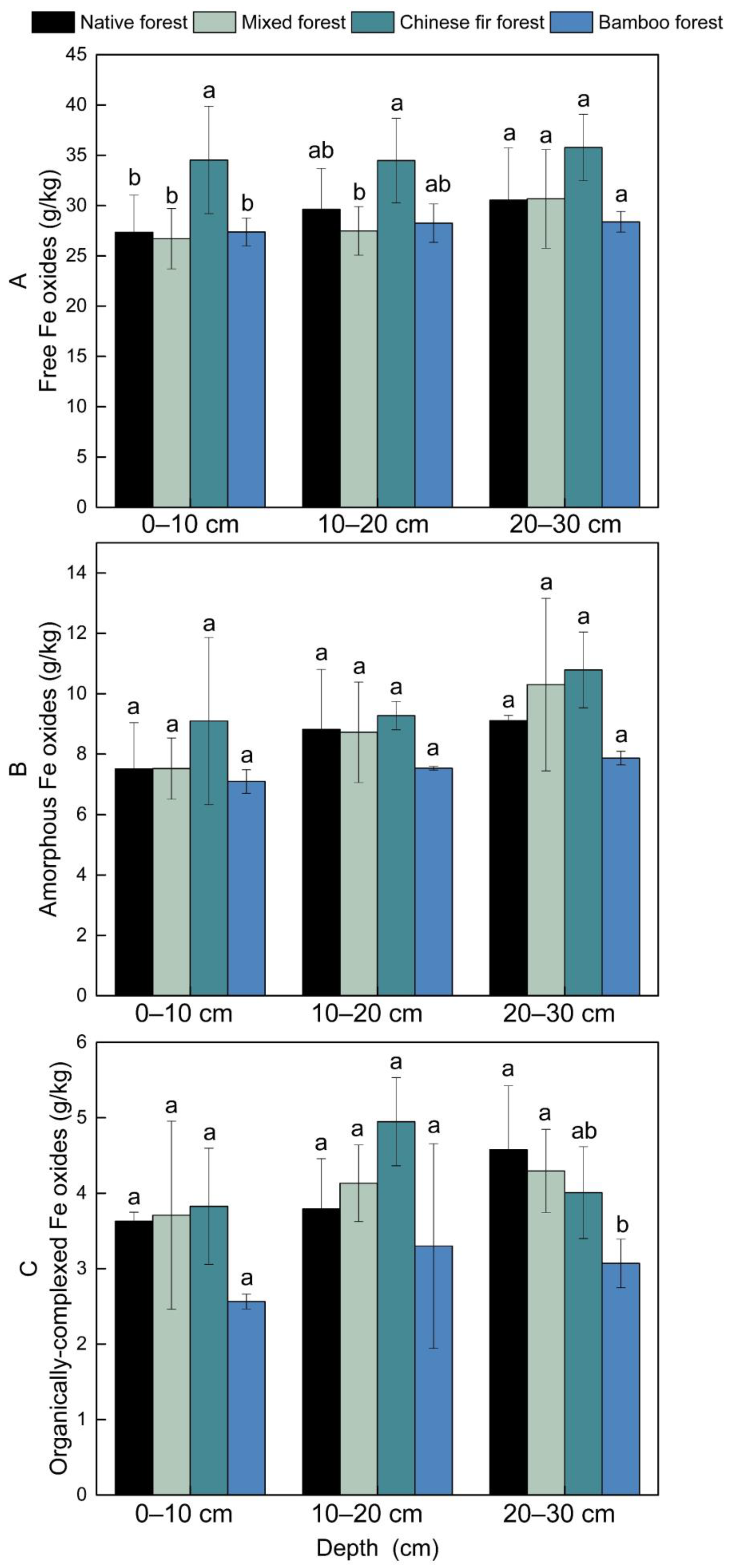
3.4. Soil Inorganic Colloid
3.5. Correlation and SEM Analysis
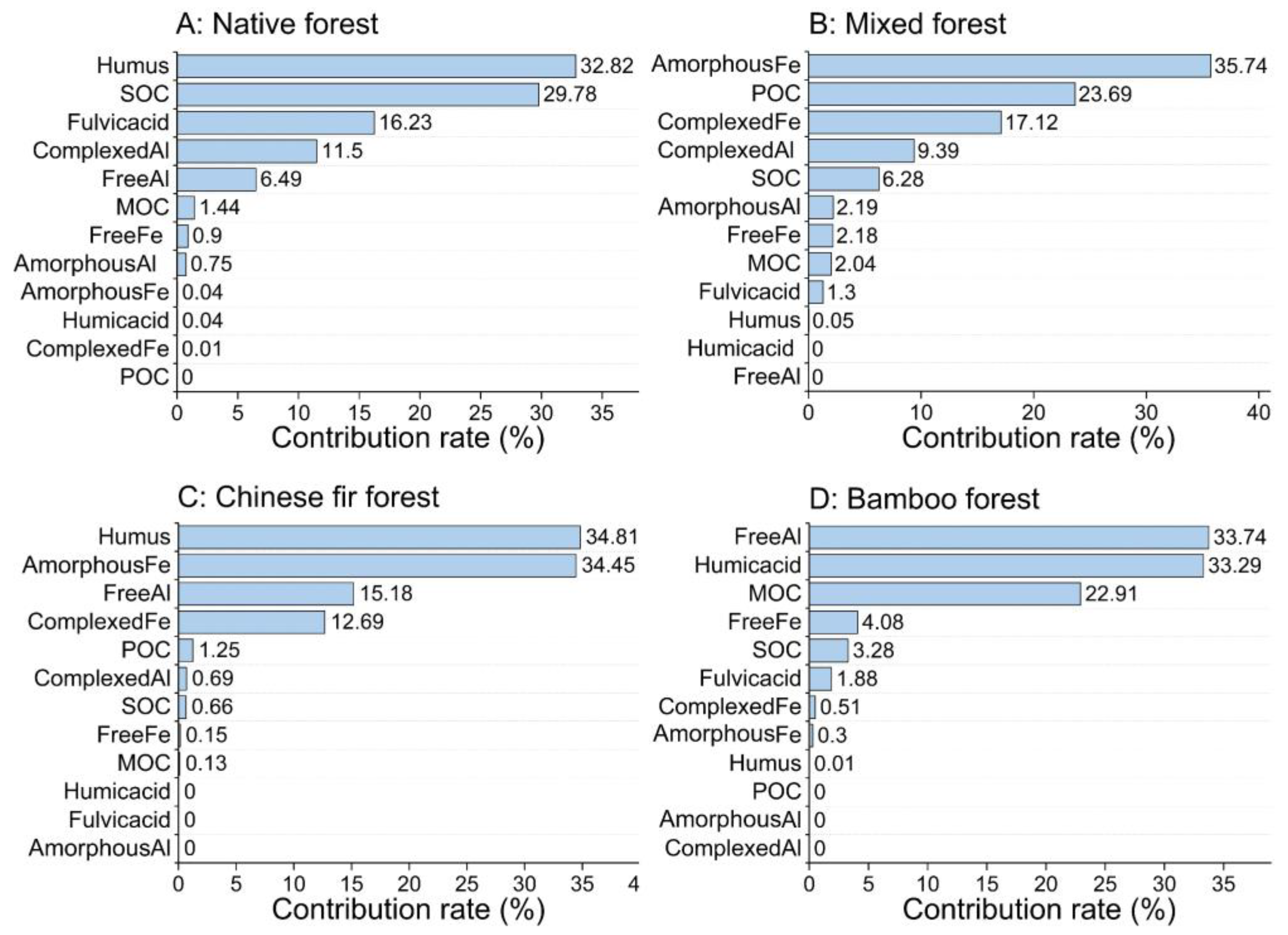
3.6. Main Factors Affecting Soil Aggregate Stability for Different Forest Types and Soil Depths
4. Discussion
4.1. Effects of Forest Conversion and Soil Depth on Soil Properties and Enzyme Activities
4.2. Effects of Forest Conversion and Soil Depth on Organic Soil Colloids
4.3. Effects of Forest Conversion and Soil Depth on Inorganic Soil Colloids
4.4. Effects of Forest Conversion and Soil Depth on Distribution and Stability of Soil Aggregates
4.5. Relationships between Soil Properties, Enzyme Activities, Organic and Inorganic Soil Colloids, Soil Aggregate Distribution, and Stability in Subtropical Forests
4.6. Main Factors Affecting Soil Aggregate Stability for Different Forest Types and Soil Depths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, X.; Yan, X.; Zhou, H.; Zhang, Y.Z.; Sun, H. Assessing the contributions of sesquioxides and soil organic matter to aggregation in an Ultisol under long-term fertilization. Soil Tillage Res. 2015, 146, 89–98. [Google Scholar] [CrossRef]
- Nweke, I.A.; Nnabude, P.C. Aggregate size distribution and stability of aggregate fractions of fallow and cultivated soils. J. Exp. Biol. Agric. Sci. 2014, 7, 514–520. [Google Scholar]
- Gao, Y.; Song, X.; Liu, K.; Li, T.; Zheng, W.; Wang, Y.; Liu, Z.; Zhang, M.; Chen, Q.; Li, Z.; et al. Mixture of controlled-release and conventional urea fertilizer application changed soil aggregate stability, humic acid molecular composition, and maize nitrogen uptake. Sci. Total Envrion. 2021, 789, 147778. [Google Scholar] [CrossRef] [PubMed]
- Nsabimana, G.; Bao, Y.; He, X.; Nambajimana, J.d.D.; Yang, L.; Li, J.; Uwiringiyimana, E.; Nsengumuremyi, P.; Ntacyabukura, T. Soil aggregate stability response to hydraulic conditions in water level fluctuation zone of the Three Gorges Reservoir, China. Catena 2021, 204, 105387. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.; Zhou, F.; Li, F.; Kubar, K.A.; Li, X.; Lu, J.; Zhu, J. Roles of soil organic carbon and iron oxides on aggregate formation and stability in two paddy soils. Soil Tillage Res. 2019, 187, 161–171. [Google Scholar] [CrossRef]
- Sun, Q.; Meng, J.; Lan, Y.; Shi, G.; Yang, X.; Cao, D.; Chen, W.; Han, X. Long-term effects of biochar amendment on soil aggregate stability and biological binding agents in brown earth. Catena 2021, 205, 105460. [Google Scholar] [CrossRef]
- An, S.; Huang, Y.; Zheng, F.; Yang, J. Aggregate characteristics during natural revegetation on the loess plateau. Pedosphere 2008, 6, 809–816. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.; Yin, Z.; Li, X.; Lu, J. Effects of organic carbon and iron oxides on soil aggregate stability under different tillage systems in a rice–rape cropping system. Catena 2019, 177, 1–12. [Google Scholar] [CrossRef]
- Sander, S.; Mosley, L.M.; Hunter, K.A. Investigation of Interparticle Forces in Natural Waters: Effects of Adsorbed Humic Acids on Iron Oxide and Alumina Surface Properties. Environ. Sci. Technol. 2004, 38, 4791–4796. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Hu, R.; Li, Y. Aggregate stability and size distribution of red soils under different land uses integrally regulated by soil organic matter, and iron and aluminum oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xu, X.; Han, N.; Sun, S.; Gao, G.; Chen, L. Assimilating leaf area index of three typical types of subtropical forest in China from MODIS time series data based on the integrated ensemble Kalman filter and PROSAIL model. ISPRS J. Photogramm. Remote Sens. 2017, 126, 68–78. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Lin, C.; Liu, X.; Chen, G.; Yang, Y. Conversion of a natural evergreen broadleaved forest into coniferous plantations in a subtropical area: Effects on composition of soil microbial communities and soil respiration. Biol. Fertil. Soils 2016, 52, 799–809. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Liu, X.; Xiong, D.; Xu, C.; Arthur, M.A.; McCulley, R.L.; Shi, S.; Yang, Y. Loss of soil organic carbon following natural forest conversion to Chinese fir plantation. For. Ecol. Manag. 2019, 449, 117476. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Li, Y.; Wang, Q.; Zhong, X.; Yang, Z.; Lin, C.; Yang, Y. Conversion of Natural Evergreen Broadleaved Forests Decreases Soil Organic Carbon but Increases the Relative Contribution of Microbial Residue in Subtropical China. Forests 2019, 10, 468. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yang, Z.; Zhong, X.; Xu, C.; Lin, Y.; Fan, Y.; Wang, M.; Chen, G.; Yang, Y. Decreases in soil P availability are associated with soil organic P declines following forest conversion in subtropical China. Catena 2021, 205, 105459. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.; Yu, P.; Fu, W.; Morrison, L.; Lin, H.; Meng, M.; Zhou, X.; Lv, Y.; Wu, J. Converting evergreen broad-leaved forests into tea and Moso bamboo plantations affects labile carbon pools and the chemical composition of soil organic carbon. Sci. Total Envrion. 2020, 711, 135225. [Google Scholar] [CrossRef]
- Meng, M.; Chen, H.Y.H.; Lin, J.; Liu, X.; Guo, X.; Yuan, Y.; Zhang, J. Long term forest conversion affected soil nanoscale pores in subtropical China. Catena 2020, 185, 104289. [Google Scholar] [CrossRef]
- Meng, M.; Lin, J.; Guo, X.; Liu, X.; Wu, J.; Zhao, Y.; Zhang, J. Impacts of forest conversion on soil bacterial community composition and diversity in subtropical forests. Catena 2019, 175, 167–173. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Ge, L.; Li, Q.; Li, Z.; Wang, L.; Liu, Y. Rhizosphere effects promote soil aggregate stability and associated organic carbon sequestration in rocky areas of desertification. Agric. Ecosyst. Environ. 2020, 304, 107126. [Google Scholar] [CrossRef]
- Ndzelu, B.S.; Dou, S.; Zhang, X.; Zhang, Y.; Ma, R.; Liu, X. Tillage effects on humus composition and humic acid structural characteristics in soil aggregate-size fractions. Soil Tillage Res. 2021, 213, 105090. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, D.; Zhou, B.; Zhang, L.; Hao, X.; Zhao, S.; Xu, X.; He, P.; Zhao, Y.; Qiu, S.; et al. Responses of soil aggregation and aggregate-associated carbon and nitrogen in black soil to different long-term fertilization regimes. Soil Tillage Res. 2021, 213, 105157. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Prieto, I.; Roumet, C.; Nespoulous, J.; Metayer, J.; Huon, S.; Villatoro, M.; Stokes, A. Soil aggregate stability in Mediterranean and tropical agro-ecosystems: Effect of plant roots and soil characteristics. Plant Soil 2017, 424, 303–317. [Google Scholar] [CrossRef]
- Dorji, T.; Field, D.J.; Odeh, I.O.A.; Bhogal, A. Soil aggregate stability and aggregate-associated organic carbon under different land use or land cover types. Soil Use Manag. 2020, 36, 308–319. [Google Scholar] [CrossRef]
- Sheng, M.; Xiong, K.; Wang, L.; Li, X.; Li, R.; Tian, X. Response of soil physical and chemical properties to Rocky desertification succession in South China Karst. Carbonates Evaporites 2016, 33, 15–28. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Peng, X.; Zhai, L.; Zhang, B.; Liu, X.; Zhang, J. Functions of mineral-solubilizing microbes and a water retaining agent for the remediation of abandoned mine sites. Sci. Total Environ. 2021, 761, 143215. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, X.; Zeng, R.; Cai, C.; Guo, Z. Spatial variations of aggregate-associated humic substance in heavy-textured soils along a climatic gradient. Soil Tillage Res. 2020, 197, 104497. [Google Scholar] [CrossRef]
- Cui, H.; Ma, K.; Fan, Y.; Peng, X.; Mao, J.; Zhou, D.; Zhang, Z.; Zhou, J. Stability and heavy metal distribution of soil aggregates affected by application of apatite, lime, and charcoal. Environ. Sci. Pollut. Res. 2016, 23, 10808–10817. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, W.; Zheng, C.; Zhu, B. Nitrogen addition has contrasting effects on particulate and mineral-associated soil organic carbon in a subtropical forest. Soil Biol. Biochem. 2020, 142, 107708. [Google Scholar] [CrossRef]
- He, Y.; Gu, F.; Xu, C.; Wang, Y. Assessing of the influence of organic and inorganic amendments on the physical-chemical properties of a red soil (Ultisol) quality. Catena 2019, 183, 104231. [Google Scholar] [CrossRef]
- Kamamia, A.W.; Vogel, C.; Mwangi, H.M.; Feger, K.-H.; Sang, J.; Julich, S. Mapping soil aggregate stability using digital soil mapping: A case study of Ruiru reservoir catchment, Kenya. Geoderma Reg. 2021, 24, e0035. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Edwards, D.P.; Mendes, L.W.; Kim, M.; Dong, K.; Kim, H.; Adams, J.M. The impact of tropical forest logging and oil palm agriculture on the soil microbiome. Mol. Ecol. 2016, 25, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hou, E.; Zhang, L.; Zang, X.; Yi, Y.; Zhang, G.; Wen, D. Effects of forest conversion on carbon-degrading enzyme activities in subtropical China. Sci. Total Environ. 2019, 696, 133968. [Google Scholar] [CrossRef] [PubMed]
- Hou, E.; Chen, C.; Wen, D.; Liu, X. Phosphatase activity in relation to key litter and soil properties in mature subtropical forests in China. Sci. Total Environ. 2015, 515–516, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, X.; Wu, J.; Zhang, Q.; Zhang, D.; Li, Q.; Long, C.; Chen, Q.; Chen, J.; Cheng, X. Inhibited enzyme activities in soil macroaggregates contribute to enhanced soil carbon sequestration under afforestation in central China. Sci. Total Environ. 2018, 640–641, 653–661. [Google Scholar] [CrossRef]
- Zhou, W.; Han, G.; Liu, M.; Zeng, J.; Liang, B.; Liu, J.; Qu, R. Determining the Distribution and Interaction of Soil Organic Carbon, Nitrogen, pH and Texture in Soil Profiles: A Case Study in the Lancangjiang River Basin, Southwest China. Forests 2020, 11, 532. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, G.; Liu, Q.; Huang, C.; Li, H.; Wu, C. Distribution Characteristics and Seasonal Variation of Soil Nutrients in the Mun River Basin, Thailand. Int. J. Environ. Res. Public Health 2018, 15, 1818. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.E.; Archontoulis, S.; Schachtman, D.P. The Effects of Soil Depth on the Structure of Microbial Communities in Agricultural Soils in Iowa (United States). Appl. Environ. Microbiol. 2021, 87, e02673-20. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, W.; Zhang, S.; Gao, W.; Fan, Y. Soil microbial community variation with time and soil depth in Eurasian Steppe (Inner Mongolia, China). Ann. Microbiol. 2021, 71, 21. [Google Scholar] [CrossRef]
- Truong, T.H.H.; Marschner, P. Respiration, available N and microbial biomass N in soil amended with mixes of organic materials differing in C/N ratio and decomposition stage. Geoderma 2018, 319, 167–174. [Google Scholar] [CrossRef]
- Avazpoor, Z.; Moradi, M.; Basiri, R.; Mirzaei, J.; Taghizadeh-Mehrjardi, R.; Kerry, R. Soil enzyme activity variations in riparian forests in relation to plant species and soil depth. Arab. J. Geosci. 2019, 12, 708. [Google Scholar] [CrossRef]
- Xiao, S.; You, H.; You, W.; Liu, J.; Cai, C.; Wu, J.; Ji, Z.; Zhan, S.; Hu, Z.; Zhang, Z.; et al. Rhizosphere and bulk soil enzyme activities in a Nothotsuga longibracteata forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J. For. Res. 2016, 28, 521–528. [Google Scholar] [CrossRef]
- Davari, M.; Gholami, L.; Nabiollahi, K.; Homaee, M.; Jafari, H.J. Deforestation and cultivation of sparse forest impacts on soil quality (case study: West Iran, Baneh). Soil Tillage Res. 2020, 198, 104504. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.L.; Binkley, D.; Mendes, J.C.T.; Stape, J.L. Soil carbon stocks and forest biomass following conversion of pasture to broadleaf and conifer plantations in southeastern Brazil. For. Ecol. Manag. 2014, 324, 37–45. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Chen, G.; Yin, Y.; Gao, R.; Lin, C. Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 2009, 323, 153–162. [Google Scholar] [CrossRef]
- Achilles, F.; Tischer, A.; Bernhardt-Römermann, M.; Heinze, M.; Reinhardt, F.; Makeschin, F.; Michalzik, B. European beech leads to more bioactive humus forms but stronger mineral soil acidification as Norway spruce and Scots pine—Results of a repeated site assessment after 2020, 63 and 82 years of forest conversion in Central Germany. For. Ecol. Manag. 2021, 483, 118769. [Google Scholar] [CrossRef]
- Lyu, M.; Xie, J.; Ukonmaanaho, L.; Jiang, M.; Li, Y.; Chen, Y.; Yang, Z.; Zhou, Y.; Lin, W.; Yang, Y. Land use change exerts a strong impact on deep soil C stabilization in subtropical forests. J. Soils Sediments 2016, 17, 2305–2317. [Google Scholar] [CrossRef]
- Lyu, M.; Noormets, A.; Ukonmaanaho, L.; Li, Y.; Yang, Y.; Xie, J. Stability of soil organic carbon during forest conversion is more sensitive in deep soil than in topsoil in subtropical forests. Pedobiologia 2021, 84, 150706. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Ni, H.; Gai, X.; Huang, Z.; Du, X.; Zhong, Z. Soil carbon and associated bacterial community shifts driven by fine root traits along a chronosequence of Moso bamboo (Phyllostachys edulis) plantations in subtropical China. Sci. Total Environ. 2021, 752, 142333. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, R.; Fang, D.; Wang, H.; Dong, Y.; Xu, R.; Jiang, J. Paddy cultivation significantly alters the forms and contents of Fe oxides in an Oxisol and increases phosphate mobility. Soil Tillage Res. 2018, 184, 176–180. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Li, B.; Yang, X. The production of phytolith-occluded carbon in China’s forests: Implications to biogeochemical carbon sequestration. Glob. Chang. Biol. 2013, 19, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Hobara, S.; Fukunaga-Yoshida, S.; Suzuki, T.; Matsumoto, S.; Matoh, T.; Ae, N. Plant silicon uptake increases active aluminum minerals in root-zone soil: Implications for plant influence on soil carbon. Geoderma 2016, 279, 45–52. [Google Scholar] [CrossRef]
- Li, X.; Gao, B.; Xu, H.; Sun, Y.; Shi, X.; Wu, J. Effect of root exudates on the stability and transport of graphene oxide in saturated porous media. J. Hazard. Mater. 2021, 413, 125362. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lu, Z.; Zhang, L.; Fan, H.; Wang, Y.; Li, J.; Lin, Y.; Liu, H.; Guo, S.; Xu, M.; et al. Red mud based passivator reduced Cd accumulation in edible amaranth by influencing root organic matter metabolism and soil aggregate distribution. Env. Pollut. 2021, 275, 116543. [Google Scholar] [CrossRef] [PubMed]
- Che, M.; Gong, Y.; Xu, M.; Kang, C.; Lv, C.; He, S.; Zheng, J. Effects of elevation and slope aspect on the distribution of the soil organic carbon associated with Al and Fe mineral phases in alpine shrub-meadow soil. Sci. Total Envrion. 2021, 753, 141933. [Google Scholar] [CrossRef]
- Xu, Z.; Ward, S.; Chen, C.; Blumfield, T.; Prasolova, N.; Liu, J. Soil carbon and nutrient pools, microbial properties and gross nitrogen transformations in adjacent natural forest and hoop pine plantations of subtropical Australia. J. Soils Sediments 2008, 8, 99–105. [Google Scholar] [CrossRef]
- Yang, C.; Ni, H.; Zhong, Z.; Zhang, X.; Bian, F. Changes in soil carbon pools and components induced by replacing secondary evergreen broadleaf forest with Moso bamboo plantations in subtropical China. Catena 2019, 180, 309–319. [Google Scholar] [CrossRef]
- Feng, H.; Wang, S.; Gao, Z.; Pan, H.; Zhuge, Y.; Ren, X.; Hu, S.; Li, C. Aggregate stability and organic carbon stock under different land uses integrally regulated by binding agents and chemical properties in saline-sodic soils. Land Degrad. Dev. 2021, 32, 4151–4161. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.-H.; Yang, Y.-F.; Li, P.-P.; Liu, J.-X. The effects of varied soil properties induced by natural grassland succession on the process of soil detachment. Catena 2018, 166, 192–199. [Google Scholar] [CrossRef]
- Bottinelli, N.; Angers, D.A.; Hallaire, V.; Michot, D.; Le Guillou, C.; Cluzeau, D.; Heddadj, D.; Menasseri-Aubry, S. Tillage and fertilization practices affect soil aggregate stability in a Humic Cambisol of Northwest France. Soil Tillage Res. 2017, 170, 14–17. [Google Scholar] [CrossRef]
- Yu, X.; Fu, Y.; Lu, S. Characterization of the pore structure and cementing substances of soil aggregates by a combination of synchrotron radiation X-ray micro-computed tomography and scanning electron microscopy. Eur. J. Soil Sci. 2017, 68, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Egan, G.; Crawley, M.J.; Fornara, D.A. Effects of long-term grassland management on the carbon and nitrogen pools of different soil aggregate fractions. Sci. Total Environ. 2018, 613–614, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Verchot, L.V.; Dutaur, L.; Shepherd, K.D.; Albrecht, A. Organic matter stabilization in soil aggregates: Understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 2011, 161, 182–193. [Google Scholar] [CrossRef]
- Jakšík, O.; Kodešová, R.; Kubiš, A.; Stehlíková, I.; Drábek, O.; Kapička, A. Soil aggregate stability within morphologically diverse areas. Catena 2015, 127, 287–299. [Google Scholar] [CrossRef]
- Kodešová, R.; Rohošková, M.; Žigová, A. Comparison of aggregate stability within six soil profiles under conventional tillage using various laboratory tests. Biologia 2009, 64, 550–554. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Chang. 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Yamagishi, K.; Kizaki, K.; Ito, S.; Hirata, R.; Mitsuda, Y. Effect of surface soil conservation by litter from shelterbelts on Chamaecyparis obtusa plantation. J. For. Res. 2017, 22, 69–73. [Google Scholar] [CrossRef]
- Shahzad, T.; Anwar, F.; Hussain, S.; Mahmood, F.; Arif, M.S.; Sahar, A.; Nawaz, M.F.; Perveen, N.; Sanaullah, M.; Rehman, K.; et al. Carbon dynamics in surface and deep soil in response to increasing litter addition rates in an agro-ecosystem. Geoderma 2019, 333, 1–9. [Google Scholar] [CrossRef]
- Laskar, S.Y.; Sileshi, G.W.; Pathak, K.; Debnath, N.; Nath, A.J.; Laskar, K.Y.; Singnar, P.; Das, A.K. Variations in soil organic carbon content with chronosequence, soil depth and aggregate size under shifting cultivation. Sci. Total Environ. 2021, 762, 143114. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yu, Z.; Lin, J.; Meng, M.; Zhao, Y.; Jia, Z.; Peng, X.; Liu, X.; Zhang, J. Forest Conversion and Soil Depth Can Modify the Contributions of Organic and Inorganic Colloids to the Stability of Soil Aggregates. Forests 2022, 13, 546. https://doi.org/10.3390/f13040546
Li C, Yu Z, Lin J, Meng M, Zhao Y, Jia Z, Peng X, Liu X, Zhang J. Forest Conversion and Soil Depth Can Modify the Contributions of Organic and Inorganic Colloids to the Stability of Soil Aggregates. Forests. 2022; 13(4):546. https://doi.org/10.3390/f13040546
Chicago/Turabian StyleLi, Chong, Zizhou Yu, Jie Lin, Miaojing Meng, Youpeng Zhao, Zhaohui Jia, Xiaonan Peng, Xin Liu, and Jinchi Zhang. 2022. "Forest Conversion and Soil Depth Can Modify the Contributions of Organic and Inorganic Colloids to the Stability of Soil Aggregates" Forests 13, no. 4: 546. https://doi.org/10.3390/f13040546






