Effects of Forest Fragmentation on the Volume of Wood Resources in Managed, Pine-Dominated Forests in Poland
Abstract
:1. Introduction
1.1. The Size of the Forest Area and Fragmentation
1.2. Forest Connectivity and Forest Patches
1.3. Edge Effect
1.4. Wood Resources and Carbon Accumulation in Biomass
1.5. Management of Forests in Poland
1.6. Aim of Study
2. Materials and Methods
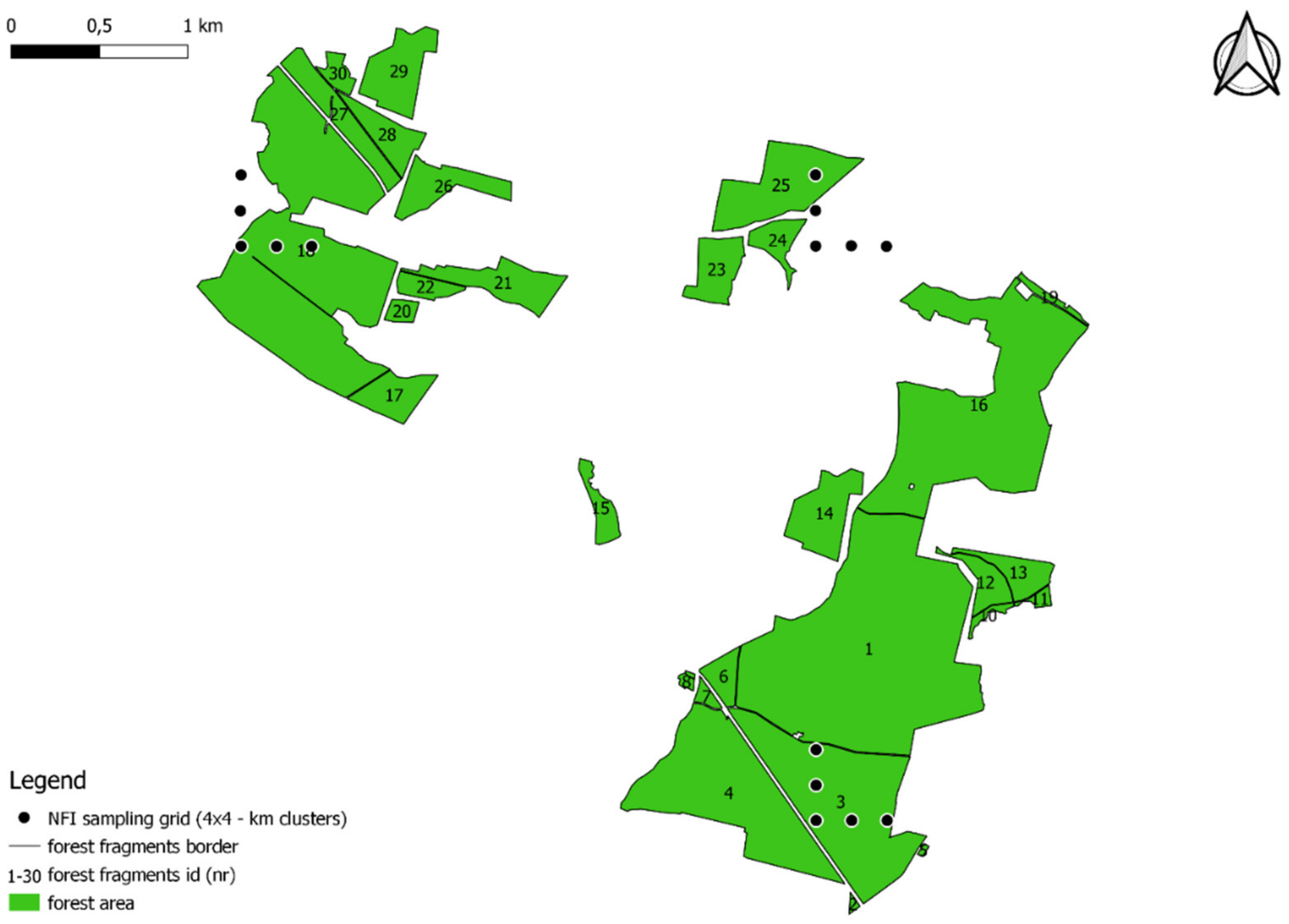
2.1. Object of Study and Material
2.2. Spatial Analysis and Database Analysis
- Species composition—Plots were assigned the name of the dominant species (species with the highest growing stock volume on the plot);
- Stand age—Each plot was assigned an age class number, determined by the age of the dominant species. The following age class rules were adopted: the age of the dominant species 1–10 years, 1 age class; the age of the dominant species 11–20 years, 2 age class, etc.;
- Fertility and humidity of the habitat, based on the forest site type.
2.3. Statistical Analysis and Calculations
- y–is the n data vector (dependent value),
- X–is a matrix of n x p which columns are p independent variables that are permanent effects. In our calculations, X is the location of the plot in the forest patch (a variable taking two possible values: edge or interior),
- β–is the p vector of permanent effects parameters,
- Z–is a matrix of n x q which columns are q independent variables that are random effects. In our calculations, Z is the id of the forest patch (a qualitative variable denoting the identification code of the patch),
- u–is the q vector of random effects parameters,
- ε–is the n random vector.
- C–carbon in tonnes,
- V–growing stock volume (m3),
- BCEF–biomass conversion and expansion factor,
- R–root factor,
- CF–carbon fraction [tonne C (tonne dry matter)-1].
3. Results
4. Discussion
4.1. Forest Patches
4.2. Forest Structure
4.3. Stands Age
4.4. Carbon Accumulation
5. Conclusions
- The effects of fragmentation depend on both the structure of the forest and the landscape metrics of the forest patches, especially the area of the fragments and the distance between them. To reduce the impact of fragmentation (edge effect) on the volume of wood resources, large forest patches of at least 3000 ha should be established.
- The effects of fragmentation on the volume of wood resources in managed pine-dominated forests depend on the age of the stands and the quality of the site. To maintain a high volume of wood resources, care should be taken to counteract the occurrence of the edge effect, especially in older pine stands on moderately fertile sites (FMBF).
- In highly barren habitats (FCF), pine-dominated stands within edge zones are characterized by a higher ability to accumulate carbon in living biomass than stands in the interior of forest patches. This means that under certain site conditions, fragmentation may increase the potential of managed forested areas to mitigate climate change.
- The use of comparative studies is an effective way to obtain generalizations about the effects of habitat fragmentation for planning and managing fragmented forest areas. Our results provide a framework for interpreting empirical findings on forest fragmentation. However, it is also clear that more detailed experimental studies are needed. This is due to the need to identify the underlying mechanisms responsible for the fragmentation patterns observed in stands with greater species richness. This is all the more important because some species of trees may be less sensitive to the edge effect [55].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Wilcove, D.S.; McLellan, C.H.; Dobson, A.P. Habitat fragmentation in the temperate zone. Conserv. Biol. 1986, 6, 237–256. [Google Scholar]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.C.; Fearnside, P.M.; Ribeiro, J.E.L.; Capretz, R.L. Rain forest fragmentation and the proliferation of successional trees. Ecology 2006, 87, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Rivas, A.; De-Nova, J.A.; Munguía-Rosas, M.A. Patch isolation and shape predict plant functional diversity in a naturally fragmented forest. J. Plant Ecol. 2016, 23, 119. [Google Scholar] [CrossRef]
- Santo-Silva, E.E.; Almeida, W.R.; Tabarelli, M.; Peres, C.A. Habitat fragmentation and the future structure of tree assemblages in a fragmented Atlantic forest landscape. Plant Ecol. 2016, 217, 1129–1140. [Google Scholar] [CrossRef]
- Echeverría, C.; Newton, A.C.; Lara, A.; Benayas, J.M.R.; Coomes, D.A. Impacts of forest fragmentation on species composition and forest structure in the temperate landscape of southern Chile. Glob. Ecol. Biogeogr. 2007, 16, 426–439. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, N.R.; Asner, G.P.; Giardina, C.P. Centennial impacts of fragmentation on the canopy structure of tropical montane forest. Ecol. Appl. 2014, 24, 1638–1650. [Google Scholar] [CrossRef]
- Islam, M.; Deb, G.P.; Rahman, M. Forest fragmentation reduced carbon storage in a moist tropical forest in Bangladesh: Implications for policy development. Land Use Policy 2017, 65, 15–25. [Google Scholar] [CrossRef]
- Raši, R.; Schwarz, M. Forest Fragmentation Indicator; FOREST EUROPE Liaison Unit Bratislava: Zvolen, Slovakia, 2020. [Google Scholar]
- Bera, B.; Saha, S.; Bhattacharjee, S. Estimation of Forest Canopy Cover and Forest Fragmentation Mapping Using Landsat Satellite Data of Silabati River Basin (India). KN J. Cartogr. Geogr. Inf. 2020, 70, 181–197. [Google Scholar] [CrossRef]
- Çakir, G.; Sivrikaya, F.; Keleş, S. Forest cover change and fragmentation using Landsat data in Maçka State Forest Enterprise in Turkey. Environ. Monit. Assess. 2008, 137, 51–66. [Google Scholar] [CrossRef]
- Andronache, I.; Marin, M.; Fischer, R.; Ahammer, H.; Radulovic, M.; Ciobotaru, A.-M.; Jelinek, H.F.; Di Ieva, A.; Pintilii, R.-D.; Drăghici, C.-C.; et al. Dynamics of Forest Fragmentation and Connectivity Using Particle and Fractal Analysis. Sci. Rep. 2019, 9, 12228. [Google Scholar] [CrossRef] [Green Version]
- Muluneh, M.G.; Feyissa, M.T.; Wolde, T.M. Effect of forest fragmentation and disturbance on diversity and structure of woody species in dry Afromontane forests of northern Ethiopia. Biodivers. Conserv. 2021, 30, 1753–1779. [Google Scholar] [CrossRef]
- Zięba, S.; Banaś, J.; Zygmunt, R.; Bujoczek, L. Postać strefy brzegowej różnych typów drzewostanów. Stud. Mater. Cent. Edukac. Przyr. Leśnej 2014, 16, 98–108. [Google Scholar]
- Veselkin, D.V.; Shavnin, S.A.; Vorobeichik, E.L.; Galako, V.A.; Vlasenko, V.E. Edge effects on pine stands in a large city. Russ. J. Ecol. 2017, 48, 499–506. [Google Scholar] [CrossRef]
- Erasmi, S.; Klinge, M.; Dulamsuren, C.; Schneider, F.; Hauck, M. Modelling the productivity of Siberian larch forests from Landsat NDVI time series in fragmented forest stands of the Mongolian forest-steppe. Environ. Monit. Assess. 2021, 193, 200. [Google Scholar] [CrossRef]
- Shen, C.; Shi, N.; Fu, S.; Ye, W.; Ma, L.; Guan, D. Decline in Aboveground Biomass Due to Fragmentation in Subtropical Forests of China. Forests 2021, 12, 617. [Google Scholar] [CrossRef]
- Numata, I.; Cochrane, M.A.; Roberts, D.A.; Soares, J.V.; Souza, C.M.; Sales, M.H. Biomass collapse and carbon emissions from forest fragmentation in the Brazilian Amazon. J. Geophys. Res. 2010, 115, 999. [Google Scholar] [CrossRef] [Green Version]
- Numata, I.; Cochrane, M.A.; Souza, C.M., Jr.; Sales, M.H. Carbon emissions from deforestation and forest fragmentation in the Brazilian Amazon. Environ. Res. Lett. 2011, 6, 44003. [Google Scholar] [CrossRef]
- Rolo, V.; Olivier, P.I.; Pfeifer, M.; van Aarde, R.J. Functional diversity mediates contrasting direct and indirect effects of fragmentation on below- and above-ground carbon stocks of coastal dune forests. For. Ecol. Manag. 2018, 407, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Ziter, C.; Bennett, E.M.; Gonzalez, A. Functional diversity and management mediate aboveground carbon stocks in small forest fragments. Ecosphere 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Ordway, E.M.; Asner, G.P. Carbon declines along tropical forest edges correspond to heterogeneous effects on canopy structure and function. Proc. Natl. Acad. Sci. USA 2020, 117, 7863–7870. [Google Scholar] [CrossRef]
- Laurance, W.F.; Laurance, S.G.; Delamonica, P. Tropical forest fragmentation and greenhouse gas emissions. For. Ecol. Manag. 1998, 110, 173–180. [Google Scholar] [CrossRef]
- Laurance, W.; Delamônica, P.; Laurance, S.; Vasconcelos, H.; Lovejoy, T. Conservation: Rainforest fragmentation kills big trees. Nature 2000, 404, 836. [Google Scholar] [CrossRef]
- Ranta, P.; Blom, T.O.M.; Niemela, J.; Joensuu, E.; Siitonen, M. The fragmented Atlantic rain forest of Brazil: Size, shape and distribution of forest fragments. Biodivers. Conserv. 1998, 7, 385–403. [Google Scholar] [CrossRef]
- Iida, S.; Nakashizuka, T. Forest fragmentation and its effect on species diversity in sub-urban coppice forests in Japan. For. Ecol. Manag. 1995, 73, 197–210. [Google Scholar] [CrossRef]
- Kozak, J.; Ziółkowska, E.; Vogt, P.; Dobosz, M.; Kaim, D.; Kolecka, N.; Ostafin, K. Forest-Cover Increase Does Not Trigger Forest-Fragmentation Decrease: Case Study from the Polish Carpathians. Sustainability 2018, 10, 1472. [Google Scholar] [CrossRef] [Green Version]
- Garmendia, A.; Arroyo-Rodríguez, V.; Estrada, A.; Naranjo, E.J.; Stoner, K.E. Landscape and patch attributes impacting medium- and large-sized terrestrial mammals in a fragmented rain forest. J. Trop. Ecol. 2013, 29, 331–344. [Google Scholar] [CrossRef]
- Fahrig, L.; Triantis, K. Rethinking patch size and isolation effects: The habitat amount hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Bähner, K.W.; Tabarelli, M.; Büdel, B.; Wirth, R. Habitat fragmentation and forest management alter woody plant communities in a Central European beech forest landscape. Biodivers. Conserv. 2020, 29, 2729–2747. [Google Scholar] [CrossRef]
- Vogt, P. Quantifying landscape fragmentation. Simpósio Sens. Remoto 2015, 17, 1239–1246. [Google Scholar]
- Li, M.; Zhu, Z.; Vogelmann, J.E.; Xu, D.; Wen, W.; Liu, A. Characterizing fragmentation of the collective forests in southern China from multitemporal Landsat imagery: A case study from Kecheng district of Zhejiang province. Appl. Geogr. 2011, 31, 1026–1035. [Google Scholar] [CrossRef]
- Estreguil, C.; Mouton, C. Measuring and Reporting on Forest Landscape Pattern, Fragmentation and Connectivity in Europe: Methods and Indicators; JRC Scientific and Technical Reports; Office for Official Publications of the European Communities: Luxembourg, 2009. [Google Scholar]
- Meddens, A.J.H.; Hudak, A.T.; Evans, J.S.; Gould, W.A.; González, G. Characterizing forest fragments in boreal, temperate, and tropical ecosystems. Ambio 2008, 37, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F.; Carvalho, R.; Mira, A.; Beja, P. Assessing landscape functional connectivity in a forest carnivore using path selection functions. Landsc. Ecol. 2016, 31, 1021–1036. [Google Scholar] [CrossRef] [Green Version]
- Siderhurst, L.A.; Griscom, H.P.; Kyger, C.; Stutzman, J.; Trumbo, B. Tree Species Composition and Diversity and the Abundance of Exotics in Forest Fragments of the Shenandoah Valley, Virginia. Castanea 2012, 77, 348–363. [Google Scholar] [CrossRef]
- Estreguil, C.; Caudullo, G.; de Rigo, D.; San-Miguel-Ayanz, J. Forest Landscape in Europe: Pattern, Fragmentation and Connectivity; JRC77295; Publications Office of the European Union: Luxembourg, 2012; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC77295#:~:text=Forest%20edges%20are%20also%20mainly,by%20mainly%20intensive%20land%20uses (accessed on 14 December 2021).
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.C.; Silva, J.N. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Meeussen, C.; Govaert, S.; Vanneste, T.; Calders, K.; Bollmann, K.; Brunet, J.; Cousins, S.A.O.; Diekmann, M.; Graae, B.J.; Hedwall, P.-O.; et al. Structural variation of forest edges across Europe. For. Ecol. Manag. 2020, 462, 117929. [Google Scholar] [CrossRef] [Green Version]
- Mendes, P.G.A.; Silva, M.A.M.; Guerra, T.N.F.; Lins-e-Silva, A.C.B.; Cavalcanti, A.d.D.C.; Sampaio, E.V.d.S.B.; Rodal, M.J.N. Dynamics and Edge Effect of an Atlantic Forest Fragment in Brazil. Floresta Ambient. 2016, 23, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Didham, R.K.; Lawton, J.H. Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments 1. Biotropica 1999, 31, 17–30. [Google Scholar] [CrossRef]
- Dodonov, P.; Harper, K.A.; Silva-Matos, D.M. The role of edge contrast and forest structure in edge influence: Vegetation and microclimate at edges in the Brazilian cerrado. Plant Ecol. 2013, 214, 1345–1359. [Google Scholar] [CrossRef]
- Almoussawi, A.; Lenoir, J.; Jamoneau, A.; Hattab, T.; Wasof, S.; Gallet-Moron, E.; Garzon-Lopez, C.X.; Spicher, F.; Kobaissi, A.; Decocq, G.; et al. Forest fragmentation shapes the alpha–gamma relationship in plant diversity. J. Veg. Sci. 2019, 31, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Riutta, T.; Clack, H.; Crockatt, M.; Slade, E.M. Landscape-Scale Implications of the Edge Effect on Soil Fauna Activity in a Temperate Forest. Ecosystems 2016, 19, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Hargis, C.D.; Bissonette, J.A.; Turner, D.L. The influence of forest fragmentation and landscape pattern on American martens. J. Appl. Ecol. 1999, 36, 157–172. [Google Scholar] [CrossRef]
- McIntyre, N.E. Effects of forest patch size on avian diversity. Landsc. Ecol. 1995, 10, 85–99. [Google Scholar] [CrossRef]
- Ewers, R.M.; Thorpe, S.; Didham, R.K. Synergistic interactions between edge and area effects in a heavily fragmented landscape. Ecology 2007, 88, 96–106. [Google Scholar] [CrossRef]
- Bender, D.J.; Contreras, T.A.; Fahrig, L. Habitat loss and population decline: A meta-analysis of the patch size effect. Ecology 1998, 79, 517–533. [Google Scholar] [CrossRef]
- Riutta, T.; Slade, E.M.; Morecroft, M.D.; Bebber, D.P.; Malhi, Y. Living on the edge: Quantifying the structure of a fragmented forest landscape in England. Landsc. Ecol. 2014, 29, 949–961. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.A.R.; Jaiteh, M.S.; Esseen, P.-A. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Ylisirniö, A.-L.; Mönkkönen, M.; Hallikainen, V.; Ranta-Maunus, T.; Kouki, J. Woodland key habitats in preserving polypore diversity in boreal forests: Effects of patch size, stand structure and microclimate. For. Ecol. Manag. 2016, 373, 138–148. [Google Scholar] [CrossRef]
- Pinto, S.R.R.; Mendes, G.; Santos, A.M.M.; Dantas, M.; Tabarelli, M.; Melo, F.P.L. Landscape attributes drive complex spatial microclimate configuration of Brazilian Atlantic forest fragments. Trop. Conserv. Sci. 2010, 3, 389–402. [Google Scholar] [CrossRef]
- Baker, S.C.; Spies, T.A.; Wardlaw, T.J.; Balmer, J.; Franklin, J.F.; Jordan, G.J. The harvested side of edges: Effect of retained forests on the re-establishment of biodiversity in adjacent harvested areas. For. Ecol. Manag. 2013, 302, 107–121. [Google Scholar] [CrossRef]
- Armenteras, D.; González, T.M.; Retana, J. Forest fragmentation and edge influence on fire occurrence and intensity under different management types in Amazon forests. Biol. Conserv. 2013, 159, 73–79. [Google Scholar] [CrossRef]
- Sampaio, A.B.; Scariot, A. Edge effect on tree diversity, composition and structure in a deciduous dry forest in central Brazil. Revista Árvore 2011, 35, 1121–1134. [Google Scholar] [CrossRef]
- Nascimento, H.E.M.; Laurance, W.F. Biomass dynamics in Amazonian forest fragments. Ecol. Appl. 2004, 14, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Talkkari, A.; Peltola, H.; Kellomäki, S.; Strandman, H. Integration of component models from the tree, stand and regional levels to assess the risk of wind damage at forest margins. For. Ecol. Manag. 2000, 135, 303–313. [Google Scholar] [CrossRef]
- Żmihorski, M.; Chylarecki, P.; Rejt, Ł.; Mazgajski, T.D. The effects of forest patch size and ownership structure on tree stand characteristics in a highly deforested landscape of central Poland. Eur. J. For. Res. 2010, 129, 393–400. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of Forestry 2021. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-forestry-2021,12,4.html (accessed on 4 February 2022).
- Łonkiewicz, B. Rola i węzłowe problemy planowania przestrzennego w leśnictwie. Prace IBL B 1986, 5, 46–50. [Google Scholar]
- Budniak, P. Metoda wyróżniania kompleksów leśnych na podstawie ciągłości obszarów leśnych i zadrzewionych. Sylwan 2020, 164, 820–830. [Google Scholar] [CrossRef]
- Jabłoński, M.; Wysocka-Fijorek, E.; Budniak, P. Struktura lasów w Polsce na podstawie danych wielkoobszarowej inwentaryzacji stanu lasu. Sylwan 2017, 161, 267–276. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2021. Available online: https://qgis.org/en/site/ (accessed on 18 May 2021).
- Zielony, R.; Rabenda, M. Lesny obszar funkcjonalny Puszcza Kozienicka. Stud. I Mater. Cent. Edukac. Przyr. Leśnej 2008, 10, 119–129. [Google Scholar]
- Laurance, W.F. Theory meets reality: How habitat fragmentation research has transcended island biogeographic theory. Biol. Conserv. 2008, 141, 1731–1744. [Google Scholar] [CrossRef]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Core Team R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2013. Available online: https://www.r-project.org/ (accessed on 12 October 2021).
- Harald, A.; Gonzalez, P.; Gytarsky, M.; Krug, T.; Kurz, W.A.; Ogle, S.; Raison, J.; Schoene, D.; Ravindranath, N.H.; Elhassan, N.G.; et al. Agriculture, Forestry and Other Land Use: Forest land (AFOLU). 2006. Available online: https://www.thegef.org/what-we-do/topics/agriculture-forestry-and-other-land-uses (accessed on 20 December 2021).
- Girvetz, E.H.; Greco, S.E. How to define a patch: A spatial model for hierarchically delineating organism-specific habitat patches. Landsc. Ecol. 2007, 22, 1131–1142. [Google Scholar] [CrossRef]
- Mesquita, R.C.G.; Delamônica, P.; Laurance, W.F. Effect of surrounding vegetation on edge-related tree mortality in Amazonian forest fragments. Biol. Conserv. 1999, 91, 129–134. [Google Scholar] [CrossRef]
- Mohandass, D.; Campbell, M.J.; Davidar, P. Impact of patch size on woody tree species richness and abundance in a tropical montane evergreen forest patches of south India. J. For. Res. 2018, 29, 1675–1687. [Google Scholar] [CrossRef]
- Majumdar, K.; Datta, B.K. Effects of patch size, disturbances on diversity and structural traits of tropical semi-evergreen forest in the lowland Indo Burma hotspot: Implication on conservation of the threatened tree species. J. Mt. Sci. 2016, 13, 1397–1410. [Google Scholar] [CrossRef]
- Hill, J.L.; Curran, P.J. Species composition in fragmented forests: Conservation implications of changing forest area. Appl. Geogr. 2001, 21, 157–174. [Google Scholar] [CrossRef]
- Estevan, H.; Lloret, F.; Vayreda, J.; Terradas, J. Determinants of woody species richness in Scot pine and beech forests: Climate, forest patch size and forest structure. Acta Oecolog. 2007, 31, 325–331. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Uriarte, M.; DeFries, R.; Bedka, K.M.; Fernandes, K.; Gutiérrez-Vélez, V.; Pinedo-Vasquez, M.A. Fragmentation increases wind disturbance impacts on forest structure and carbon stocks in a western Amazonian landscape. Ecol. Appl. 2017, 27, 1901–1915. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Franklin, J.F.; Spies, T.A. Vegetation responses to edge environments in old-growth Douglas-fir forests. Ecol. Appl. 1992, 2, 387–396. [Google Scholar] [CrossRef]
- Gonzalez, M.; Ladet, S.; Deconchat, M.; Cabanettes, A.; Alard, D.; Balent, G. Relative contribution of edge and interior zones to patch size effect on species richness: An example for woody plants. For. Ecol. Manag. 2010, 259, 266–274. [Google Scholar] [CrossRef]
- Kupfer, J.A.; Malanson, G.P.; Franklin, S.B. Not seeing the ocean for the islands: The mediating influence of matrix-based processes on forest fragmentation effects. Glob. Ecol. Biogeogr. 2006, 15, 8–20. [Google Scholar] [CrossRef]
- King, A.J.; Melbourne, B.A.; Davies, K.F.; Nicholls, A.O.; Austin, M.P.; Tuff, K.T.; Evans, M.J.; Hardy, C.M.; Cunningham, S.A. Spatial and temporal variability of fragmentation effects in a long term, eucalypt forest fragmentation experiment. Landsc. Ecol. 2018, 33, 609–623. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Aragão, L.E.O.C.; Anderson, L.O.; Fonseca, M.G.; Shimabukuro, Y.E.; Vancutsem, C.; Achard, F.; Beuchle, R.; Numata, I.; Silva, C.A.; et al. Persistent collapse of biomass in Amazonian forest edges following deforestation leads to unaccounted carbon losses. Sci. Adv. 2020, 6, eaaz8360. [Google Scholar] [CrossRef]


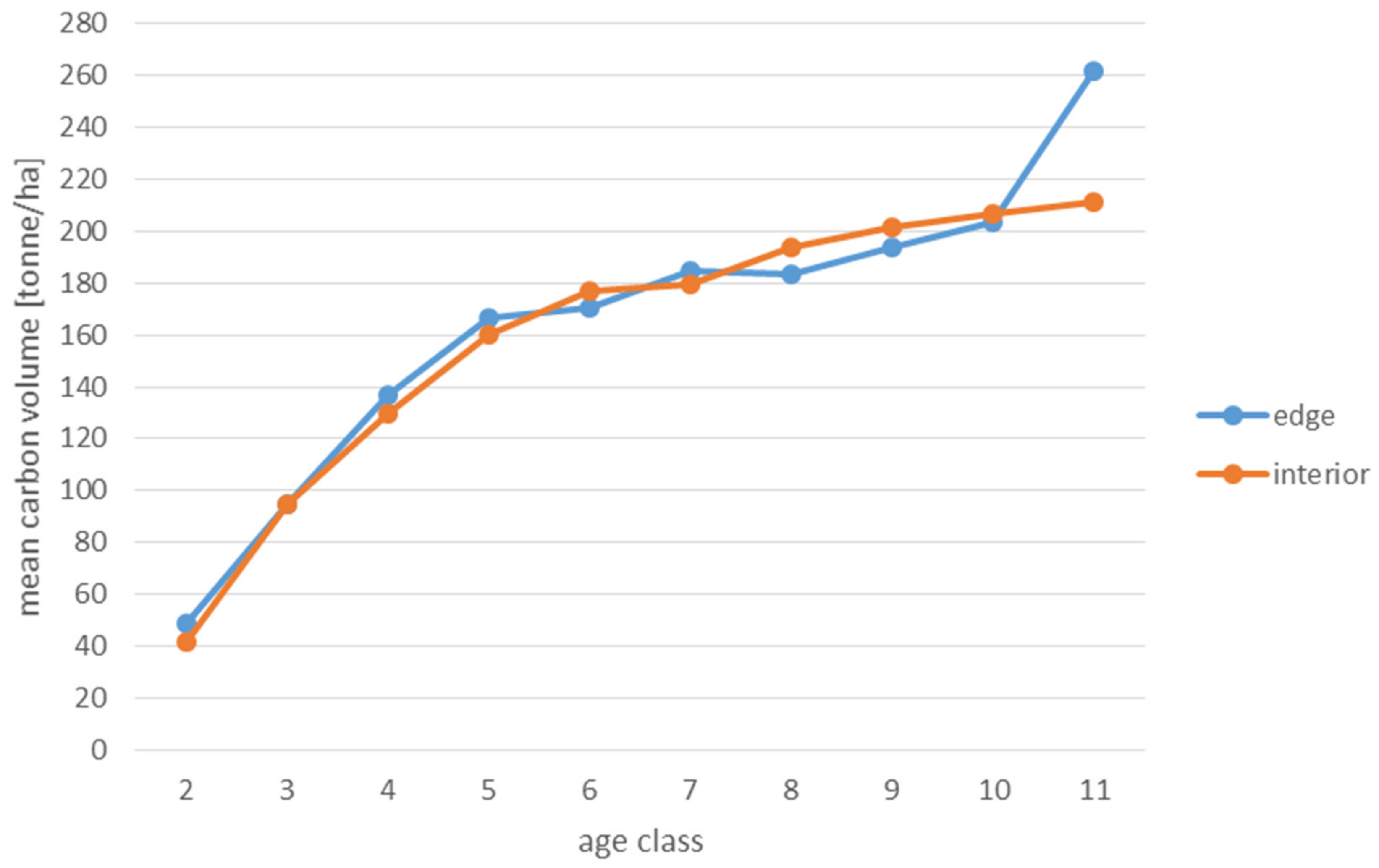

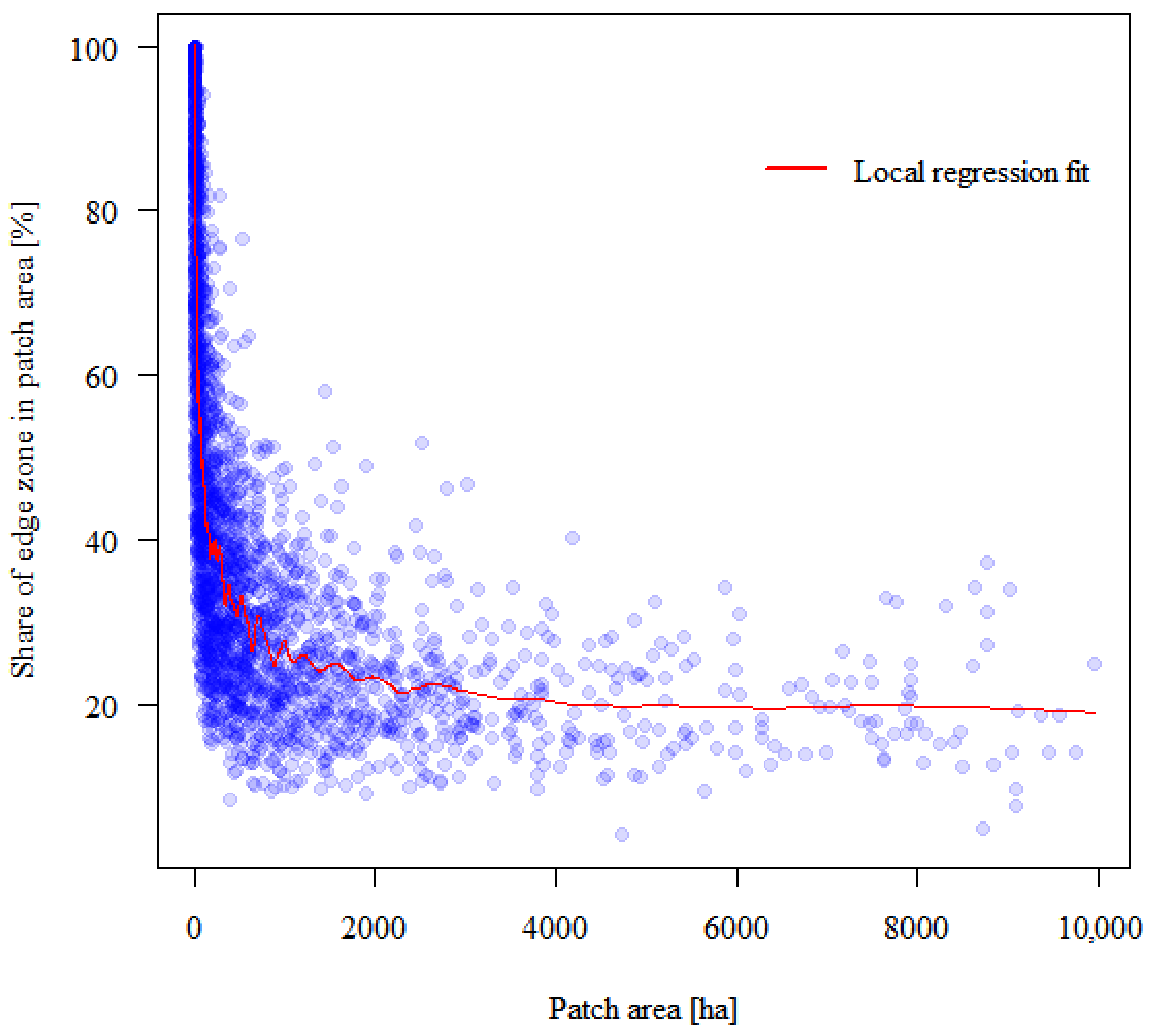
| Feature | v | g | zv |
|---|---|---|---|
| v | - | r = 0.918, p < 0.001 | r = 0.683, p < 0.001 |
| g | r = 0.918, p < 0.001 | - | r = 0.601, p < 0.001 |
| zv | r = 0.683, p < 0.001 | r = 0.601, p < 0.001 | - |
| Forest Site Type | Age Class | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| FCF | 0.001 * | 0.681 | 0.002 * | 0.209 | 0.036 * | 0.529 | 0.446 | 0.176 | 0.043 ** | 0.617 |
| FMCF | 0.033 * | 0.126 | 0.640 | 0.513 | 0.129 | 0.592 | 0.029 ** | 0.030 ** | 0.376 | 0.245 |
| FMBF | 0.524 | 0.612 | 0.447 | 0.442 | 0.030 ** | 0.260 | 0.430 | 0.025 ** | 0.371 | 0.011 ** |
| Forest Site Type | Age Class | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||||||||||
| E | I | E | I | E | I | E | I | E | I | E | I | E | I | E | I | E | I | E | I | |
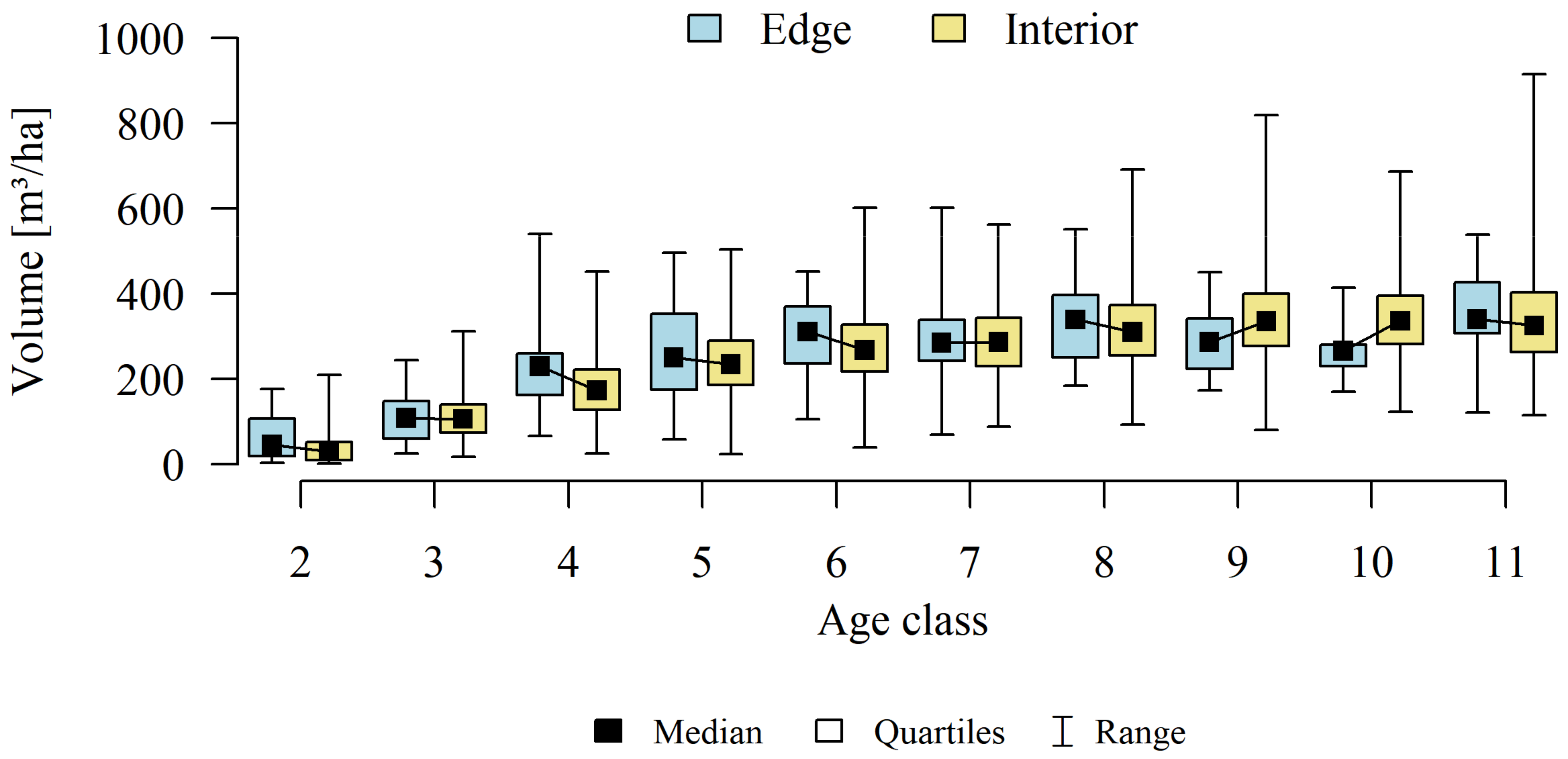
| FCF | 45 | 30 | 108 | 106 | 229 | 173 | 249 | 234 | 311 | 268 | 284 | 287 | 339 | 310 | 286 | 334 | 266 | 336 | 339 | 325 |
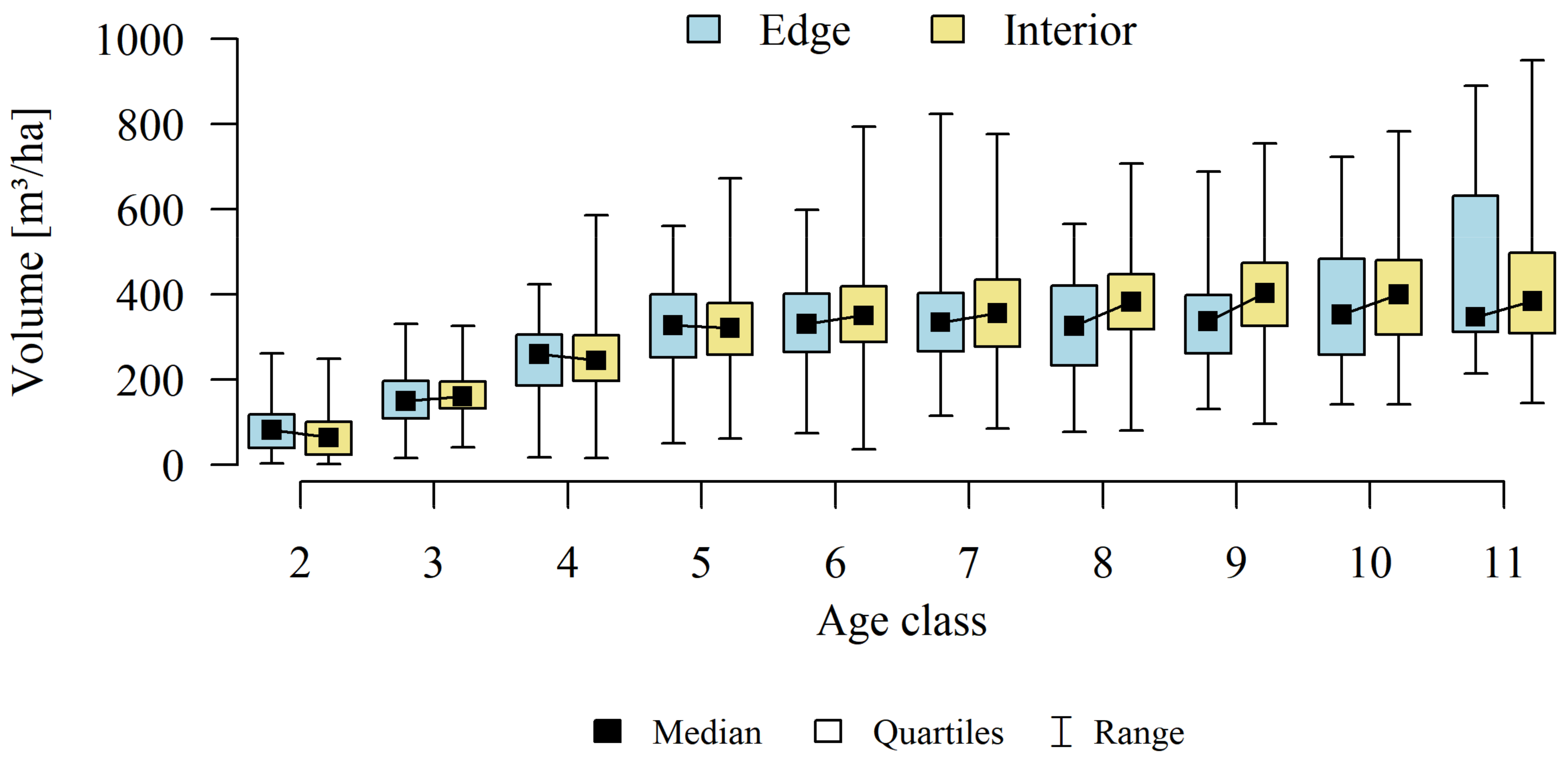
| FMCF | 81 | 63 | 149 | 160 | 259 | 244 | 327 | 321 | 330 | 350 | 334 | 356 | 325 | 382 | 337 | 403 | 353 | 399 | 347 | 384 |
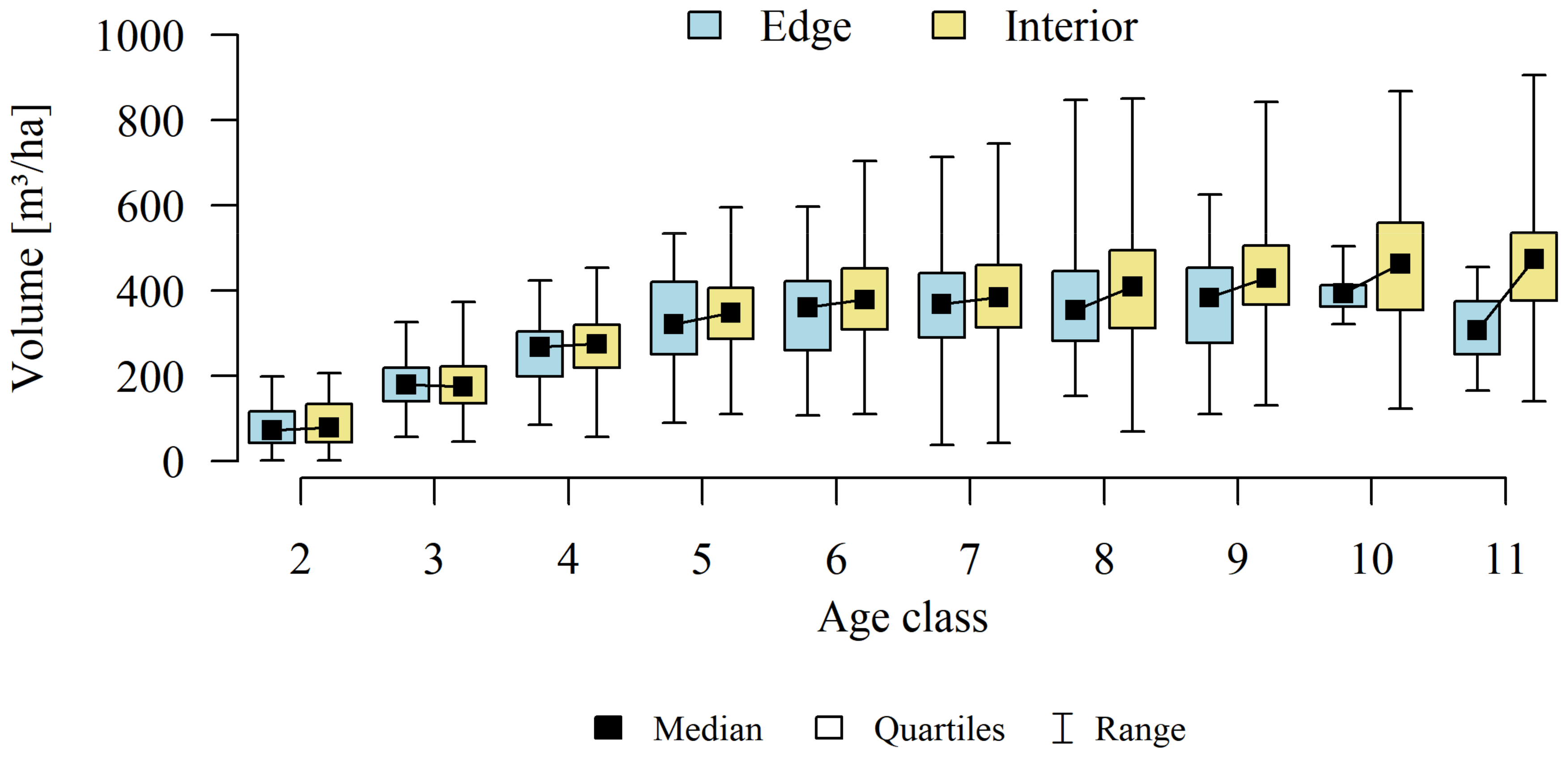
| FMBF | 72 | 78 | 179 | 175 | 267 | 274 | 321 | 348 | 360 | 378 | 368 | 384 | 354 | 409 | 383 | 429 | 394 | 463 | 306 | 474 |
| Forest Site Type | Age Class | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| FCF | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.03 | <0.01 | 0.03 | 0.01 | 0.06 |
| FMCF | 0.02 | 0.01 | <0.01 | <0.01 | 0.02 | 0.02 | 0.06 | 0.04 | 0.06 | 0.02 |
| FMBF | <0.01 | <0.01 | <0.01 | <0.01 | 0.05 | 0.03 | 0.04 | 0.09 | 0.02 | 0.06 |
| Forest Site Type | Age Class | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| FCF | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
| FMCF | 0.03 | <0.01 | 0.02 | <0.01 | 0.02 | 0.02 | 0.03 | 0.05 | 0.05 | 0.03 |
| FMBF | <0.01 | <0.01 | 0.03 | <0.01 | 0.07 | 0.07 | 0.03 | 0.10 | <0.01 | 0.12 |
| Forest Site Type | Variable | Age Class | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||||||||||
| E | I | E | I | E | I | E | I | E | I | E | I | E | I | E | I | E | I | E | I | ||
| FCF | CI− | 33 | 23 | 57 | 60 | 101 | 87 | 115 | 113 | 131 | 128 | 122 | 135 | 139 | 144 | 131 | 153 | 109 | 153 | 123 | 151 |
| M | 43 | 26 | 71 | 63 | 118 | 90 | 131 | 117 | 142 | 131 | 145 | 139 | 167 | 148 | 156 | 157 | 134 | 158 | 164 | 159 | |
| CI+ | 53 | 30 | 85 | 66 | 136 | 94 | 148 | 120 | 152 | 134 | 167 | 142 | 195 | 151 | 180 | 161 | 158 | 162 | 205 | 167 | |
| FMCF | CI− | 43 | 38 | 87 | 91 | 125 | 125 | 156 | 156 | 162 | 173 | 173 | 175 | 160 | 188 | 175 | 196 | 173 | 199 | 153 | 200 |
| M | 48 | 42 | 95 | 95 | 137 | 130 | 166 | 160 | 171 | 177 | 185 | 179 | 183 | 194 | 194 | 202 | 204 | 206 | 262 | 211 | |
| CI+ | 54 | 46 | 102 | 99 | 149 | 135 | 176 | 164 | 180 | 181 | 196 | 183 | 206 | 200 | 212 | 207 | 234 | 213 | 370 | 223 | |
| FMBF | CI− | 37 | 44 | 100 | 103 | 134 | 149 | 143 | 177 | 170 | 201 | 195 | 212 | 196 | 224 | 187 | 240 | 209 | 251 | 172 | 255 |
| M | 49 | 52 | 116 | 111 | 149 | 158 | 163 | 184 | 183 | 207 | 213 | 219 | 220 | 232 | 225 | 247 | 239 | 261 | 217 | 271 | |
| CI+ | 61 | 60 | 132 | 119 | 164 | 167 | 183 | 192 | 196 | 213 | 230 | 226 | 243 | 240 | 263 | 254 | 269 | 271 | 262 | 287 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budniak, P.; Zięba, S. Effects of Forest Fragmentation on the Volume of Wood Resources in Managed, Pine-Dominated Forests in Poland. Forests 2022, 13, 590. https://doi.org/10.3390/f13040590
Budniak P, Zięba S. Effects of Forest Fragmentation on the Volume of Wood Resources in Managed, Pine-Dominated Forests in Poland. Forests. 2022; 13(4):590. https://doi.org/10.3390/f13040590
Chicago/Turabian StyleBudniak, Piotr, and Stanisław Zięba. 2022. "Effects of Forest Fragmentation on the Volume of Wood Resources in Managed, Pine-Dominated Forests in Poland" Forests 13, no. 4: 590. https://doi.org/10.3390/f13040590
APA StyleBudniak, P., & Zięba, S. (2022). Effects of Forest Fragmentation on the Volume of Wood Resources in Managed, Pine-Dominated Forests in Poland. Forests, 13(4), 590. https://doi.org/10.3390/f13040590






