Assessing the Hydric Deficit on Two Polylepis Species from the Peruvian Andean Mountains: Xylem Vessel Anatomic Adjusting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Tree-Ring Data and Chronology Development
2.3. Drought Effect on Tree-Ring Width
2.4. Spatial Correlation Polylepis Tree-Rings’ vs. SST
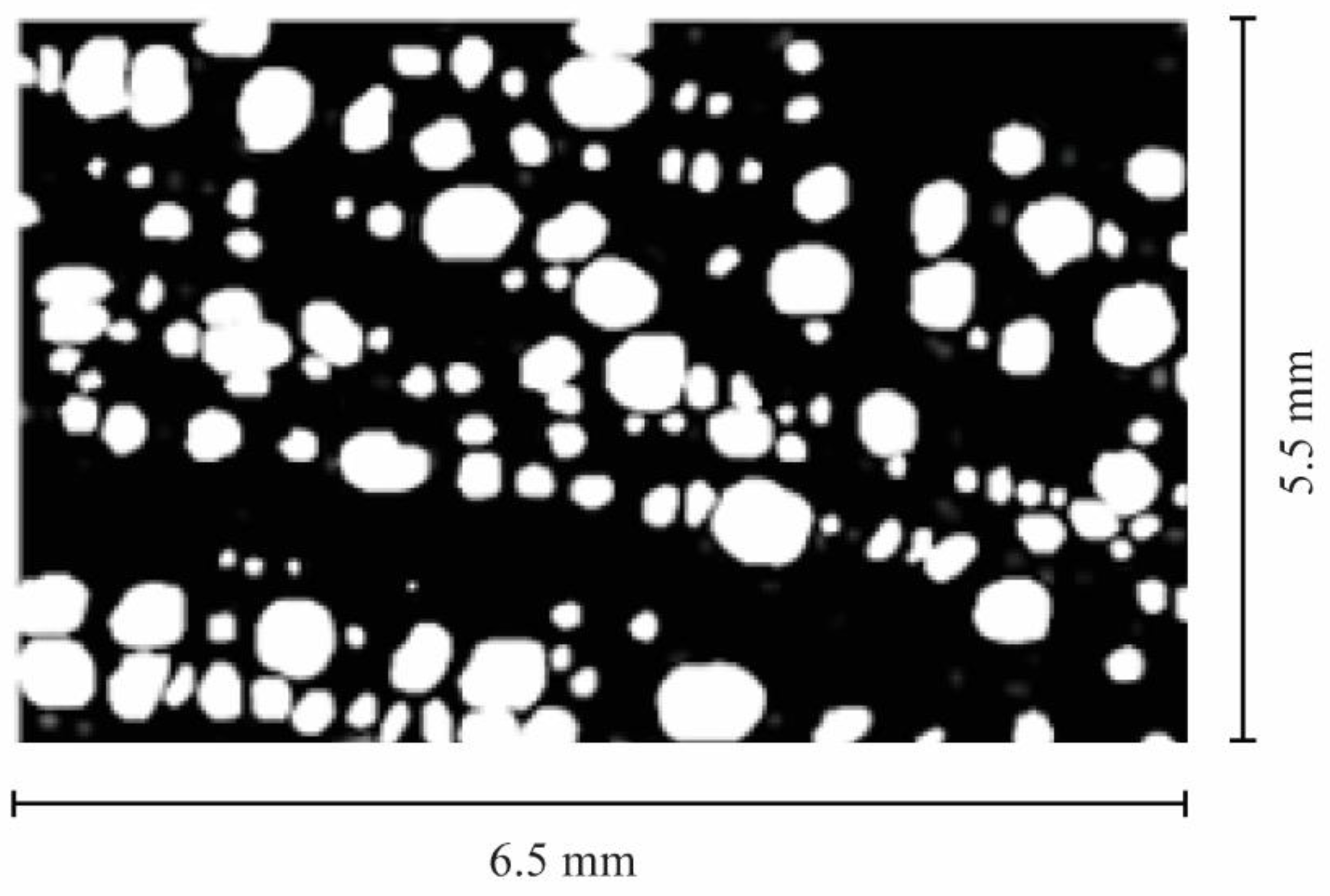
2.5. Digitalization of Xylem Vessel Anatomical Traits
2.6. Xylem Vessel Data Analysis
3. Results
3.1. Tree-Ring Chronology
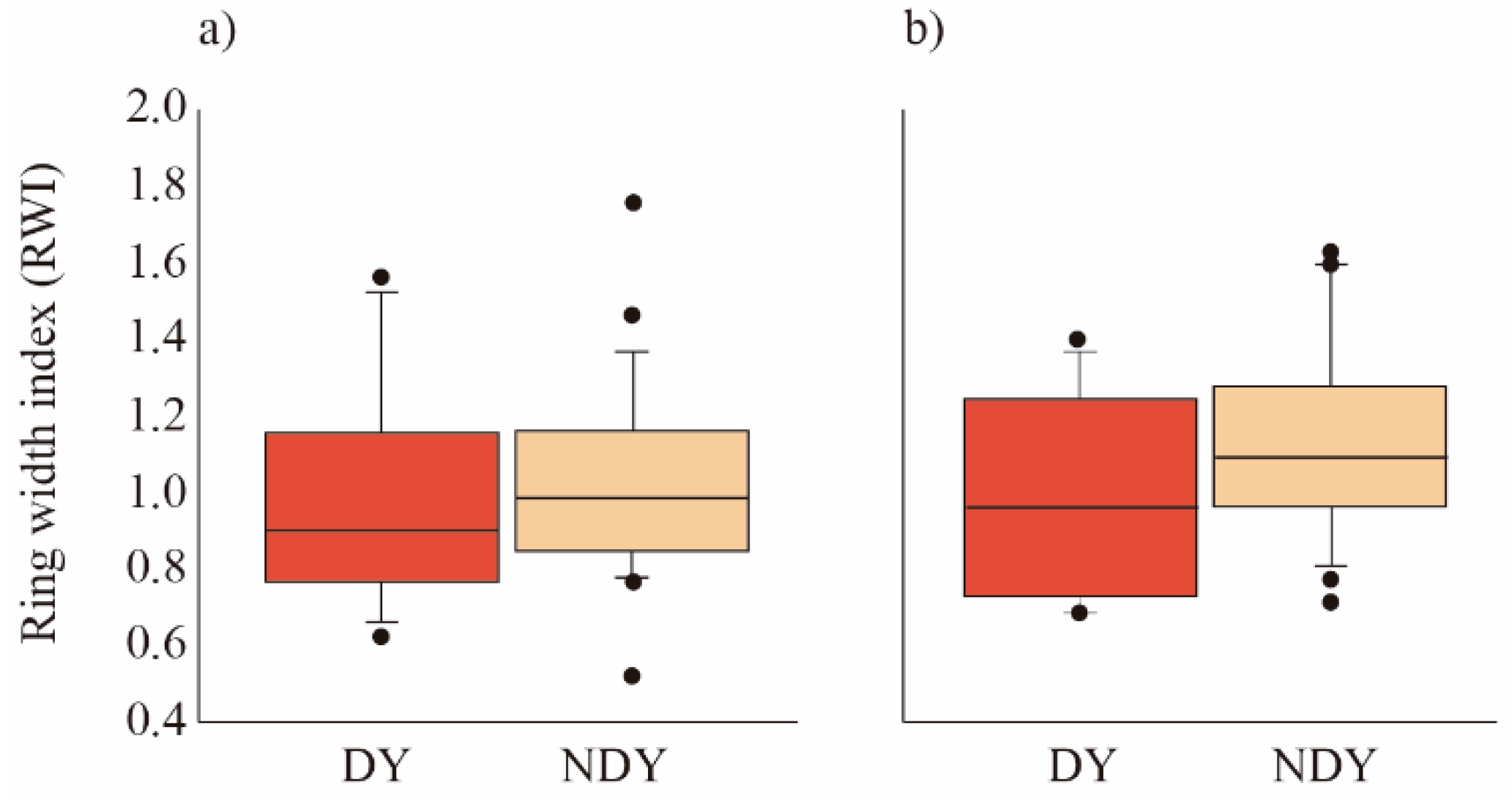
3.2. Drought Effect on RWI
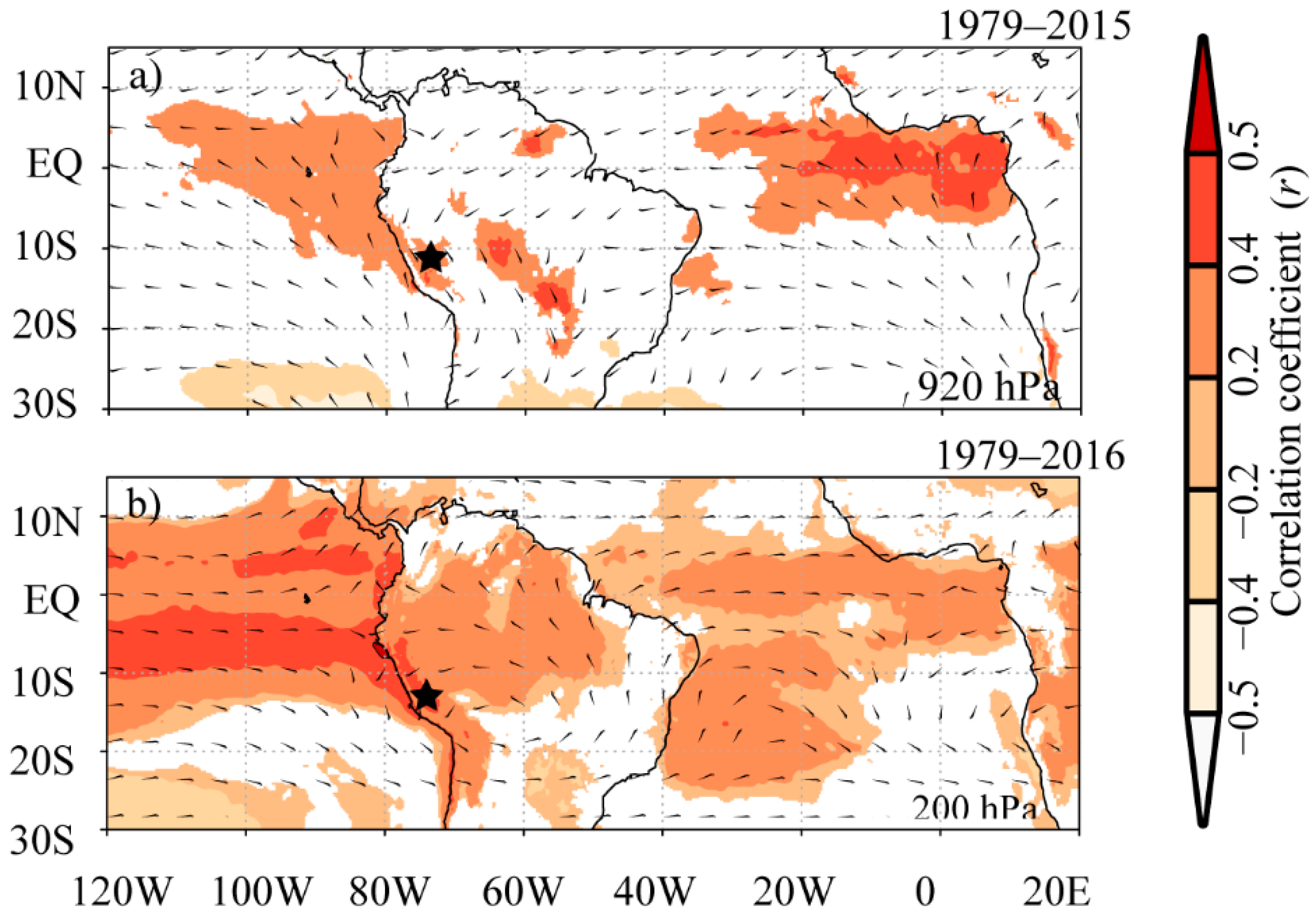
3.3. Spatial Linking between Tree-Ring Chronologies and High Temperature
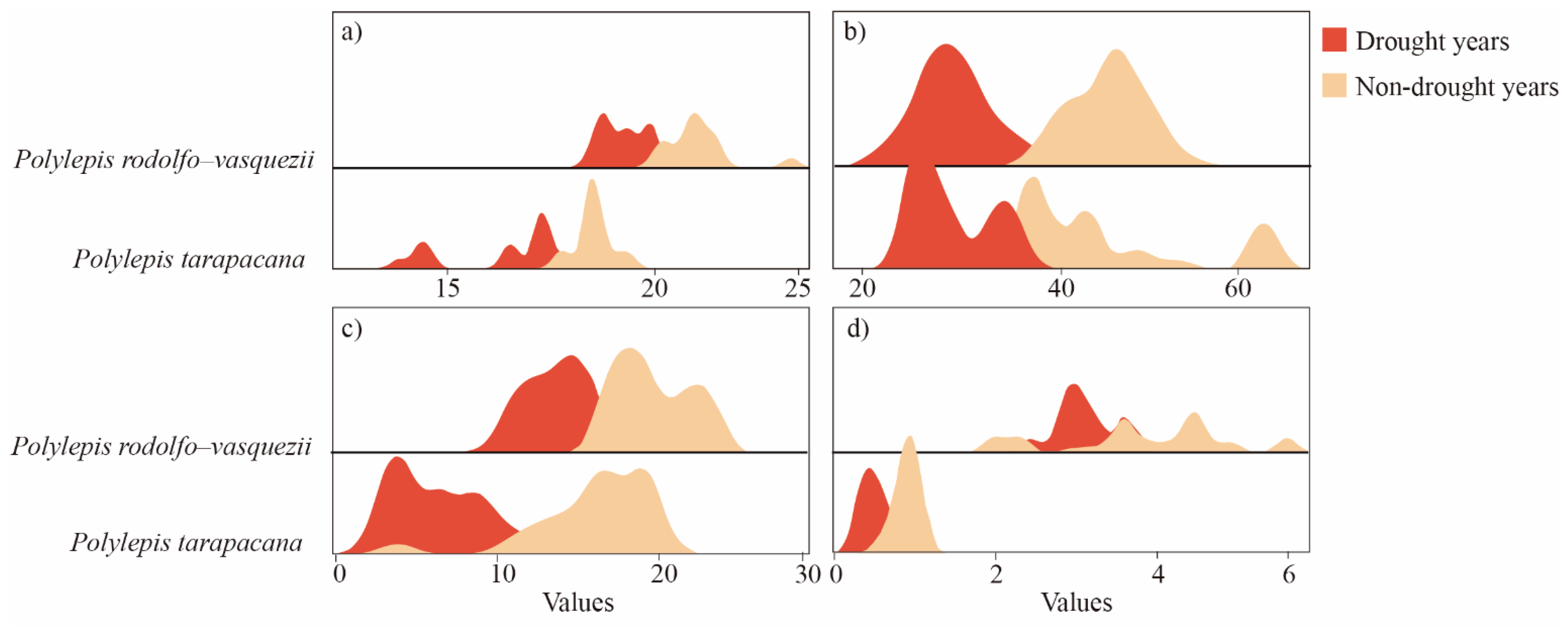
3.4. Xylem Anatomical Adjusting of Polylepis Species to Hydric Deficit
4. Discussion
5. Conclusions
- We highlight that vessel traits prove to have a better climatic signal than growth-ring width in the face of drought events.
- This study exhibits the usefulness of ring-width index and vessel traits as climate-resilience indicators during specific drought conditions.
- Local climatic oscillations can influence vessel anatomical plasticity adaptations.
- Our results suggest that specific climatic events influence each Polylepis species’ adaptability to drought periods and could also restrict them from remaining as a part of the Peruvian Andean mountain ecosystems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, M. Bosques de Polylepis; Universidad Mayor de San Andrés: La Paz, Bolivia, 2006; ISBN 9788449109119. [Google Scholar]
- Simpson, B.B. A revision of the genus Polylepis (Rosaceae: Sanguisorbeae). Smithson. Contrib. Bot. 1979, 43, 1–62. [Google Scholar] [CrossRef]
- Requena-Rojas, E.J.; Morales, M.; Villalba, R. Dendroclimatological assessment of Polylepis rodolfo-vasquezii: A novel Polylepis species in the Peru highlands. Dendrochronologia 2020, 62, 125722. [Google Scholar] [CrossRef]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.A.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J.; et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Moya, J.; Lara, A. Cronologías de ancho de anillos de queñoa (Polylepis tarapacana) para los últimos 500 años en el Altiplano de la región de Arica y Parinacota, Chile. Bosque 2011, 32, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Mamani Salas, M.S. Diversidad Genética de Poblaciones de Polylepis canoi W. Mendoza y Polylepis rodolfo-vasquezii L. Valenzuela & L. Villalba mediante el uso de Microsatélites (SRR) en la Región Junín. Master’s Thesis, Universidad Nacional del Centro del Perú, Huancayo, Peru, 2018. [Google Scholar]
- Camel, V.; Quispe-Melgar, H.R.; Ames-Martínez, F.N.; Navarro-Romo, W.C.; Segovia-Salcedo, M.C.; Kessler, M. Forest structure of three endemic species of the genus Polylepis (Rosaceae) in central Peru. Ecol. Austral 2019, 29, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Boza, T.E.; Quispe-Melgar, H.R.; Kessler, M. Taxonomic reevaluation of the Polylepis sericea complex (Rosaceae), with the description of a new species. Syst. Bot. 2019, 44, 324–334. [Google Scholar] [CrossRef]
- Requena-Rojas, E.J.; Crispín-DelaCruz, D.B.; Ticse-Otarola, G.; Quispe-Melgar, H.R.; Inga, J.G.; Camel, V.; Guerra, A.; Ames-Martínez, F.N.; Morales, M. Temporal growth variation in high-elevation forests: Case study of Polylepis forests in Central Andes. In Latin American Dendroecology: Combining Tree-Ring Sciences and Ecology in a Megadiverse Territory; Pompa-García, M., Camarero, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 263–279. [Google Scholar] [CrossRef]
- Solíz, C.; Villalba, R.; Argollo, J.; Morales, M.S.; Christie, D.A.; Moya, J.; Pacajes, J. Spatio-temporal variations in Polylepis tarapacana radial growth across the Bolivian Altiplano during the 20th century. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 281, 296–308. [Google Scholar] [CrossRef]
- Requena-Rojas, E.J.; Taquire-Arroyo, A. Anatomía del leño y caracterización de los anillos de crecimiento en individuos de Polylepis tarapacana en el Altiplano-Tacna-Perú. Quebracho 2019, 27, 66–75. [Google Scholar]
- Camel, V.; Arizapana-Almonacid, M.; Pyles, M.; Galeano, E.; Quispe-Melgar, H.R.; Ninanya-Parra, Z.; Ames-Martínez, F.N.; Requena-Rojas, E.; Kessler, M. Using dendrochronology to trace the impact of the hemiparasite Tristerix chodatianus on Andean Polylepis trees. Plant Ecol. 2019, 220, 873–886. [Google Scholar] [CrossRef]
- Jomelli, V.; Pavlova, I.; Guin, O.; Soliz-Gamboa, C.; Contreras, A.; Toivonen, J.M.; Zetterberg, P. Analysis of the dendroclimatic potential of Polylepis pepei, P. subsericans and P. rugulosa in the tropical andes (Peru-Bolivia). Tree-Ring Res. 2012, 68, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, J.; Requeña-Rojas, E.J.; Mark, B.G.; Fernández, A. Assessing the dendroclimatological potential of Polylepis rodolfo-vasquezii in the Peruvian Andes. In AGU Fall Meeting Abstracts; The Ohio State University: Columbus, OH, USA, 2018. [Google Scholar]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Rita, A.; Cherubini, P.; Leonardi, S.; Todaro, L.; Borghetti, M. Functional adjustments of xylem anatomy to climatic variability: Insights from long-term Ilex aquifolium tree-ring series. Tree Physiol. 2015, 35, 817–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Ames-Martínez, F.N.; Quispe, H.; Zuñiga, D.; Segovía, M.; Kessler, M. Bosques de Polylepis: Biodiversidad en la Región Central del Perú; Universidad Continental: Huancayo, Peru, 2019; p. 200. [Google Scholar]
- Crispín-DelaCruz, D.B.; Morales, M.S.; Andreu-Hayles, L.; Christie, D.A.; Guerra, A.; Requena-Rojas, E.J. High ENSO sensitivity in tree rings from a northern population of Polylepis tarapacana in the Peruvian Andes. Dendrochronologia 2022, 71, 125902. [Google Scholar] [CrossRef]
- Stokes, M.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Speer, B.J.H. Fundamentals of Tree-Ring Research; Cambridge University Press: Cambridge, UK, 2010; p. 509. [Google Scholar] [CrossRef]
- Schulman, E. (Ed.) Dendroclimatic Changes in Semiarid America, 1st ed.; University of Arizona Press: Tucson, AZ, USA, 1956. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press Inc.: London, UK, 1976. [Google Scholar]
- Melvin, T.M.; Briffa, K.R. A “signal-free” approach to dendroclimatic standardisation. Dendrochronologia 2008, 26, 71–86. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Cook, E.; Kairiukstis, L. Methods of dendrochronology. Sci. Total Environ. 1991, 104, 249. [Google Scholar] [CrossRef]
- Briffa, K.R. Interpreting high-resolution proxy climate data: The example of dendroclimatology. In Analysis of Climate Variability; Von Storch, H., Navarra, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 0500, pp. 77–94. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Imfeld, N.; Barreto Schuler, C.; Correa-Marrou, K.M.; Jacques-Coper, M.; Sedlmeier, K.; Gubler, S.; Huerta, A.; Brönnimann, S. Summertime precipitation deficits in the southern Peruvian highlands since 1964. Int. J. Climatol. 2019, 39, 4497–4513. [Google Scholar] [CrossRef]
- El Servicio Nacional de Meteorología e Hidrología del Perú. Caracterización Espacio Temporal de la Sequía en los Departamentos Altoandinos del Perú (1981–2018); El Servicio Nacional de Meteorología e Hidrología del Perú: Lima, Peru, 2019. [Google Scholar]
- van der Schrier, G.; Barichivich, J.; Briffa, K.R.; Jones, P.D. A scPDSI-based global data set of dry and wet spells for 1901–2009. J. Geophys. Res. Atmos. 2013, 118, 4025–4048. [Google Scholar] [CrossRef]
- Kalnay, E.; Kanamitsu, M.; Kistler, R.; Collins, W.; Deaven, D.; Gandin, L. The NCEP/NCAR 40-Year Reanalysis Project. Bull. Am. Meteorol. Soc. 1996, 77, 437–472. [Google Scholar] [CrossRef] [Green Version]
- Vuille, M.; Bradley, R.S.; Keimig, F. Climate variability in the Andes of Ecuador and its relation to tropical Pacific and Atlantic Sea Surface temperature anomalies. J. Clim. 2000, 13, 2520–2535. [Google Scholar] [CrossRef]
- Segura, H.; Espinoza, J.C.; Junquas, C.; Lebel, T.; Vuille, M.; Garreaud, R. Recent changes in the precipitation-driving processes over the southern tropical Andes/western Amazon. Clim. Dyn. 2020, 54, 2613–2631. [Google Scholar] [CrossRef]
- da Fonseca Júnior, S.F.; Piedade, M.T.F.; Schöngart, J. Wood growth of Tabebuia barbata (E. Mey.) Sandwith (Bignoniaceae) and Vatairea guianensis Aubl. (Fabaceae) in Central Amazonian black-water (igapó) and white-water (várzea) floodplain forests. Trees 2009, 23, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Souto-Herrero, M.; Rozas, V.; García-González, I. Earlywood vessels and latewood width explain the role of climate on wood formation of Quercus pyrenaica Willd. across the Atlantic-Mediterranean boundary in NW Iberia. For. Ecol. Manag. 2018, 425, 126–137. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Luna-Vega, I. Dendroecology as a research tool to investigate climate change resilience on Magnolia vovidesii, a threatened Mexican cloud forest tree species of eastern Mexico. In Latin American Dendroecology: Combining Tree-Ring Sciences and Ecology in a Megadiverse Territory; Pompa-García, M., Camarero, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–20. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sperry, J.S.; Saiendra, N.Z. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant Cell Environ. 1994, 17, 1233–1241. [Google Scholar] [CrossRef]
- Carlquist, S. Ecological factors in wood evolution: A floristic approach. Am. J. Bot. 1977, 64, 887–896. [Google Scholar] [CrossRef]
- Wilke, C.O. Ridgeline Plots in ‘ggplot2′. R Package Ggridges Version 0.5.3. 2021. Available online: https://cran.r-project.org/web/packages/ggridges/index.html (accessed on 12 October 2021).
- Zimmermann, M.H. The Vessel Network in the Stem. In Xylem Structure and the Ascent of Sap; Springer Series in Wood Science: Berlin/Heidelberg, Germany, 1983; ISBN 978-3-662-22627-8. [Google Scholar]
- García-González, I.; Souto-Herrero, M.; Campelo, F. Ring-porosity and earlywood vessels: A review on extracting environmental information through time. IAWA J. 2016, 37, 295–314. [Google Scholar] [CrossRef]
- Gil, R.; Villalba, R. Tree rings as a surrogate for economic stress: An example from the Puna of Jujuy, Argentina in the 19th century. Dendrochronologia 2005, 22, 141–147. [Google Scholar] [CrossRef]
- Rodriguez-Caton, M.; Andreu-Hayles, L.; Morales, M.S.; Daux, V.; Christie, D.A.; Coopman, R.E.; Alvarez, C.; Rao, M.P.; Aliste, D.; Flores, F.; et al. Different climate sensitivity for radial growth, but uniform for tree-ring stable isotopes along an aridity gradient in Polylepis tarapacana, the world’s highest elevation tree-species. Tree Physiol. 2021, 41, 1353–1371. [Google Scholar] [CrossRef] [PubMed]
- Humanes-Fuente, V.; Ferrero, M.E.; Muñoz, A.A.; González-Reyes; Requena-Rojas, E.J.; Barichivich, J.; Inga, J.G.; Layme-Huaman, E.T. Two centuries of hydroclimatic variability reconstructed from tree-ring records over the Amazonian Andes of Peru. J. Geophys. Res. Atmos. 2020, 125, e2020JD032565. [Google Scholar] [CrossRef]
- Kessler, M.; Toivonen, J.M.; Sylvester, S.P.; Kluge, J.; Hertel, D. Elevational patterns of Polylepis tree height (Rosaceae) in the high Andes of Peru: Role of human impact and climatic conditions. Front. Plant Sci. 2014, 5, 194. [Google Scholar] [CrossRef] [Green Version]
- Alvites, C.; Battipaglia, G.; Santopuoli, G.; Hampel, H.; Vázquez, R.F.; Matteucci, G.; Tognetti, R. Dendrochronological analysis and growth patterns of Polylepis reticulata (Rosaceae) in the Ecuadorian Andes. IAWA J. 2019, 40, 331–351. [Google Scholar] [CrossRef]
- Ault, T.R.; Mankin, J.S.; Cook, B.I.; Smerdon, J.E. Relative impacts of mitigation, temperature, and precipitation on 21st-century megadrought risk in the American Southwest. Sci. Adv. 2016, 2, e1600873. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.S.; Cook, E.R.; Barichivich, J.; Christie, D.A.; Villalba, R.; LeQuesne, C.; Srur, A.M.; Ferrero, M.E.; González-Reyes, Á.; Couvreux, F.; et al. Six hundred years of South American tree rings reveal an increase in severe hydroclimatic events since mid-20th century. Proc. Natl. Acad. Sci. USA 2020, 117, 16816–16823. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- García-González, I.; Fonti, P. Selecting earlywood vessels to maximize their environmental signal. Tree Physiol. 2006, 26, 1289–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonti, P.; von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, E.C.; Vázquez-García, J.A.; García-González, I.; Alcántara-Ayala, O.; Luna-Vega, I. Drought effects on the plasticity in vessel traits of two endemic Magnolia species in the tropical montane cloud forests of eastern Mexico. J. Plant Ecol. 2020, 13, 331–340. [Google Scholar] [CrossRef]
- Hertel, D.; Wesche, K. Tropical moist Polylepis stands at the treeline in East Bolivia: The effect of elevation on stand microclimate, above and below-ground structure, and regeneration. Trees 2008, 22, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]


| Polylepis Species | Climate Classification (sensu Peel et al. [18]) | Dry Season | Wet Season | Mean Temperature (°C) | Mean Precipitation (mm) |
|---|---|---|---|---|---|
| P. rodolfo-vasquezii | Semidry with abundant moisture; C(r)B’ | June–August | December–March | 8.8 | 913 |
| P. tarapacana | Semiarid with dry winters; D(i)C’ | April–October | December–March | 10.9 | 535 |
| Polylepis Species | Time Spanned | Mean RWI | Samples Live Trees/Stump Cross–Sections | EPS | Rbar |
|---|---|---|---|---|---|
| P. rodolfo-vasquezii | 1891–2015 | 1.21 | 24/6 | 0.82 | 0.30 |
| P. tarapacana | 1665–2015 | 0.91 | 25/6 | 0.91 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ramírez, E.C.; Crispín-DelaCruz, D.B.; Ticse-Otarola, G.; Requena-Rojas, E.J. Assessing the Hydric Deficit on Two Polylepis Species from the Peruvian Andean Mountains: Xylem Vessel Anatomic Adjusting. Forests 2022, 13, 633. https://doi.org/10.3390/f13050633
Rodríguez-Ramírez EC, Crispín-DelaCruz DB, Ticse-Otarola G, Requena-Rojas EJ. Assessing the Hydric Deficit on Two Polylepis Species from the Peruvian Andean Mountains: Xylem Vessel Anatomic Adjusting. Forests. 2022; 13(5):633. https://doi.org/10.3390/f13050633
Chicago/Turabian StyleRodríguez-Ramírez, Ernesto C., Doris B. Crispín-DelaCruz, Ginette Ticse-Otarola, and Edilson J. Requena-Rojas. 2022. "Assessing the Hydric Deficit on Two Polylepis Species from the Peruvian Andean Mountains: Xylem Vessel Anatomic Adjusting" Forests 13, no. 5: 633. https://doi.org/10.3390/f13050633







