Changes in Understory Composition of Rural North American Temperate Forests after a 14-Year Period with Focus on Exotic and Sensitive Plant Species
Abstract
1. Introduction
2. Materials and Methods
3. Results
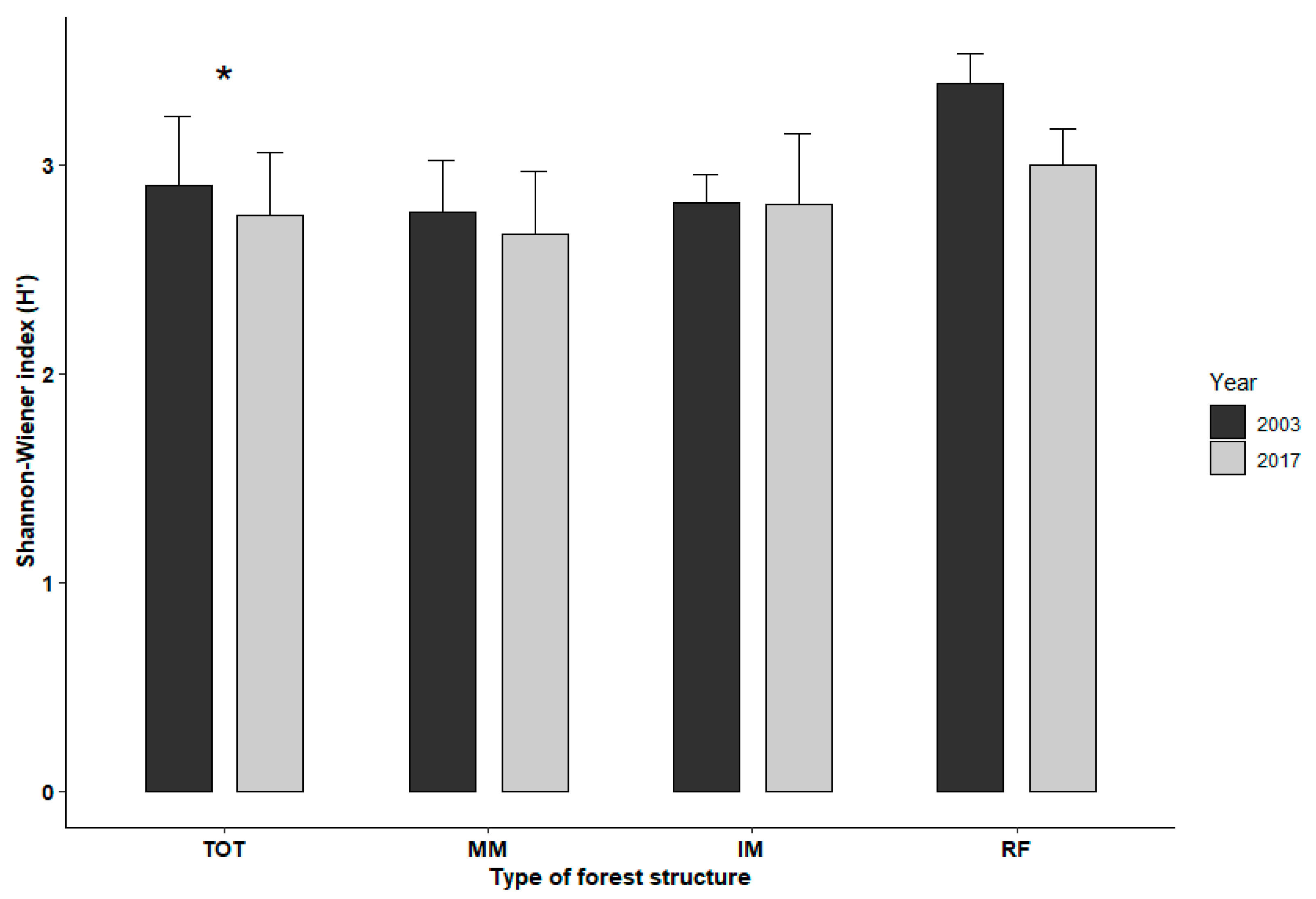
3.1. Changes in Community Diversity and Composition after 14 Years (Q1)
3.2. Identification of Biotic and Anthropogenic Filters Influencing Changes in Species Occurrence (Q2)
3.3. Detecting Occurrence Changes for Shade Tolerant Exotic Species (Q3) and for Sensitive Spring Geophytes (Q4) after 14 Years
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; Proesmans, W.; Ma, S.; Proesmans, W.; Blondeel, H.; et al. The Functional Role of Temperate Forest Understorey Vegetation in a Changing World. Glob. Chang. Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Bierzychudek, P. Life Histories and Demography of Shade-Tolerant Temperate Forest Herbs: A Review. New Phytol. 1982, 90, 757–776. [Google Scholar] [CrossRef]
- Antos, J.A. Understory Plants in Temperate Forests. In Forests and Forest Plants Volume 1; Owens, J.N., Lund, H.G., Eds.; EOLSS Publications: Paris, France, 2009; pp. 262–279. [Google Scholar]
- Fischer, A.; Marshall, P.; Camp, A. Disturbances in Deciduous Temperate Forest Ecosystems of the Northern Hemisphere: Their Effects on Both Recent and Future Forest Development. Biodivers. Conserv. 2013, 22, 1863–1893. [Google Scholar] [CrossRef]
- Chun, J.; Ali, A.; Lee, C. Topography and Forest Diversity Facets Regulate Overstory and Understory Aboveground Biomass in a Temperate Forest of South Korea. Sci. Total Environ. 2020, 744, 140783. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through Reassembly: Plant Traits and Invasion Resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Debille, S.; Lortie, C.J. Biotic Resistance and Island Susceptibility Hypotheses. In Invasion Biology: Hypotheses and Evidence; Jeschke, J.M., Heger, T., Eds.; CABI: Wallingford, UK, 2018; pp. 60–70. [Google Scholar]
- Yu, M.; Sun, O.J. Effects of Forest Patch Type and Site on Herb-Layer Vegetation in a Temperate Forest Ecosystem. For. Ecol. Manag. 2013, 300, 14–20. [Google Scholar] [CrossRef]
- Collins, B.S.; Dunne, K.P.; Pickett, S.T. Responses of Forest Herbs to Canopy Gaps. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T., White, P.S., Eds.; Academic Press: Fredericton, NB, Canada, 1985; pp. 217–234. [Google Scholar]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of Tree Species on Understory Vegetation Diversity and Mechanisms Involved—A Critical Review for Temperate and Boreal Forests. For. Ecol. Manag. 2008, 254, 38. [Google Scholar] [CrossRef]
- Lenière, A.; Houle, G. Response of Herbaceous Plant Diversity to Reduced Structural Diversity in Maple-Dominated (Acer Saccharum marsh) Forests Managed for Sap Extraction. For. Ecol. Manag. 2006, 231, 94–104. [Google Scholar] [CrossRef]
- Meier, A.J.; Bratton, S.P.; Duffy, D.C. Possible Ecological Mechanisms for Loss of Vernal-Herb Diversity in Logged Eastern Deciduous Forests. Ecol. Appl. 1995, 5, 935–946. [Google Scholar] [CrossRef]
- de Blois, S.; Domon, G.; Bouchard, A. Factors Affecting Plant Species Distribution in Hedgerows of Southern Quebec. Biol. Conserv. 2002, 105, 355–367. [Google Scholar] [CrossRef]
- Bellemare, J.; Motzkin, G.; Foster, D.R. Legacies of the Agricultural Past in the Forested Present: An Assessment of Historical Land-Use Effects on Rich Mesic Forests. J. Biogeogr. 2002, 29, 1401–1420. [Google Scholar] [CrossRef]
- Aubin, I.; Gachet, S.; Messier, C.; Bouchard, A. How Resilient Are Northern Hardwood Forests to Human Disturbance ? An Evaluation Using a Plant Functional Group Approach. Ecoscience 2007, 14, 259–271. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the Formation of Dense Understory Layers in Forests Worldwide: Consequences and Implications for Forest Dynamics, Biodiversity, and Succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Graae, B.J.; Sunde, P.B. The Impact of Forest Continuity and Management on Forest Floor Vegetation Evaluated by Species Traits. Ecography 2000, 23, 720–731. [Google Scholar] [CrossRef]
- Mosher, E.S.; Silander, J.A.; Latimer, A.M. The Role of Land-Use History in Major Invasions by Woody Plant Species in the Northeastern North American Landscape. Biol. Invasions 2009, 11, 2317–2328. [Google Scholar] [CrossRef]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why Forests Appear Resistant to Exotic Plant Invasions: Intentional Introductions, Stand Dynamics, and the Role of Shade Tolerance. Front. Ecol. Environ. 2009, 7, 142–149. [Google Scholar] [CrossRef]
- Loewenstein, N.J.; Loewenstein, E.F. Non-Native Plants in the Understory of Riparian Forests across a Land Use Gradient in the Southeast. Urban Ecosyst. 2005, 8, 79–91. [Google Scholar] [CrossRef]
- Whitfeld, T.J.S.; Lodge, A.G.; Roth, M.; Reich, P.B. Community Phylogenetic Diversity and Abiotic Site Characteristics Influence Abundance of the Invasive Plant Rhamnus cathartica L. J. Plant Ecol. 2014, 7, 202–209. [Google Scholar] [CrossRef]
- Kurylo, A.J.S.; Knight, K.S.; Stewart, J.R.; Endress, A.G.; The, S.; Society, B. Rhamnus cathartica: Native and Naturalized Distribution and Habitat Preferences. J. Torrey Bot. Soc. 2007, 134, 420–430. [Google Scholar] [CrossRef]
- Prati, D.; Bossdorf, O. Allelopathic Inhibition of Germination by Alliaria petiolata (Brassicaceae). Am. J. Bot. 2004, 91, 285–288. [Google Scholar] [CrossRef]
- Burke, D.J. Effects of Alliaria petiolata (Garlic Mustard; Brassicaceae) on Mycorrhizal Colonization and Community Structure in Three Herbaceous Plants in a Mixed Deciduous Forest. Am. J. Bot. 2008, 95, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.; Sanchez-ortiz, K.; Martin, P.A.; Chadwick, A.; Gilbert, G.; Bates, A.E.; Luca, B.; Contu, S.; Hill, S.L.L.; Purvis, A. Challenges with Inferring How Land-Use Affects Terrestrial Biodiversity: Study, Design, Time, Space and Synthesis. Adv. Ecol. Res. 2018, 58, 163–199. [Google Scholar] [CrossRef]
- Rolo, V.; Olivier, P.I.; Guldemond, R.A.R.; Van Aarde, R.J. Validating Space-for-Time Substitution in a New-Growth Coastal Dune Forest. Appl. Veg. Sci. 2016, 19, 235–243. [Google Scholar] [CrossRef]
- Damgaard, C. A Critique of the Space-for-Time Substitution Practice in Community Ecology. Trends Ecol. Evol. 2019, 34, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Nantel, P.; Gagnon, D.; Nault, A. Population Viability Analysis of American Ginseng and Wild Leek Harvested in Stochastic Environments. Conserv. Biol. 1996, 10, 608–621. [Google Scholar] [CrossRef]
- Benjamin, K.; Domon, G.; Bouchard, A. Vegetation Composition and Succession of Abandoned Farmland: Effects of Ecological, Historical and Spatial Factors. Landsc. Ecol. 2005, 20, 627–647. [Google Scholar] [CrossRef]
- Robitaille, A.; Saucier, J.-P. Paysages Régionaux Du Québec Méridional; Les publications du Québec: Sainte-Foy, France, 1998. [Google Scholar]
- Brisson, J.; Bouchard, A. In the Past Two Centuries, Human Activities Have Caused Major Changes in the Tree Species Composition of Southern Québec, Canada. Ecoscience 2003, 10, 236–246. [Google Scholar] [CrossRef]
- Meilleur, A.; Bouchard, A.; Bergeron, Y. The Relation between Geomorphology and Forest Community Types of the Haut-Saint-Laurent, Quebec. Vegetatio 1994, 111, 173–192. [Google Scholar] [CrossRef]
- de Blois, S.; Boisvert-Marsh, L.; Schmucki, R.; Lovat, C.-A.; Byun, C.; Gomez-garcia, P.; Otfinowski, R.; Groeneveld, E.; Lavoie, C. Outils Pour Évaluer Les Risques D’invasion Biologique Dans un Contexte de Changements Climatiques; Université McGill: Montréal, QC, Canada, 2013. [Google Scholar]
- Lavoie, C.; Saint-Louis, A.; Guay, G.; Groeneveld, E. Les Plantes Vasculaires Exotiques Naturalisées: Une Nouvelle Liste Pour Le Québec. Le Nat. Can. 2012, 136, 6–32. [Google Scholar] [CrossRef]
- CFIA. Invasive Alien Plants in Canada; CFIA: Ottawa, ON, Canada, 2008. [Google Scholar]
- Haber, E. Impact of Invasive Plants on Species and Habitats at Risk in Canada; National Botanical Services: Ottawa, ON, Canada, 2000. [Google Scholar]
- Marie-Victorin, É.C. Flore Laurentienne, 3rd ed.; Morin, G., Ed.; Gaëtan Morin Éditeur ltée: Montréal, QC, Canada, 2002. [Google Scholar]
- Cleophas, T.J.; Zwinderman, A.H. Paired Continuous Data (Paired T-Test, Wilcoxon Signed Rank Test). In Clinical Data Analysis on a Pocket Calculator; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Lisboa, F.J.G.; Peres-Neto, P.R.; Chaer, G.M.; Jesus, E.d.C.; Mitchell, R.J.; Chapman, S.J.; Luis, R.; Berbara, L. Much beyond Mantel: Bringing Procrustes Association Metric to the Plant and Soil Ecologist’s Toolbox. PLoS ONE 2014, 9, e101238. [Google Scholar] [CrossRef]
- Uyanik, G.K.; Güler, N. A Study on Multiple Linear Regression Analysis. Procedia Soc. Behav. Sci. 2013, 106, 234–240. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Moola, F.M.; Vasseur, L. The Maintenance of Understory Residual Flora with Even-Aged Forest Management: A Review of Temperate Forests in Northeastern North America. Environ. Rev. 2008, 16, 141–155. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Woods, K.D.; Hicks, D.J.; Schultz, J. Losses in Understory Diversity over Three Decades in an Old-Growth Cool-Temperate Forest in Michigan, USA. Can. J. For. Res. 2012, 549, 532–549. [Google Scholar] [CrossRef]
- Rooney, T.P.; Wiegmann, S.M.; Rogers, D.A.; Waller, D.M. Biotic Impoverishment and Homogenization in Unfragmented Forest Understory Communities. Conserv. Biol. 2004, 18, 787–798. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and Process in the Plant Community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef]
- Holmes, M.A.; Matlack, G.R. Agricultural History Drives Structure and Tree-Species Composition of Second Growth Forest over 100 Years in Southeastern Ohio, USA. J. Veg. Sci. 2017, 28, 736–746. [Google Scholar] [CrossRef]
- Wesolowski, T.; Rowinski, P.; Maziarz, M. Interannual Variation in Tree Seed Production in a Primeval Temperate Forest: Does Masting Prevail ? Eur. J. For. Res. 2014, 134, 99–112. [Google Scholar] [CrossRef]
- Reddoch, J.M.; Reddoch, A.H. The Impact of Deer Herbivory and Drought on Population Growth of Goodyera pubescens (Orchidaceae) in Southwestern Quebec. Can. Field Nat. 2012, 126, 242–244. [Google Scholar] [CrossRef][Green Version]
- White, M.A. Long-Term Effects of Deer Browsing: Composition, Structure and Productivity in a Northeastern Minnesota Old-Growth Forest. For. Ecol. Manag. 2012, 269, 222–228. [Google Scholar] [CrossRef]
- Sonnier, G.; Johnson, S.E.; Waller, D.M. Fragmentation Reduces the Importance of Niche-based Factors Relative to Dispersal Traits in Structuring Temperate Forest Understories. J. Veg. Sci. 2020, 31, 75–83. [Google Scholar] [CrossRef]
- Kumordzi, B.B.; Aubin, I.; Cardou, F.; Shipley, B.; Violle, C.; Johnstone, J.; Anand, M.; Arsenault, A.; Wayne Bell, F.; Bergeron, Y.; et al. Geographic Scale and Disturbance Influence Intraspecific Trait Variability in Leaves and Roots of North American Understorey Plants. Funct. Ecol. 2019, 33, 1771–1784. [Google Scholar] [CrossRef]
- Wiegmann, S.M.; Waller, D.M. Fifty Years of Change in Northern Upland Forest Understories: Identity and Traits of “winner” and “loser” Plant Species. Biol. Conserv. 2005, 129, 109–123. [Google Scholar] [CrossRef]
- Vellend, M.; Verheyen, K.; Flinn, K.M.; Jacquemyn, H.; Kolb, A.; Calster, H.V.A.N.; Peterken, G.; Graae, B.J.; Bellemare, J.; Honnay, O.; et al. Homogenization of Forest Plant Communities and Weakening of Species—Environment Relationships via Agricultural Land Use. J. Ecol. 2007, 95, 565–573. [Google Scholar] [CrossRef]
- Webster, C.R.; Jenkins, M.A. Age Structure and Spatial Patterning of Trillium Populations in Old-Growth Forests. Plant Ecol. 2008, 199, 43–54. [Google Scholar] [CrossRef]
- Stokes, R.L.; Philpott, M.; Culley, T.M. Clonality and Genetic Diversity in the Eastern North American Spring Ephemeral Erythronium americanum Ker-Gawl. (American trout Lily). J. Torrey Bot. Soc. 2019, 146, 143–154. [Google Scholar] [CrossRef]
- Ruhren, S.; Dudash, M.R. Consequences of the Timing of Seed Release of Erythronium americanum (Liliaceae), a Deciduous Forest Myrmecochore. Am. J. Bot. 1996, 83, 633–640. [Google Scholar] [CrossRef]
- Handel, S.N.; Fisch, S.B.; Schatz, G.E. Ants Disperse a Majority of Herbs in a Mesic Forest Community in New York State. Bull. Torrey Bot. Club 1981, 108, 430–437. [Google Scholar] [CrossRef]
- Hermy, M.; Verheyen, K. Legacies of the Past in the Present-Day Forest Biodiversity: A Review of Past Land-Use Effects on Forest Plant Species Composition and Diversity. In Sustainability and Diversity of Forest Ecosystems; Nakashizuka, T., Ed.; Springer: Tokyo, Japan, 2007; pp. 361–371. [Google Scholar] [CrossRef]
- Schmucki, R.; de Blois, S. Population Structures and Individual Performances of Trillium Grandiflorum in Hedgerow and Forest Habitats. Plant Ecol. 2008, 202, 67–78. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A Meta-Analysis of Biotic Resistance to Exotic Plant Invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Pan, D.; Domon, G.; de Blois, S.; Bouchard, A. Temporal (1958–1993) and Spatial Patterns of Land Use Changes in Haut-Saint-Laurent (Quebec, Canada) and Their Relation to Landscape Physical Attributes. Landsc. Ecol. 1999, 14, 35–52. [Google Scholar] [CrossRef]
- Charles-Dominique, T.; Edelin, C.; Brisson, J.; Bouchard, A. Architectural Strategies of Rhamnus cathartica (Rhamnaceae) in Relation to Canopy Openness. Botany 2012, 90, 976–989. [Google Scholar] [CrossRef]
- Chapman, J.I.; Myers, A.L.; Burky, A.J.; Mcewan, R.W. Edge Effects, Invasion, and the Spatial Pattern of Herb-Layer Biodiversity in an Old-Growth Deciduous Forest Fragment. Nat. Areas J. 2015, 35, 439–451. [Google Scholar] [CrossRef]
- Knight, T.M.; Dunn, J.L.; Smith, L.A.; Davis, J.; Kalisz, S. Deer Facilitate Invasive Plant Success in a Pennsylvania Forest Understory. Nat. Areas J. 2009, 29, 110–116. [Google Scholar] [CrossRef]
| Forest Structure | N | Stand Age in 2017 | Characteristics | Basal Area (m2 ha−1) | Stem Density (N ha−1) |
|---|---|---|---|---|---|
| Mature maple (MM) | 13 | >70 years old | Mature canopy dominated by maple trees | 24.6 | 600 |
| Immature maple (IM) | 3 | 35–50 years old | Closed canopydominated by young maple trees | 21.5 | 550 |
| Reforested (RF) | 4 | 25–45 years old | Closed canopy originating from abandoned pasture | 18.8 | 1018 |
| Variable Name | Units | Description | Filter |
|---|---|---|---|
| Biotic variables | |||
| H′03/H′17 | - | Shannon–Wiener index for species richness of the entire understory plant community, calculated separately for the 2003 (H′03) and the 2017 (H′17) inventories | Understory structure and diversity |
| Cover | % | Relative proportion of sampling points (52 in total) with at least one occurring species | Understory structure and diversity |
| BArea | m2/ha | Basal area per hectare of late successional tree species with DBH > 5 cm | Canopy structure and composition |
| Open | % | Relative proportion of canopy openness based on 52 sampling points (open—1 point; semi-open—0.5 point; closed—0 point) | Canopy structure |
| Anthropogenic variables | |||
| Maple | Binary | Maple syrup production: 0—Never recorded since 1964, 1—Recorded at least once in 2003 or still ongoing | Disturbance— minor intensity |
| UndCut | Binary | Recent clearing of undergrowth vegetation: 0—Never recorded since 2003, 1—Recorded at least once in 2003 or 2017 | Disturbance— minor intensity |
| ParCut | Binary | Partial wood harvesting: 0—Never recorded since 1964, 1—Recorded at least once in 2003 or 2017 | Disturbance— major intensity |
| Graze | Ordinal | Pastureland grazing: 0—Never recorded since 1964, 1—Recorded at least once between 1964 and 1975, 2—Recorded at least once between 1975 and 2003 but not in 2017 | Disturbance— major intensity |
| Age | Years | Time since last anthropogenic disturbance | Disturbance frequency |
| Anth | % | Relative proportion of surrounding lands with anthropogenic activities evaluated with regional satellite maps in 2017 | Fragmentation and habitat loss |
| Species | 2003 | 2017 | Procrustes Residuals | ||||
|---|---|---|---|---|---|---|---|
| %TOT | %AVG | SD | %TOT | %AVG | SD | ||
| A. Occurrence gains (24 species) | |||||||
| Circaea canadensis | 2.2 | 2.44 | 2.59 | 3.6 | 3.42 | 5.41 | 54.71 |
| Erythronium americanum 1 | 6.6 | 6.18 | 7.38 | 13.9 | 14.3 | 12.1 | 51.89 |
| Ostrya virginiana | 5.9 | 5.84 | 5.51 | 6.4 | 5.89 | 6.46 | 48.80 |
| Carya cordiformis | 1.7 | 1.81 | 1.70 | 5.1 | 5.79 | 5.22 | 47.62 |
| Onoclea sensibilis | 0.9 | 0.51 | 1.63 | 1.7 | 0.86 | 3.21 | 32.00 |
| Zanthoxylum americanum | 1.5 | 1.41 | 2.61 | 1.8 | 1.66 | 2.56 | 31.03 |
| Trillium grandiflorum 1 | 4.3 | 4.19 | 6.03 | 5.0 | 4.56 | 6.47 | 27.66 |
| Cornus alternifolia | 1.7 | 1.94 | 3.28 | 2.9 | 2.91 | 3.97 | 27.02 |
| Dryopteris carthusiana | 0.9 | 0.95 | 2.18 | 1.6 | 1.80 | 2.63 | 26.03 |
| Rubus occidentalis | 0.4 | 0.39 | 0.91 | 0.9 | 0.79 | 1.75 | 23.03 |
| Maianthemum canadense | 0.8 | 0.74 | 1.12 | 1.4 | 1.42 | 2.09 | 22.84 |
| Poaceae spp. | 0.9 | 0.75 | 1.18 | 1.1 | 0.98 | 1.59 | 20.78 |
| Caulophyllum thalictroides | 2.1 | 2.49 | 4.38 | 2.2 | 3.21 | 7.47 | 19.16 |
| Thuja occidentalis | 0.6 | 0.52 | 1.10 | 0.7 | 0.55 | 1.43 | 19.07 |
| Prunus virginiana | 1.4 | 1.47 | 1.31 | 2.0 | 1.97 | 2.83 | 18.46 |
| Rubus allegheniensis | 0.7 | 0.66 | 1.09 | 0.9 | 0.69 | 1.11 | 18.42 |
| Polystichum acrostichoides | 0.5 | 0.42 | 1.18 | 0.6 | 0.51 | 0.78 | 17.10 |
| Ulmus rubra | 0.8 | 0.81 | 2.39 | 1.1 | 1.06 | 2.71 | 16.92 |
| Populus tremuloides | 0.2 | 0.13 | 0.49 | 0.3 | 0.16 | 0.48 | 16.39 |
| Parthenocissus quinquefolia | 0.8 | 0.69 | 1.23 | 1.0 | 0.82 | 1.17 | 16.32 |
| Rubus pubescens | 0.6 | 0.33 | 1.44 | 0.6 | 0.34 | 1.14 | 15.04 |
| Prunus serotina | 0.7 | 0.75 | 0.68 | 0.8 | 0.77 | 1.29 | 14.21 |
| Cardamine concatenata 1 | 0.06 | 0.08 | 0.23 | 0.4 | 0.52 | 1.72 | 13.04 |
| Athyrium filix-femina | 0.7 | 0.68 | 0.98 | 0.6 | 0.73 | 1.39 | 12.24 |
| B. Occurrence losses (21 species) | |||||||
| Acer saccharum | 17.7 | 20.2 | 10.6 | 6.7 | 8.53 | 7.63 | 194.03 |
| Claytonia caroliniana 1 | 2.1 | 1.67 | 4.95 | 0.7 | 0.87 | 2.35 | 83.03 |
| Fagus grandifolia | 1.6 | 1.42 | 3.24 | 0.3 | 0.40 | 0.51 | 60.97 |
| Fraxinus americana | 7.4 | 7.48 | 6.38 | 7.8 | 6.96 | 6.52 | 42.19 |
| Carpinus caroliniana | 1.1 | 1.21 | 4.25 | 0.3 | 0.35 | 1.44 | 42.14 |
| Acer rubrum | 0.8 | 0.66 | 2.64 | 0.3 | 0.25 | 0.88 | 34.43 |
| Tilia americana | 1.6 | 1.65 | 1.96 | 0.5 | 0.55 | 0.61 | 26.15 |
| Ulmus americana | 1.3 | 1.18 | 1.32 | 0.1 | 0.10 | 0.24 | 25.67 |
| Rubus hispidus | 0.5 | 0.39 | 1.73 | 0.1 | 0.14 | 0.34 | 25.11 |
| Carex spp. | 3.4 | 3.08 | 2.84 | 3.6 | 2.94 | 2.70 | 23.06 |
| Rubus idaeus | 0.7 | 0.64 | 1.63 | 0.1 | 0.14 | 0.45 | 22.87 |
| Equisetum pratense | 0.4 | 0.37 | 1.61 | 0.2 | 0.16 | 0.60 | 20.30 |
| Cornus obliqua | 1.3 | 0.75 | 2.88 | 1.4 | 0.64 | 2.72 | 20.03 |
| Potentilla reptans 2 | 0.3 | 0.15 | 0.69 | 0.02 | 0.01 | 0.04 | 17.73 |
| Galium palustre | 0.3 | 0.17 | 0.75 | 0.07 | 0.03 | 0.15 | 17.12 |
| Acer nigrum | 0.7 | 0.64 | 1.63 | 0.1 | 0.14 | 0.45 | 16.94 |
| Hepatica acutiloba 1 | 0.7 | 0.79 | 1.56 | 0.7 | 0.57 | 0.93 | 16.23 |
| Symphyotrichum lateriflorum var. lateriflorum | 0.9 | 0.87 | 1.15 | 0.5 | 0.40 | 0.67 | 15.02 |
| Vitis riparia | 0.8 | 0.83 | 1.05 | 0.7 | 0.74 | 0.78 | 14.44 |
| Cornus sericea | 0.3 | 0.23 | 0.75 | 0.04 | 0.02 | 0.07 | 13.53 |
| Solidago flexicaulis | 0.5 | 0.57 | 1.03 | 0.4 | 0.32 | 0.52 | 12.20 |
| Model | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| A. Species with the largest occurrence gains | ||||
| Age + H′17 * | 4 | 163.12 | 0.00 | 0.48 |
| Age + BArea + H′17 | 5 | 164.54 | 1.42 | 0.24 |
| Age + Open + H′17 | 5 | 166.30 | 3.19 | 0.10 |
| Age + Anth + H′17 | 5 | 166.31 | 3.19 | 0.10 |
| Age + UndCut + H′17 | 5 | 166.48 | 3.37 | 0.08 |
| B. Species with the largest occurrence losses | ||||
| Null * | 2 | 171.68 | 0.00 | 0.31 |
| Age | 3 | 172.33 | 0.65 | 0.23 |
| Age + ParCut | 4 | 172.54 | 0.86 | 0.20 |
| Maple | 3 | 173.18 | 1.50 | 0.15 |
| Age + UndCut | 4 | 173.86 | 2.18 | 0.11 |
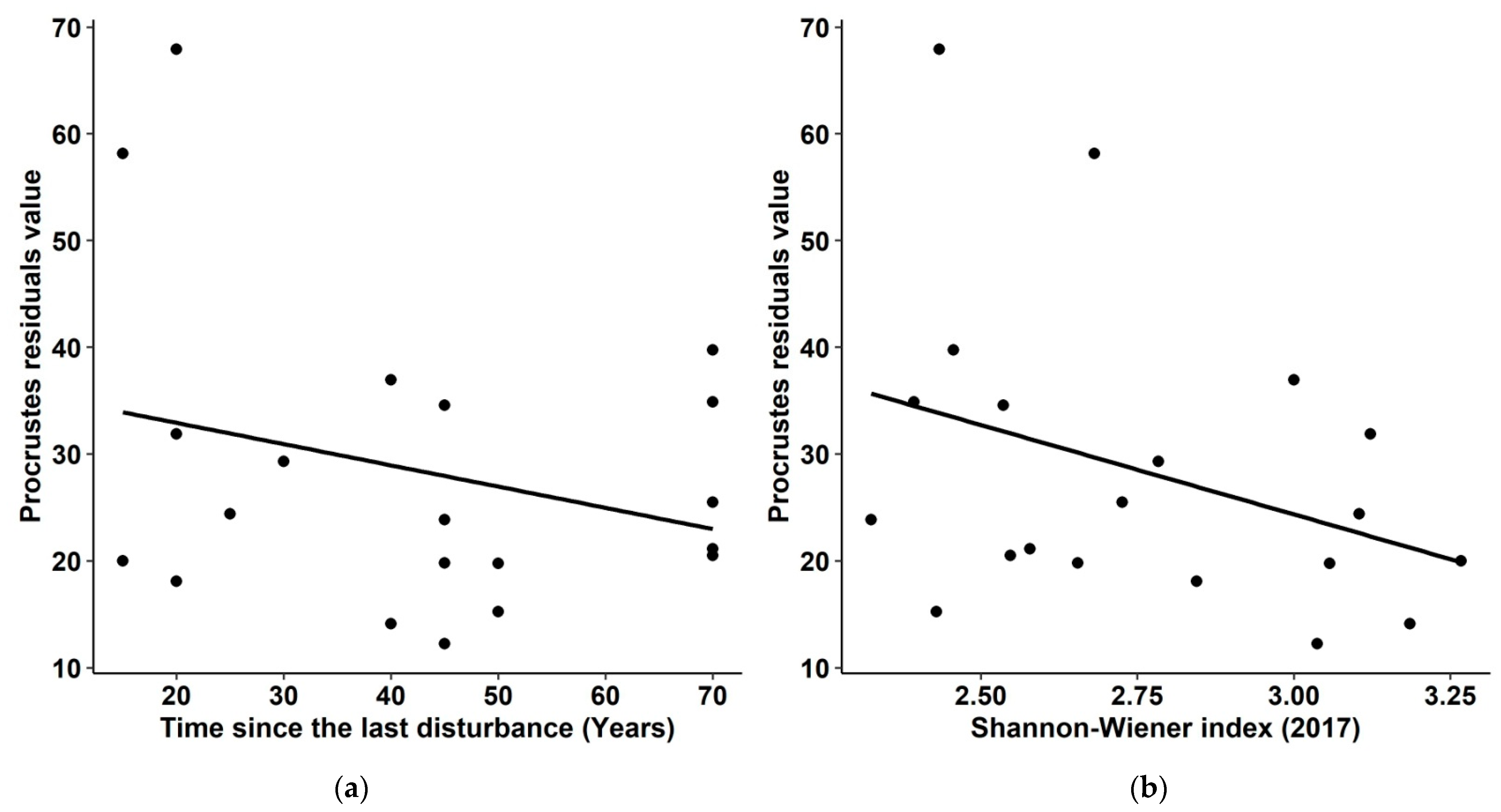
| Variable | R2adj | Estimate (±SE) | t Value (df = 17) | p |
|---|---|---|---|---|
| Model | 0.31 | 0.017 | ||
| Intercept | 129.3 (±32.6) | 3.97 | <0.001 | |
| Age | −0.42 (±0.16) | −2.66 | 0.016 | |
| H′17 | −30.1 (±10.4) | −2.89 | 0.01 |
| Group | 2003 | 2017 | Direction | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NSp | %TOT | %AVG | SD | NSp | %TOT | %AVG | SD | |||
| Shade tolerant exotics | 7 | 1.6 | 1.88 | 3.60 | 10 | 2.4 | 2.80 | 5.46 | Gain | 0.040 |
| Sensitive spring geophytes | 31 | 20 | 19.8 | 13.7 | 30 | 27 | 30.1 | 17.9 | Gain | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellerose, J.; Dupuch, A.; Aubin, I. Changes in Understory Composition of Rural North American Temperate Forests after a 14-Year Period with Focus on Exotic and Sensitive Plant Species. Forests 2022, 13, 678. https://doi.org/10.3390/f13050678
Bellerose J, Dupuch A, Aubin I. Changes in Understory Composition of Rural North American Temperate Forests after a 14-Year Period with Focus on Exotic and Sensitive Plant Species. Forests. 2022; 13(5):678. https://doi.org/10.3390/f13050678
Chicago/Turabian StyleBellerose, Julien, Angélique Dupuch, and Isabelle Aubin. 2022. "Changes in Understory Composition of Rural North American Temperate Forests after a 14-Year Period with Focus on Exotic and Sensitive Plant Species" Forests 13, no. 5: 678. https://doi.org/10.3390/f13050678
APA StyleBellerose, J., Dupuch, A., & Aubin, I. (2022). Changes in Understory Composition of Rural North American Temperate Forests after a 14-Year Period with Focus on Exotic and Sensitive Plant Species. Forests, 13(5), 678. https://doi.org/10.3390/f13050678






