PIN3 from Liriodendron May Function in Inflorescence Development and Root Elongation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.1.1. Hybrid Liriodendron
2.1.2. Arabidopsis thaliana
2.2. LhPIN3 Gene Cloning
2.3. Sequence Analysis of LhPIN3
2.4. Quantitative qPCR Analysis
2.5. Vector Construction
2.6. Genetic Transformation
3. Results
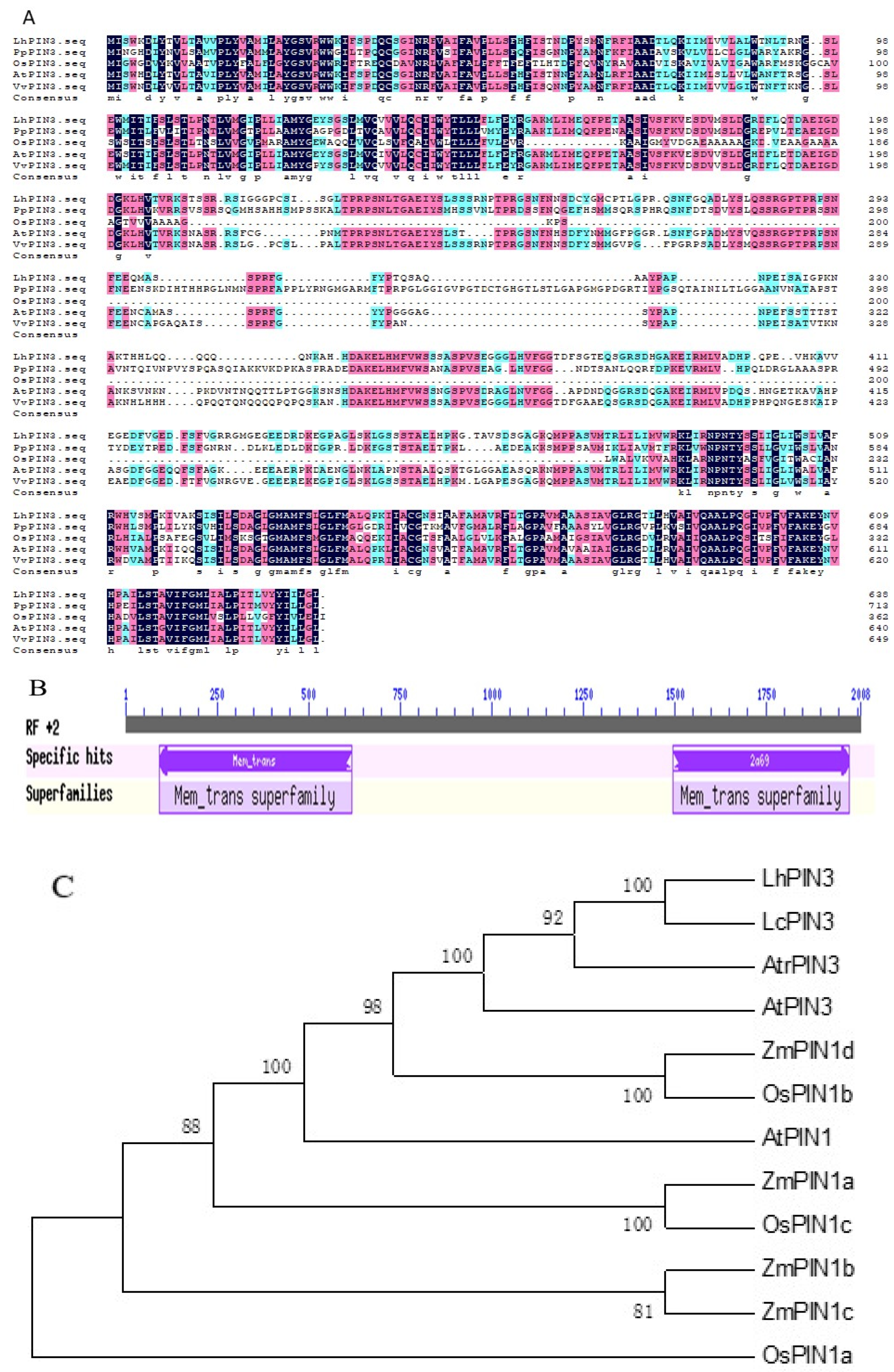
3.1. Cloning and Sequence Analysis
3.2. Expression Analysis in Hybrid Liriodendron
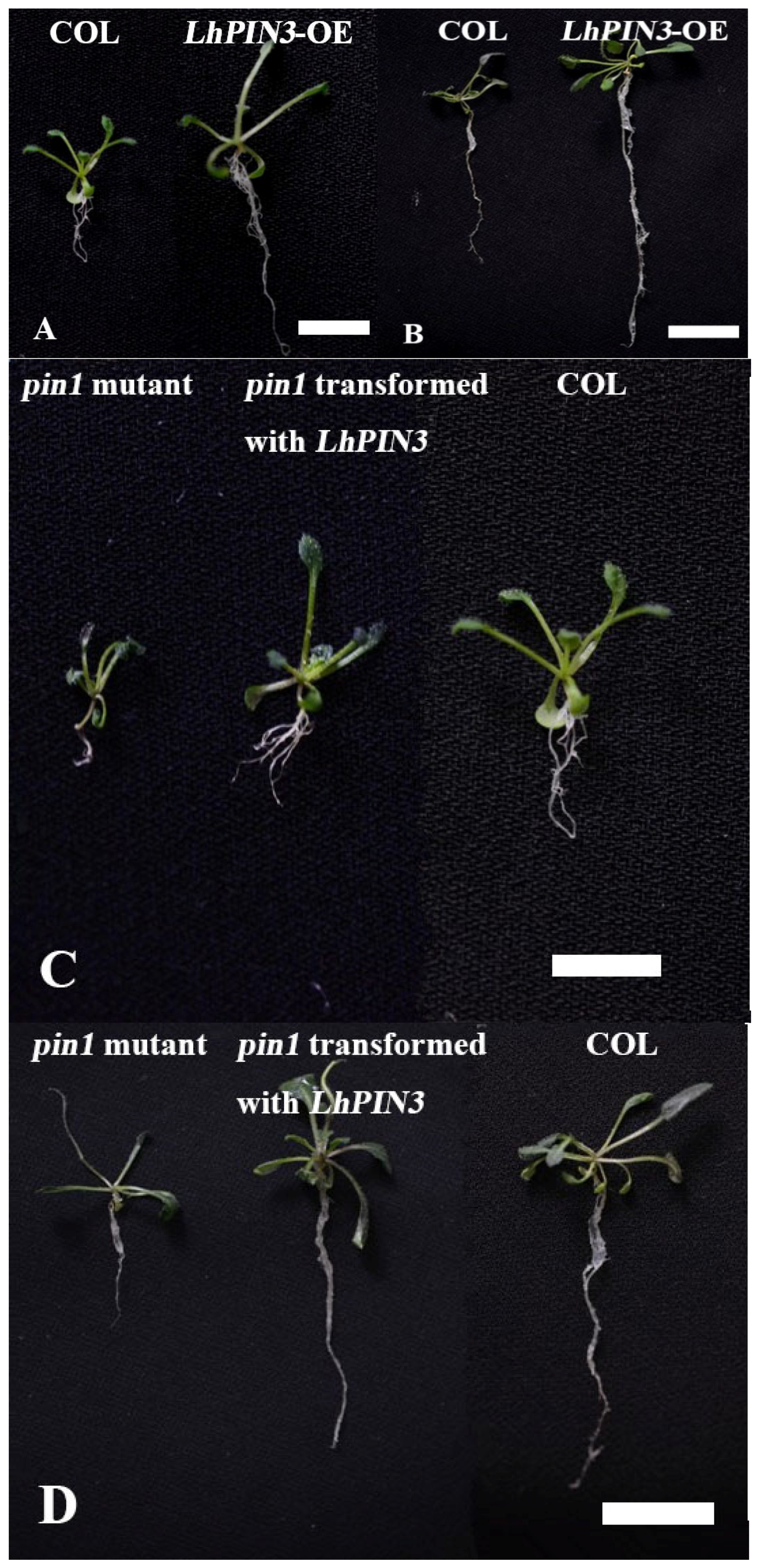
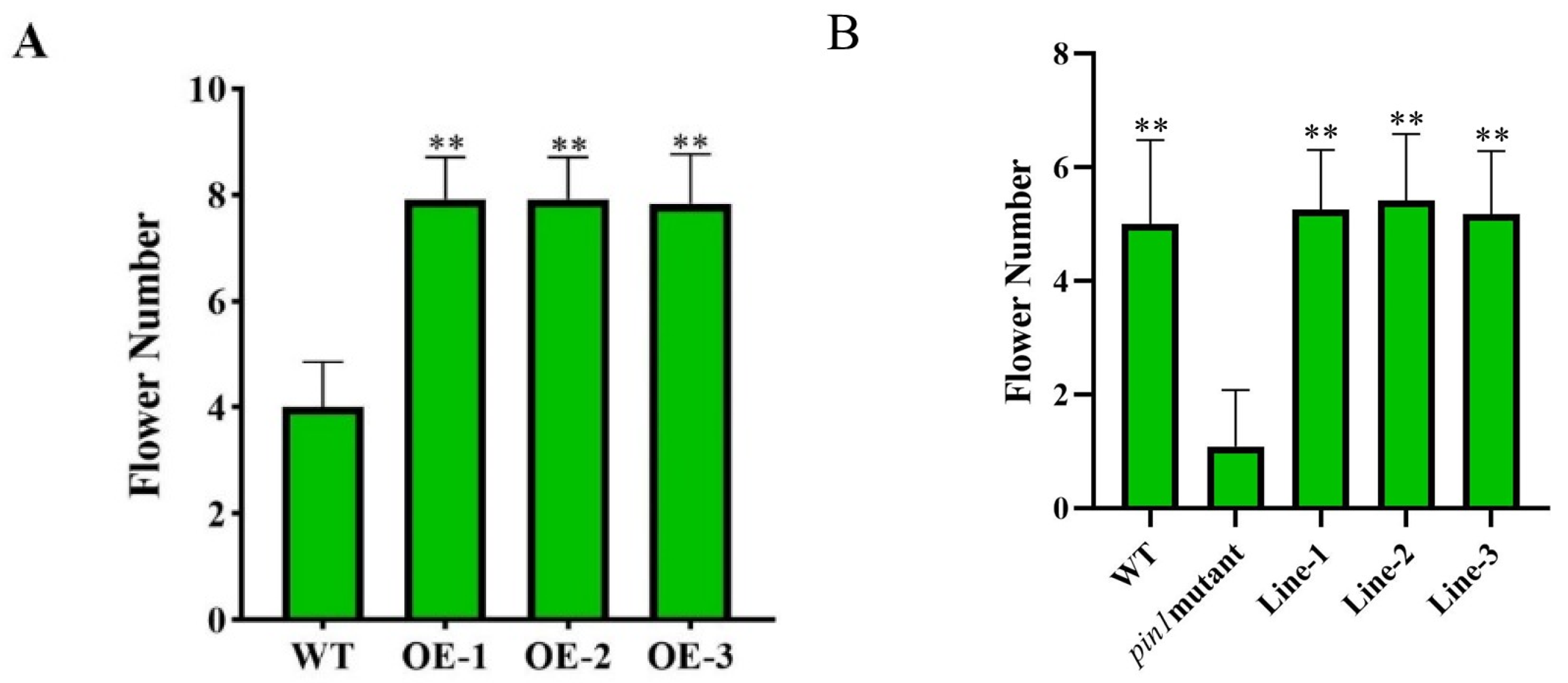
3.3. LhPIN3 Might Rescue the Root Length of the Pin1 in A. thaliana
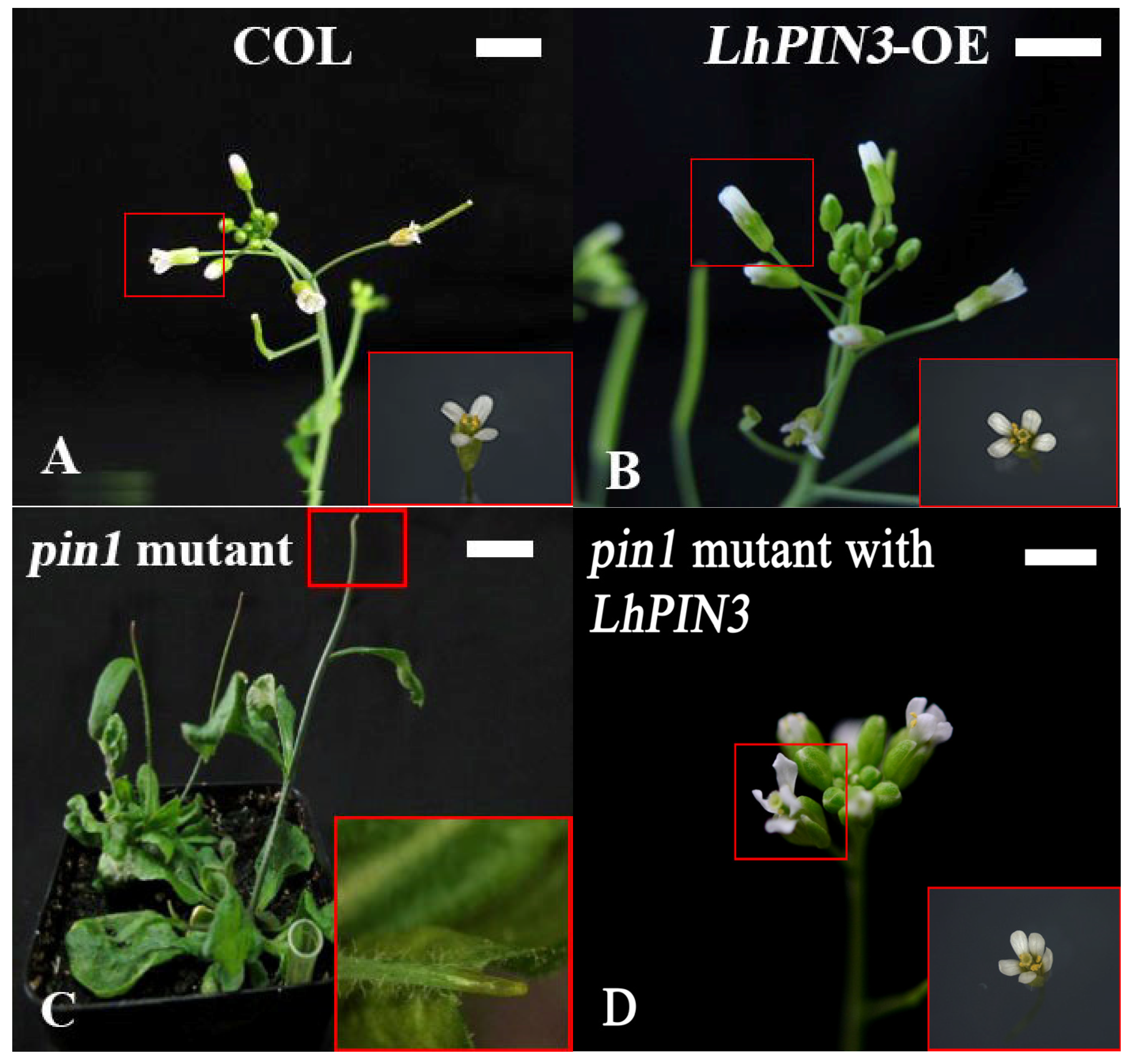
3.4. LhPIN3 May Have Function in Inflorescence Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauer, M.; Kleine-Vehn, J. PIN-FORMED and PIN-LIKES auxin transport facilitators. Development 2019, 146, dev168088. [Google Scholar] [CrossRef] [Green Version]
- Friml, J.; Palme, K. Polar auxin transport—Old questions and new concepts? Plant Mol. Biol. 2002, 3, 273–284. [Google Scholar] [CrossRef]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 2, 180–196. [Google Scholar] [CrossRef] [Green Version]
- Blakely, L.M.; Evans, T.A. Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci. Lett. 1979, 14, 79–83. [Google Scholar] [CrossRef]
- Strader, L.C.; Zhao, Y. Auxin perception and downstream events. Curr. Opin. Plant Biol. 2016, 33, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Petrásĕk, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An auxin-dependent distal organizer of pattern and polarity in the arabidopsis root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 5, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, J.; Ckurshumova, W.; Berleth, T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003, 131, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [Green Version]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef] [Green Version]
- Band, L.R.; Wells, D.M.; Fozard, J.A.; Ghetiu, T.; French, A.P.; Pound, M.P.; Wilson, M.H.; Yu, L.; Li, W.; Hijazi, H.I.; et al. Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell 2014, 26, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Paponov, I.A.; Teale, W.D.; Trebar, M.; Blilou, I.; Palme, K. The PIN auxin efflux facilitators: Evolutionary and functional perspectives. Trends Plant Sci. 2005, 10, 170–177. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Cazzonelli, C.I.; Vanstraelen, M.; Simon, S.; Yin, K.; Carron-Arthur, A.; Nisar, N.; Tarle, G.; Cuttriss, A.J.; Searle, I.R.; Benkova, E.; et al. Role of the Arabidopsis PIN6 auxin transporter in auxin homeostasis and auxin-mediated development. PLoS ONE 2013, 8, e70069. [Google Scholar] [CrossRef] [Green Version]
- Keek, P.; Skpa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zaímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar]
- Naramoto, S. Polar transport in plants mediated by membrane transporters: Focus on mechanisms of polar auxin transport. Curr. Opin. Plant Biol. 2017, 40, 8–14. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.J.; Bai, Y.H.; Wang, S.K.; Zhang, S.N.; Wu, Y.R.; Chen, M.; Jiang, D.A.; Qi, Y.H. Expression profile of PIN, AUX/LAX and PGP auxin transporter gene families in Sorghum bicolor under phytohormone and abiotic stress. FEBS J. 2010, 277, 2954–2969. [Google Scholar] [CrossRef]
- Yue, R.; Tie, S.; Sun, T.; Zhang, L.; Yang, Y.; Qi, J.; Yan, S.; Han, X.; Wang, H.; Shen, C. Genome-wide identification and ex-pression profiling analysis of ZmPIN, ZmPILS, ZmLAX and ZmABCB auxin transporter gene families in maize (Zea mays L.) under various abiotic stresses. PLoS ONE 2015, 3, 0118751. [Google Scholar]
- Huang, X.; Bai, X.; Guo, T.; Xie, Z.; Laimer, M.; Du, D.; Gbokie, T.; Zhang, Z.; He, C.; Lu, Y.; et al. Genome-wide analysis of the PIN auxin efflux carrier gene family in coffee. Plants 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hilson, P.; Sedbrook, J.; Rosen, E.; Caspar, T. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 1998, 95, 15112–15117. [Google Scholar] [CrossRef] [Green Version]
- Keuskamp, D.H.; Pollmann, S.; Voesenek, L.; Peeters, A.; Pierik, R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA 2010, 52, 22740–22744. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Yang, L.; Maere, S.; Lee, E.; Vanneste, S. A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development. Nat. Commun. 2015, 6, 8821. [Google Scholar]
- Friml, J.; Benková, E.; Blilou, I.; Wisniewska, J.; Palme, K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002, 5, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Mravec, J.; Skpa, P.; Bailly, A.; Hoyerová, K.; Keek, P.; Bielach, A.; Petráek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.D. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009, 459, 1136–1140. [Google Scholar] [CrossRef]
- Ditengou, F.A.; Gomes, D.; Nziengui, H.; Kochersperger, P.; Lasok, H.; Medeiros, V.; Paponov, I.A.; Nagy, S.K.; Nádai, T.V.; Mészáros, T.; et al. Characterization of auxin transporter PIN6 plasma membrane targeting reveals a function for PIN6 in plant bolting. New Phytol. 2017, 217, 1610–1624. [Google Scholar] [CrossRef] [Green Version]
- Michel, R.R.; Sascha, W.; Jürgen, K.V. PIN7 auxin carrier has a preferential role in terminating radial root expansion in Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 4, 1238. [Google Scholar]
- Lee, H.; Ganguly, A.; Lee, R.D.; Park, M.; Cho, H.T. Intracellularly localized PIN-FORMED8 promotes lateral root emergence in Arabidopsis. Front. Plant Sci. 2020, 10, 1808. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Vosolsobě, S.; Feraru, M.I.; Petrášek, J.; Kleine-Vehn, J. Evolution and structural diversification of PILS putative auxin carriers in plants. Front. Plant Sci. 2012, 3, 227. [Google Scholar] [CrossRef] [Green Version]
- Cristian, F.; Silvia, F.; Serena, V. The maize PIN gene family of auxin transporters. Front. Plant Sci. 2012, 3, 16. [Google Scholar]
- Vieten, A.; Vanneste, S.; Wisniewska, J.; Benkova, E.; Benjamins, R.; Beeckman, T.; Luschnig, C.; Friml, J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 2005, 20, 4521–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, G.-Q.; Mao, G.-Y.; Li, D.-W.; Wan, Y.; Zhu, L.-H. First report of Alternaria alternata causing leaf spots of Liriodendron chinense × tulipifera in China. J. Plant Pathol. 2021, 103, 689–690. [Google Scholar] [CrossRef]
- Jinhui Chen, Z.H.X.G. Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Sci. Total Environ. 2019, 5, 18–25. [Google Scholar]
- Hu, L.; Wang, P.; Long, X.; Wu, W.; Zhang, J.; Pan, Y.; Cheng, T.; Shi, J.; Chen, J. The PIN gene family in relic plant L. chinense: Genome-wide identification and gene expression profiling in different organizations and abiotic stress responses. Plant Physiol. Biochem. 2021, 162, 634–646. [Google Scholar] [CrossRef]
- Kpczyńska, E.; Orowska, A. Profiles of endogenous ABA, bioactive GAs, IAA and their metabolites in Medicago truncatula Gaertn. non-embryogenic and embryogenic tissues during induction phase in relation to somatic embryo formation. Planta 2021, 253, 67. [Google Scholar] [CrossRef]
- Zhai, S.; Cai, W.; Xiang, Z.; Chen, C.; Lu, Y.; Yuan, T. PIN3-mediated auxin transport contributes to blue light-induced adventitious root formation in Arabidopsis. Plant Sci. 2021, 312, 111044. [Google Scholar] [CrossRef]
- Yuan, T.T.; Xiang, Z.X.; Li, W.; Gao, X.; Lu, Y. Osmotic stress represses root growth by modulating the transcriptional regulation of PIN-FORMED3. New Phytol. 2021, 232, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Jinhui, C.; Jisen, S.; Qiang, Z.; Minren, H. Studies on the somatic embryogenesis of liriodendron hybrids (L. chinense × L. tulipifera). Sci. Silvae Sin. 2003, 4, 49–53. [Google Scholar]
- Wu, W.; Zhu, S.; Zhu, L.; Wang, D.; Liu, Y.; Liu, S.; Zhang, J.; Hao, Z.; Lu, Y.; Cheng, T.; et al. Characterization of the Liriodendron chinense MYB gene family and its role in abiotic stress response. Front. Plant Sci. 2021, 12, 641280. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.H.; Chen, X.N.; Wang, J. Primer design with primer premier 5.0. Northwest Med. Educ. 2008, 16, 6–8. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 4, 783–791. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, W. OLIGO 7 primer analysis software. PCR Primer Des. 2007, 402, 35–59. [Google Scholar] [CrossRef]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin response in Arabidopsis under cold stress: Underlying molecular mechanisms. Plant Cell 2009, 12, 3823–3838. [Google Scholar]
- Miyashita, Y.; Takasugi, T.; Ito, Y. Identification and expression analysis of PIN genes in rice. Plant Sci. 2010, 178, 424–428. [Google Scholar] [CrossRef]
- Habets, M.E.; Offringa, R. PIN-driven polar auxin transport in plant developmental plasticity: A key target for environmental and endogenous signals. New Phytol. 2014, 2, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Swarup, R.; Paponov, I.A.; Swarup, K.; Casimiro, I.; Lake, D.; Peret, B.; Zappala, S.; Mairhofer, S.; Whitworth, M.; et al. SHORT-ROOT regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol. 2011, 155, 384–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, I.; Tetsumura, T.; De Rybel, B.; Frey, N.F.D.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D.; et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007, 134, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friml, J.; Wiśniewska, J.; Benková, E.; Mendgen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Rodriguez, L.; Li, L.; Zhang, X.; Friml, J. Functional innovations of PIN auxin transporters mark crucial evolutionary transitions during rise of flowering plants. Sci. Adv. 2020, 50, 8895. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, C.; Valliyodan, B.; Maupin, C.; Annen, B.; Nguyen, H.T. Genome-wide analysis and expression profiling of the PIN auxin transporter gene family in soybean (Glycine max). BMC Genom. 2015, 16, 951. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991, 7, 677–684. [Google Scholar]
- Okal, M.; Miyamoto, K.; Okada, K.; Ueda, J. Auxin polar transport and flower formation in Arabidopsis thaliana transformed with indoleacetamide hydrolase (iaaH) gene. Plant Cell Physiol. 1999, 40, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Shan, T.; Fang, H.; Sun, K.; Shi, W.; Tang, B.; Wu, J.; Wang, K.; Li, P.; Wang, B. Genome-wide analysis reveals the spatiotemporal expression patterns of SOS3 genes in the maize B73 genome in response to salt stress. BMC Genom. 2022, 23, 60. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Chen, C.; Zou, L.; Yin, S.; Liu, S.; Yuan, J.; Wu, N.; Liu, N.W.X. Comparative transcriptome analysis reveals variations of bioactive constituents in Lonicera japonica flowers under salt stress. Plant Physiol. Biochem. 2022, 173, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, F.; Li, Y.; Zong, S.; Tao, J. Genome-wide identification and expression analysis of the Hsp gene superfamily in Asian long-horned beetle (Anoplophora glabripennis). Int. J. Biol. Macromol. 2022, 200, 583–592. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Pan, Y.; Hu, L.; Yang, D.; Yuan, M.; Hao, Z.; Lu, Y.; Xiao, F.; Shi, J.; Chen, J. PIN3 from Liriodendron May Function in Inflorescence Development and Root Elongation. Forests 2022, 13, 568. https://doi.org/10.3390/f13040568
Li R, Pan Y, Hu L, Yang D, Yuan M, Hao Z, Lu Y, Xiao F, Shi J, Chen J. PIN3 from Liriodendron May Function in Inflorescence Development and Root Elongation. Forests. 2022; 13(4):568. https://doi.org/10.3390/f13040568
Chicago/Turabian StyleLi, Rui, Yan Pan, Lingfeng Hu, Dingjie Yang, Mengjian Yuan, Zhaodong Hao, Ye Lu, Fuming Xiao, Jisen Shi, and Jinhui Chen. 2022. "PIN3 from Liriodendron May Function in Inflorescence Development and Root Elongation" Forests 13, no. 4: 568. https://doi.org/10.3390/f13040568





