Modelling Response of Norway Spruce Forest Vegetation to Projected Climate and Environmental Changes in Central Balkans Using Different Sets of Species
Abstract
:1. Introduction
- How climate change, according to the projections, would affect vegetation structure in the Norway spruce forests of the Central Balkans;
- How changes in environmental factors and habitat suitability would affect vegetation structure in the named forest habitats;
- Defining a list of plant species as the most suitable for dynamic modelling of HSI metrics and early diagnosis of Norway spruce plant community reaction to multiple stressors.
2. Materials and Methods
2.1. Study Area
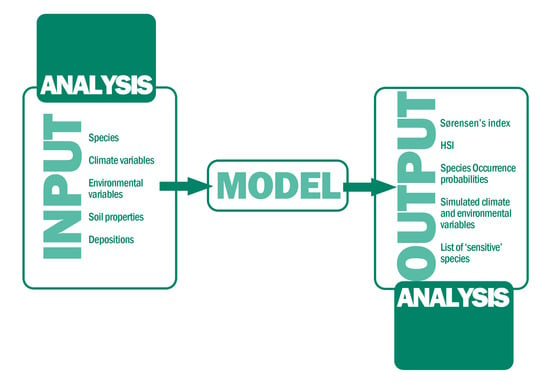
2.2. Vegetation Sampling, Data Processing, and Model Description
2.3. Climatic Data and Modelling
2.4. Soil, Deposition Data, and Modelling
3. Results
3.1. Pre-Modelling Analyses
3.2. Results of the Norway Spruce Forest Modelling
3.3. Habitat Suitability Analysis
3.4. Canonical Correspondence Analysis
4. Discussion
4.1. Norway Spruce Forests’ Response to Climate Changes
4.2. Norway Spruce Forests’ Response to Environmental Changes
4.3. The Effects of Changes in Soil Properties
4.4. The Effects of Deposition Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Väre, H.; Lampinen, R.; Humphries, C.; Williams, P. Taxonomic Diversity of Vascular Plants in the European Alpine Areas. In Alpine Biodiversity in Europe; Nagy, L., Grabherr, G., Körner, C., Thompson, D.B.A., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2003; Volume 167. [Google Scholar] [CrossRef]
- Barthlott, W.; Lauer, W.; Placke, A. Global Distribution of Species Diversity in Vascular Plants: Towards a World Map of Phytodiversity (Globale Verteilung der Artenvielfalt Höherer Pflanzen: Vorarbeiten zu einer Weltkarte der Phytodiversität). Erdkunde 1996, 50, 317–327. Available online: http://www.jstor.org/stable/25646853 (accessed on 15 April 2022). [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Harald, P.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA* master site Schrankogel, Tyrol, Austria. Glob. Change Biol. 2007, 13, 147–156. [Google Scholar] [CrossRef]
- Beniston, M. Climatic Change in Mountain Regions: A Review of Possible Impacts. Clim. Change 2003, 59, 5–31. [Google Scholar] [CrossRef]
- Ulrich, B.; Mayer, R.; Khanna, P.K. Chemical changes due to acidic precipitation in a loess-derived soils in Central Europe. Soil Sci. 1980, 130, 193–199. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts; adaptive capacity; and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Hansen, A.J.; Neilson, R.P.; Dale, V.H.; Flather, C.H.; Iverson, L.R.; Currie, D.J.; Shafer, S.; Cook, R.; Bartlein, P.J. Global change in forest: Response of species; communities and biomes: Interactions between climate change and land use are projected to cause large shifts in biodiversity. Biosci. J. 2001, 51, 765–779. [Google Scholar] [CrossRef]
- Modrzyński, J. Outline of Ecology. In Biology and Ecology of Norway Spruce; Tjoelker, M., Boratisńki, A., Bugala, A., Eds.; Forestry Science; Springer: Berlin/Heidelberg, Germany, 2007; pp. 195–273. [Google Scholar]
- Skrøppa, T. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Norway Spruce (Picea abies). Bioversity International. 2003. Available online: http://www.euforgen.org/fileadmin/templates/euforgen.org/upload/Publications/Technical_guidelines/Technical_guidelines_Picea_abies.pdf (accessed on 15 April 2022).
- CH2014-Impacts. Toward Quantitative Scenarios of Climate Change Impacts in Switzerland; OCCR, FOEN, MeteoSwiss, C2SM, Agroscope, and ProClim, Bern, Switzerland. 2014. Available online: http://www.ch2014-impacts.ch/res/files/CH2014-Impacts_report.pdf (accessed on 22 April 2022).
- Schulze, E.D. Air Pollution and Forest Decline in a Spruce (Picea abies) Forest. Science 1989, 19, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Hlásny, T.; Sitková, Z. (Eds.) Spruce Forests Decline in the Beskids; National Forest Centre–Forest Research Institute Zvolen & Czech University of Life Sciences Prague & Forestry and Game Management Research Institute Jiloviště—Strnady: Zvolen, Slovakia, 2010; 182p, ISBN 978-80-8093-127-8. [Google Scholar]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Hettelingh, J.-P.; de Vries, W.; Posch, M.; Reinds, G.J.; Slootweg, J.; Hicks, W.K. Development of the critical loads concept and current and potential applications to different regions of the world. In Nitrogen Deposition; Critical Loads and Biodiversity; Hicks, W.K., Haeuber, R., Sutton, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 281–293. [Google Scholar]
- Phoenix, G.K.; Hicks, W.K.; Cinderby, S.; Kuylenstierna, J.C.I.; Stock, W.D.; Dentener, F.J.; Giller, K.E.; Austin, A.T.; Lefroy, R.D.B.; Gimeno, B.S.; et al. Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Glob. Chang. Biol. 2006, 12, 470–476. [Google Scholar] [CrossRef]
- Sutton, M.A.; Skiba, U.M.; van Grinsven, H.J.; Oenema, O.; Watson, C.J.; Williams, J.; Hellums, D.T.; Maas, R.; Gyldenkaerne, S.; Pathak, H.; et al. Green economy thinking and the control of nitrous oxide emissions. Environ. Dev. 2014, 9, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Rowe, E.C.; Wamelink, G.W.W.; Smart, S.M.; Butler, A.; Henrys, P.; van Dobben, H.F.; Reinds, G.J.; Evans, C.D.; Kros, J.; de Vries, W. Field survey-based models for exploring nitrogen and acidity effects on plant species diversity and assessing long-term critical loads. In Critical Loads and Dynamic Risk Assessments; de Vries, W., Hettelingh, J.P., Posch, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 297–326. [Google Scholar] [CrossRef]
- Le Duc, S.D.; Rothstein, D.E. Plant-available organic and mineral nitrogen shift in dominance with forest stand age. Ecology 2010, 91, 708–720. [Google Scholar] [CrossRef]
- Jovanović, B. Picea abies. In Flora of SR Serbia I.; Sarić, M., Ed.; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1992; pp. 183–185. (In Serbian) [Google Scholar]
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea abies in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 114–116. [Google Scholar]
- Probst, A.; Obeidy, C.; Gaudio, N.; Belyazid, S.; Gégout, J.-C.; Alard, D.; Corket, E.; Party, J.-P.; Gauquelin, T.; Mansat, A.; et al. Evaluation of Plant Responses to Atmospheric Nitrogen Deposition in France Using Integrated Soil-Vegetation Models. In Critical Loads and Dynamic Risk Assessments; de Vries, W., Hettelingh, J.P., Posch, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 359–380. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Lindner, M. Adapting to Climate Change in European Forests—Results of the MOTIVE Project; Pensoft Publishers: Sofia, Bulgaria, 2013; p. 108. [Google Scholar]
- Schmied, G.; Hilmers, T.; Uhl, E.; Pretzsch, H. The Past Matters: Previous Management Strategies Modulate Current Growth and Drought Responses of Norway Spruce (Picea abies H. Karst.). Forests 2022, 13, 243. [Google Scholar] [CrossRef]
- Wilkening, J.; Pearson-Prestera, W.; Mungi, N.A.; Bhattacharyya, S. Endangered species management and climate change: When habitat conservation becomes a moving target. Wildl. Soc. Bull. 2019, 43, 11–20. [Google Scholar] [CrossRef] [Green Version]
- European Environmental Agency. Digital Map of European Ecological Regions. 2009. Available online: https://www.eea.europa.eu/data-and-maps/figures/dmeer-digital-map-of-european-ecological-regions (accessed on 18 October 2020).
- Painho, M.; Farral, H.; Barata, F. Digital Map of European Ecological Regions (DMEER). Its concept and elaboration. In Proceedings of the Second Joint European Conference & Exhibition on Geographical Information (Vol. 1): From Research to Application through Cooperation; IOS Press: Barcelona, Spain, 1996; pp. 437–446. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springerm: Wien, Austria, 1964. [Google Scholar]
- Tomić, Z. Šumske fitocenoze Srbije; Šumarski fakultet: Beograd, Srbija, 1992. [Google Scholar]
- Tomić, Z. Šumarska fitocenologija; Šumarski fakultet: Beograd, Srbija, 2004. [Google Scholar]
- European Commission. Dg Environment Interpretation Manual of European Union Habitats—EUR28 2013. Available online: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (accessed on 21 March 2022).
- Tichý, L. JUICE; software for vegetation classification. J. Veg. Sci. 2002, 3, 451–453. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, Z. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- Reinds, G.J.; Mol-Dijkstra, J.; Bonten, L.; Wamelink, W.; de Vries, W.; Posch, M. VSD+PROPS: Recent developments. In Modelling and Mapping the Impacts of Atmospheric Deposition on Plant Species Diversity in Europe. CCE Status Report; Slootweg, J., Posch, M., Hettelingh, J.P., Mathijssen, L., Eds.; RIVM: Bilthoven, The Netherlands, 2014; pp. 47–53. Available online: http://hdl.handle.net/10029/557117 (accessed on 21 March 2022).
- Reinds, G.J.; Mol-Dijkstra, J.; Bonten, L.; Wamelink, W.; Hennekens, S.; Goedhart, P.; Posch, M. Probability of plant species (PROPS) model: Latest developments. In Modelling and Mapping the Impacts of Atmospheric Deposition of Nitrogen and Sulphur. CCE Status Report 2015; Slootweg, J., Posch, M., Hettelingh, J.-P., Eds.; National Institute for Public Health and the Environment; RIVM Report 2015-0193; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2015; pp. 55–62. Available online: https://www.umweltbundesamt.de/en/cce-status-reports?parent=68093 (accessed on 21 March 2022).
- Dirnböck, T.; Djukic, I.; Kitzler, B.; Kobler, J.; Mol-Dijkstra, J.P.; Posch, M.; Reinds, G.J.; Schlutow, A.; Starlinger, F.; Wamelink, W.G.W. Climate and air pollution impacts on habitat suitability of Austrian forest ecosystems. PLoS ONE 2017, 12, 0184194. [Google Scholar] [CrossRef] [Green Version]
- Bonten, L.T.C.; Reinds, G.J.; Posch, M. A model to calculate effects of atmospheric deposition on soil acidification; eutrophication and carbon sequestration. Environ. Model Softw. 2016, 79, 75–84. [Google Scholar] [CrossRef]
- Rowe, E.C.; Ford, A.E.S.; Smart, S.M.; Henrys, P.A.; Ashmore, M.R. Using qualitative and quantitative methods to choose a habitat quality metric for air pollution policy evaluation. PLoS ONE 2016, 11, e0161085. [Google Scholar] [CrossRef] [Green Version]
- Češka, A. Estimation of the mean floristic similarity between and within sets of vegetational relevés. Folia Geobot. Phytotaxon. 1966, 1, 93–100. [Google Scholar] [CrossRef]
- Posch, M.; Hettelingh, J.P.; Slootweg, J.; Reinds, G.J. Deriving critical loads based on plant diversity targets. In Modelling and Mapping the Impacts of Atmospheric Deposition on Plant Species Diversity in Europe: CCE Status Report 2014; Slootweg, J., Posch, M., Hettelingh, J.P., Mathijssen, L., Eds.; Report 2014–0075; RIVM: Bilthoven, The Netherlands, 2014; pp. 41–46. [Google Scholar]
- Djurdjevic, V.; Rajkovic, B. Verification of a coupled atmosphere-ocean model using satellite observations over the Adriatic Sea. Ann. Geophys. 2008, 26, 1935–1954. [Google Scholar] [CrossRef] [Green Version]
- Kržič, A.; Tošić, I.; Djurdjević, V.; Veljović, K.; Rajković, B. Changes in some indices over Serbia according to the SRES A1B and A2 scenarios. Clim. Res. 2011, 49, 73–86. [Google Scholar] [CrossRef]
- Nakićenović, N.; Davidson, D.G.; Grübler, A.; Kram, T.; La Rovere, L.E.; Bert, M.; Tsuneyuki, M.; Peper, W.; Hugh, P.; Sankovski, A.; et al. Special Report on Emissions Scenarios. A Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2000; Available online: http://ipcc.ch/pdf/special-reports/spm/sres-en.pdf (accessed on 21 March 2022).
- Ruml, M.; Vuković, A.; Vujadinović, M.; Djurdjević, V.; Ranković-Vasić, Z.; Atanacković, Z.; Sivčev, B.; Marković, N.; Matijašević, S.; Petrović, N. On the use of regional climate models: Implications of climate change for viticulture in Serbia. Agric. For. Meteorol. 2012, 158–159, 53–62. [Google Scholar] [CrossRef]
- Berrisford, P.; Dee, D.; Poli, P.; Brugge, R.; Fielding, K.; Fuentes, M.; Kållberg, P.; Kobayashi, S.; Uppala, S.; Simmons, A. The ERA-interim Archive Version 2.0. ERA Report Series 1. ECMWF. Shinfield Park. 2011. Available online: https://www.ecmwf.int/en/elibrary/8174-era-interim-archive-version-20 (accessed on 21 March 2022).
- The Norwegian Meteorological Institute, The European Monitoring and Evaluation Programme EMEP. Available online: https://www.emep.int/mscw/mscw_moddata.html (accessed on 21 March 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: https://www.nhm.uio.no/english/research/infrastructure/past/ (accessed on 18 November 2020).
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.-P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant; bryophyte; lichen; and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Mihailović, D.T.; Lalić, B.; Drešković, N.; Mimić, G.; Djurdjević, V.; Jančić, M. Climate change effects on crop yields in Serbia and related shifts of Köppen climate zones under the SRES-A1B and SRES-A2. Int. J. Climatol. 2014, 35, 3320–3334. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems; Springer: Heidelberg, Germany, 2003. [Google Scholar]
- Bech Bruun, T.; de Neergaard, A.; Lawrence, D.; Ziegler, A. Environmental consequences of the Demise in Swidden cultivation in Southeast Asia: Carbon storage and soil quality. Hum. Ecol. 2006, 37, 375–388. [Google Scholar] [CrossRef]
- IPCC. Climate Change Synthesis Report. Contribution of Working Groups I; II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; 104p, Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ar4_syr_full_report.pdf (accessed on 21 March 2022).
- IPCC. Climate Change Synthesis Report. Contribution of Working Groups I; II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Core Writing Team; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p, Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/SYR_AR5_FINAL_full.pdf (accessed on 21 March 2022).
- Theurillat, J.P.; Guisan, A. Potential impact of climate change on vegetation in the European Alps: A review. Clim. Change 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Bonan, G.B.; Van Cleve, K. Soil temperature; nitrogen mineralization; and carbon source-sink relationships in boreal forests. Can. J. For. Res. 1992, 22, 629–639. [Google Scholar] [CrossRef]
- Melillo, J.M.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global climate change and terrestrial net primary production. Nature 1993, 363, 234–240. [Google Scholar] [CrossRef]
- Houghton, J. Global Warming: The Complete Briefing, 5th ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 409–412. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Loustau, D.; Delzon, S. Temperature response of parameters of a biochemically based model of photosynthesis. I. Seasonal changes in mature maritime pine (Pinus pinaster Ait.). Plant Cell Environ. 2002, 25, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- McMurtrie, R.E.; Medlyn, B.E.; Dewar, R.E. Increased understanding of nutrient immobilization in soil organic matter is critical for predicting the carbon sink strength in forest ecosystem over the next 100 years. Tree Physiol. 2001, 21, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartík, M.; Jančo, M.; Střelcová, K.; Škvareninová, J.; Škvarenina, J.; Mikloš, M.; Vido, J.; Waldhauserová, P.D. Rainfall interception in a disturbed montane spruce (Picea abies) stand in the West Tatra Mountains. Biologia 2016, 71, 1002–1008. [Google Scholar] [CrossRef]
- Šrámek, V.; Hellebrandová, K.N.; Fadrhonsová, V. Interception and soil water relation in Norway spruce stands of different age during the contrasting vegetation seasons of 2017 and 2018. J. For. Sci. 2019, 65, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Mihailović, D.; Drešković, N.; Arsenić, I.; Ćirić, V.; Djurdjević, V.; Mimić, G.; Pap, I.; Balaž, I. Impact of climate change on soil thermal and moisture regimes in Serbia: An analysis with data from regional climate simulations under SRES-A1B. Sci. Total Environ. 2016, 571, 398–409. [Google Scholar] [CrossRef]
- Kazakis, G.; Ghosn, D.; Vogiatzakis, I.N.; Papanastasis, V.P. Vascular plant diversity and climate change in the alpine zone of the Lefka Ori; Crete. Biodivers. Conserv. 2007, 16, 1603–1615. [Google Scholar] [CrossRef]
- Read, D.J. Mycorrhizas in ecosystems. Experientia 1991, 47, 376–391. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe, 4th ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Carré, F.; Jeannée, N.; Casalegno, S.; Lemarchand, O.; Reuter, H.; Montanarella, L. Mapping the CN ratio of the forest litters in Europe-lessons for global digital soil mapping. In Digital Soil Mapping: Bridging Research; Environmental Application and Operation; Boettinger, J.L., Howell, D.W., Moore, A.C., Hartemink, A.E., Kienast-Brown, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 217–226. [Google Scholar]
- Cools, N.; Vesterdal, L.; de Vos, B.; Vanguelova, E.; Hansen, K. Tree species is the major factor explaining C:N ratios in European forest soils. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Abrahamsen, G.; Miller, H.G. Effects of Acidic Deposition on Forest Soil and Vegetation. Philos. Trans. R. Soc. Lond. 1984, 305, 369–382. [Google Scholar]
- Falkengren-Grerup, U. Soil Acidification and Vegetation Changes in deciduous Forest in Southern Sweden. Oecologia 1986, 70, 339–347. [Google Scholar] [CrossRef]
- Thimonier, A.; Dupouey, J.; Timbal, T. Floristic changes in the herb-layer vegetation of a deciduous forest in the Lorrain plain under the influence of atmospheric deposition. For. Ecol. Manag. 1992, 55, 149–167. [Google Scholar] [CrossRef]
- Lameire, S.; Hermy, M.; Honnay, O. Two decades of change in the ground vegetation of a mixed deciduous forest in an agricultural landscape. J. Veg. Sci. 2000, 11, 695–704. [Google Scholar] [CrossRef]
- De Vries, W.; Wamelink, G.W.W.; van Dobben, H.; Kros, J.; Reinds, G.J.; Mol-Dijkstra, J.P.; Smart, S.M.; Evans, C.D.; Rowe, E.C.; Belyazid, S.; et al. Use of dynamic soil-vegetation models to assess impacts of nitrogen deposition on plant species composition: An overview. Ecol. Appl. 2010, 20, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, L.B.; Van Zelm, R.; Hendriks, A.J.; Bobbink, R.; Huijbregts, M.A.J. Global assessment of the effects of terrestrial acidification on plant species richness. Environ. Pollut. 2013, 174, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Rizzetto, S.; Belyazid, S.; Gégout, J.-C.; Nicolas, M.; Alard, D.; Corcket, E.; Gaudio, N.; Sverdrup, H.; Probst, A. Modelling the impact of climate change and atmospheric N deposition on French forests biodiversity. Environ. Pollut. 2016, 213, 1016–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranger, T.; Lorenz, U.; Gregor, H.D. Manual on Methodologies and Criteria for Modelling and Mapping Critical Loads & Levels and Air Pollution Effects, Risks and Trends; The Umweltbundesamt: Berlin, Germany, 2004; p. 266. [Google Scholar]




| Site | Stara Mt. | Zlatar | Golija | Kopaonik |
|---|---|---|---|---|
| Ecoregions | Rodope montane forests | Dinaric montane forests | Balkan mixed forests | Balkan mixed forests |
| Coordinates | 43°17′34.33′ N 22°47′09.76′ E | 43°24′20.04′ N 19°48′36.19′ E | 43°20′6.38′ N 20°17′12.21′ E | 43°17′30′ N 20°48′50′ E |
| Altitude (m) | 1450–1700 | 1220–1400 | 1300–1720 | 1520–1800 |
| Precipitation (mm) * | 606 | 826 | 797 | 770 |
| Temperature (°C) * | 6.84 | 6.00 | 5.38 | 5.86 |
| Soil parent material | Permian red sandstone | Chromic Humic Cambisol/Chert | Phyllite | Granodiorite/Granite |
| Soil type (WRB) | Podzolic Cambisol | Chromic Humic Cambisol/Dystric Cambisol | Podzolic Cambisol/Dystric Cambisol | Podzolic Cambisol Humic Cambisols |
| Protection status | Nature Park | Landscape of exceptional natural beauty | Nature Park | National Park |
| EUNIS habitat type | G3.1E—Southern European P. abies forests | |||
| EU HD type | 9410 Acidophilous Picea forests of the montane to alpine levels (Vaccinio-Piceetea) | |||
| EU HD subtype | 42.24 Southern European Norway spruce forests | |||
| Sites | Variables | HSIdom | P. abies | T | P | N | pH |
|---|---|---|---|---|---|---|---|
| Stara Mt. | HSIdom | / | 0.648 | −0.579 | 0.492 | −0.638 | 0.748 |
| HSItyp | 0.584 | 0.944 | −0.736 | 0.617 | n.s. | n.s. | |
| HSIdia | 0.474 | 0.745 | −0.571 | 0.673 | n.s. | n.s. | |
| P. abies | 0.648 | / | −0.839 | 0.572 | n.s. | 0.46 | |
| Zlatar | HSIdom | / | 0.802 | −0.554 | 0.778 | −0.646 | 0.708 |
| HSItyp | 0.817 | 0.77 | −0.441 | 0.691 | −0.52 | 0.498 | |
| HSIdia | 0.539 | 0.78 | −0.501 | 0.420 | n.s. | n.s. | |
| P. abies | 0.802 | / | −0.85 | 0.592 | −0.467 | 0.608 | |
| Golija | HSIdom | / | 0.679 | n.s. | 0.674 | −0.801 | 0.855 |
| HSItyp | 0.966 | 0.7968 | n.s. | 0.611 | −0.718 | 0.742 | |
| HSIdia | 0.939 | 0.711 | −0.31 | 0.629 | −0.741 | 0.81 | |
| P. abies | 0.679 | / | n.s. | 0.533 | −0.381 | 0.375 | |
| Kopaonik | HSIdom | / | 0.588 | n.s. | 0.66 | −0.825 | 0.862 |
| HSItyp | 0.735 | 0.799 | −0.203 | 0.606 | −0.567 | 0.452 | |
| HSIdia | 0.897 | 0.571 | −0.432 | 0.55 | −0.776 | 0.886 | |
| P. abies | 0.588 | / | −0.612 | 0.703 | −0.499 | 0.484 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obratov-Petković, D.; Beloica, J.; Čavlović, D.; Djurdjević, V.; Belanović Simić, S.; Bjedov, I. Modelling Response of Norway Spruce Forest Vegetation to Projected Climate and Environmental Changes in Central Balkans Using Different Sets of Species. Forests 2022, 13, 666. https://doi.org/10.3390/f13050666
Obratov-Petković D, Beloica J, Čavlović D, Djurdjević V, Belanović Simić S, Bjedov I. Modelling Response of Norway Spruce Forest Vegetation to Projected Climate and Environmental Changes in Central Balkans Using Different Sets of Species. Forests. 2022; 13(5):666. https://doi.org/10.3390/f13050666
Chicago/Turabian StyleObratov-Petković, Dragica, Jelena Beloica, Dragana Čavlović, Vladimir Djurdjević, Snežana Belanović Simić, and Ivana Bjedov. 2022. "Modelling Response of Norway Spruce Forest Vegetation to Projected Climate and Environmental Changes in Central Balkans Using Different Sets of Species" Forests 13, no. 5: 666. https://doi.org/10.3390/f13050666