The Genetics and Ecology of Post-Fire Eucalyptus globulus Recruitment in an Isolated Stand in Central Portugal
Abstract
:1. Introduction
2. Materials and Methods
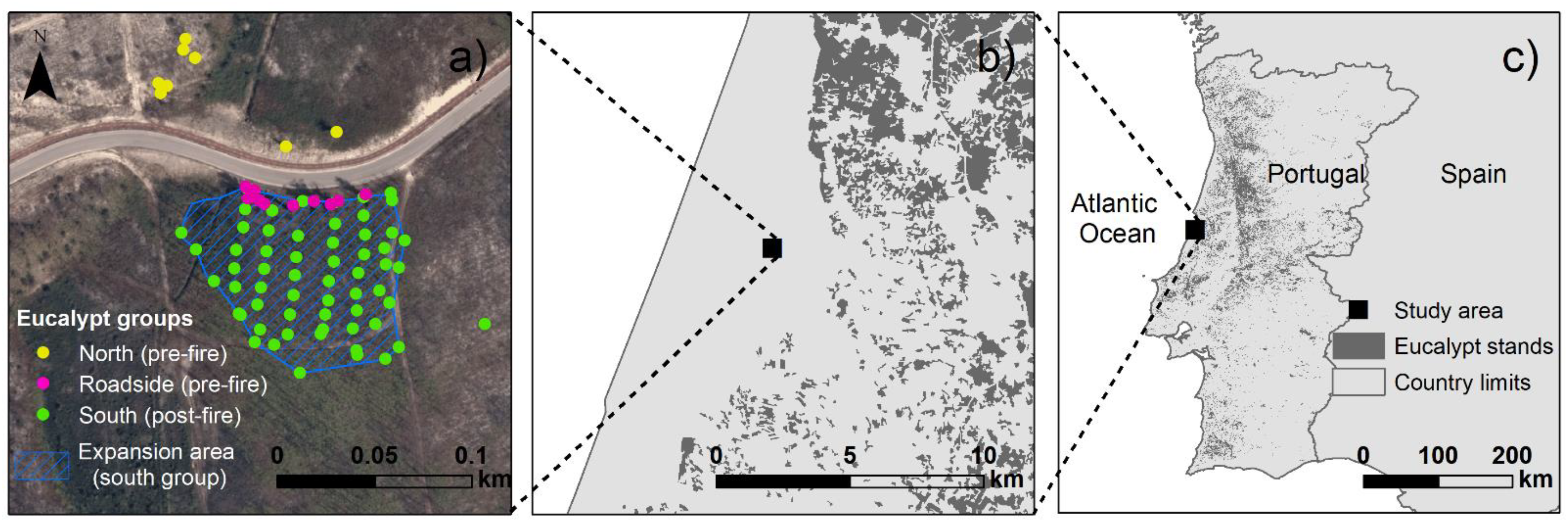
2.1. Study Area
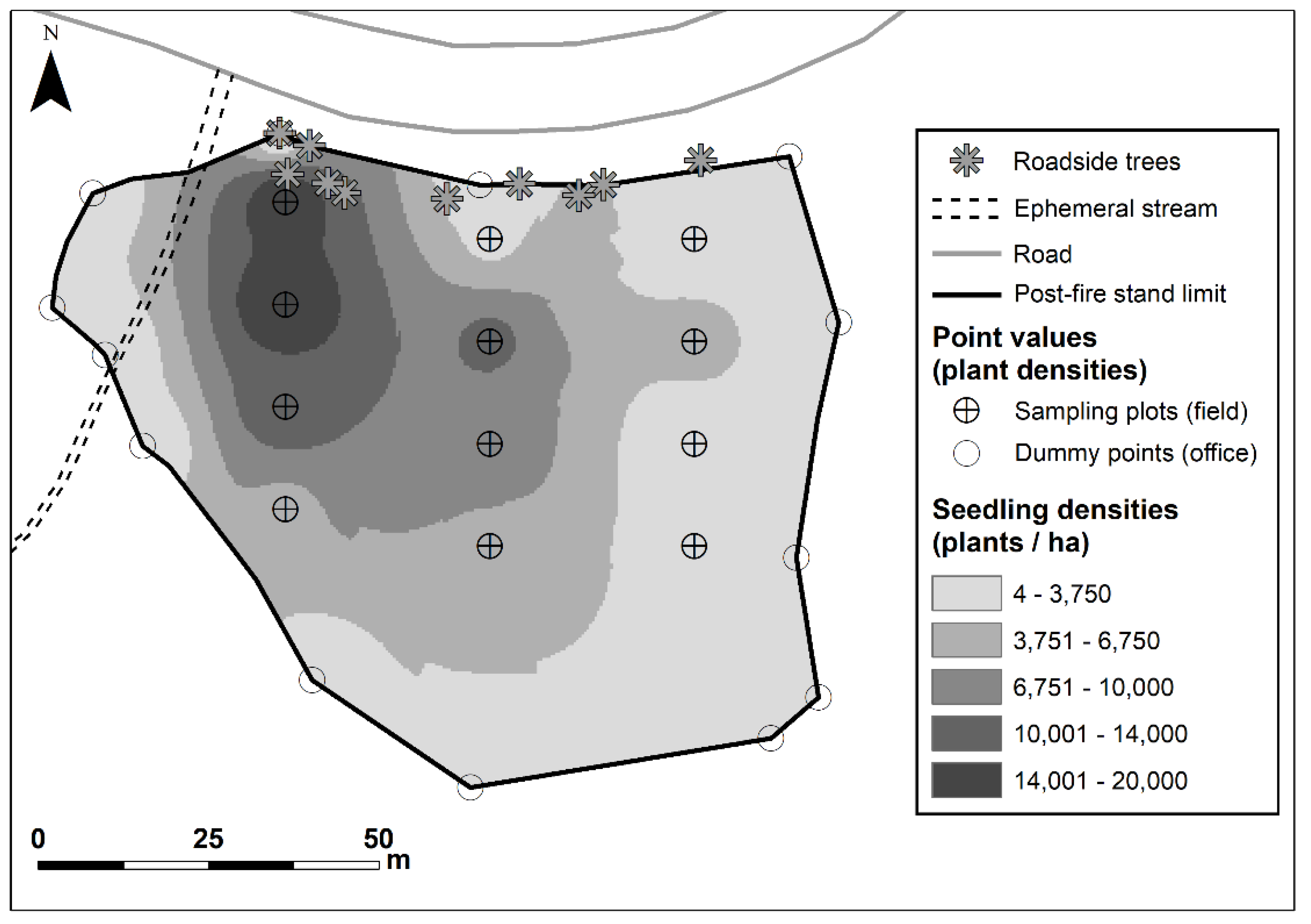
2.2. Sampling and Data Collection
2.3. DNA Extraction and Microsatellite Analysis
2.4. Genetic Analysis
2.4.1. Genetic Diversity
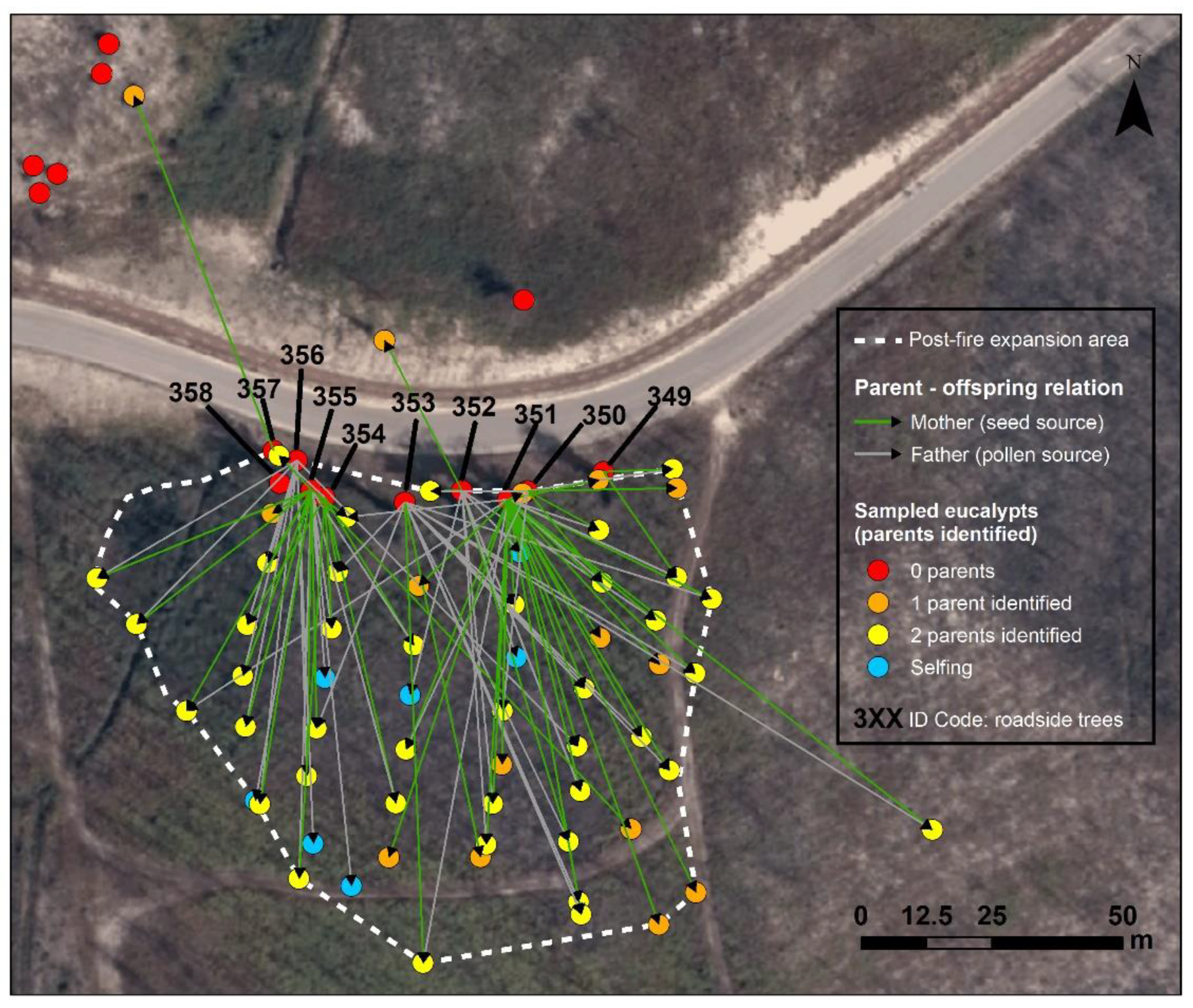
2.4.2. Paternity Assignment
2.5. Analysis of Field Data
3. Results
3.1. Population structure
3.2. Genetic Diversity Analyses
3.3. Parentage Analysis and Genetic Structure
3.4. Seed and Pollen Dispersal
4. Discussion
4.1. Genetic Diversity
4.2. Population Structure and Gene Flow
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, G.; Borralho, N.; Tilyard, P.; Potts, B. Identification of races in Eucalyptus globulus spp globulus based on growth traits in Tasmania and geographic distribution. Silvae Genet. 1994, 43, 292–298. [Google Scholar]
- Potts, B.M.; Vaillancourt, R.E.; Jordan, G.; Dutkowski, G.; Costa, J.; Mckinnon, G.; Steane, D.; Volker, P.; Lopez, G.; Apiolaza, L.; et al. Exploration of the Eucalyptus Globulus Gene Pool. In Proceedings of the Eucalyptus in a Changing World Proc. of IUFRO Conference, Aveiro, Portugal, 11–15 October 2004; pp. 11–15. [Google Scholar]
- Alves, A.M.; Pereira, J.S.; Silva, J.M.N. Introdução e expansão do eucalipto em Portugal. Pinhais E Eucaliptais—A Floresta Cultiv. Colecção Árvores E Florestas Port. 2007, 4, 151–165. [Google Scholar]
- Potts, B.M.; McGowen, M.H.; Williams, D.R.; Suitor, S.; Jones, T.H.; Gore, P.L.; Vaillancourt, R.E. Advances in reproductive biology and seed production systems of Eucalyptus: The case of Eucalyptus globulus. South. For. 2008, 70, 145–154. [Google Scholar] [CrossRef]
- ICNF. 6.0 Inventário Florestal Nacional—Resultados Portugal—NUTS I [Portuguese National Forest Inventory]; Instituto da Conservação da Natureza e das Florestas: Lisbon, Portugal, 2019. [Google Scholar]
- Silva, J.S.; Nereu, M.; Pinho, S.; Queirós, L.; Jesús, C.; Deus, E. Post-fire demography, growth, and control of eucalyptus globulus wildlings. Forests 2021, 12, 156. [Google Scholar] [CrossRef]
- Anjos, A.; Fernandes, P.; Marques, C.; Borralho, N.; Valente, C.; Correia, O.; Máguas, C.; Chozas, S. Management and fire, a critical combination for Eucalyptus globulus dispersal. For. Ecol. Manag. 2021, 490, 91–102. [Google Scholar] [CrossRef]
- Fernandes, P.; Antunes, C.; Pinho, P.; Máguas, C.; Correia, O. Natural regeneration of Pinus pinaster and Eucalyptus globulus from plantation into adjacent natural habitats. For. Ecol. Manag. 2016, 378, 91–102. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D. Eucalypts. In Encyclopedia of Biological Invasions; Simberloff, D.R.M., Rejmánek, M., Richardson, D., Eds.; University of California Press: Los Angeles, CA, USA, 2011; pp. 203–209. [Google Scholar]
- Catry, F.X.; Moreira, F.; Deus, E.; Silva, J.S.; Águas, A. Assessing the Extent and the Environmental Drivers of Eucalyptus Globulus Wildling Establishment in Portugal: Results from a Countrywide Survey. Biol. Invasions 2015, 17, 3163–3181. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Rubido-Bará, M. Invasive potential of Eucalyptus globulus: Seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For. Ecol. Manag. 2013, 305, 129–137. [Google Scholar] [CrossRef]
- dos Santos, P.; Matias, H.; Deus, E.; Águas, A.; Silva, J.S. Fire effects on capsules and encapsulated seeds from Eucalyptus globulus in Portugal. Plant Ecol. 2015, 216, 1611–1621. [Google Scholar] [CrossRef]
- Águas, A.; Ferreira, A.; Maia, P.; Fernandes, P.M.; Roxo, L.; Keizer, J.; Silva, J.S.; Rego, F.C.; Moreira, F. Natural establishment of Eucalyptus globulus Labill. in burnt stands in Portugal. For. Ecol. Manag. 2014, 323, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Calviño-Cancela, M.; Lorenzo, P.; González, L. Fire increases Eucalyptus globulus seedling recruitment in forested habitats: Effects of litter, shade and burnt soil on seedling emergence and survival. For. Ecol. Manag. 2018, 409, 826–834. [Google Scholar] [CrossRef]
- Silva, J.S.; Dos Santos, P.; Sério, A.; Gomes, F. Effects of heat on dehiscence and germination in Eucalyptus globulus Labill. Int. J. Wildl. Fire 2016, 25, 478–483. [Google Scholar] [CrossRef]
- Fernandes, P.; Máguas, C.; Correia, O. Combined effects of climate, habitat, and disturbance on seedling establishment of Pinus pinaster and Eucalyptus globulus. Plant Ecol. 2017, 218, 501–515. [Google Scholar] [CrossRef]
- Selkoe, K.A.; Toonen, R.J. Microsatellites for ecologists: A practical guide to using and evaluating microsatellite markers. Ecol. Lett. 2006, 9, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Pairon, M.; Jonard, M.; Jacquemart, A.L. Modeling seed dispersal of black cherry, an invasive forest tree: How microsatellites may help? Can. J. For. Res. 2006, 36, 1385–1394. [Google Scholar] [CrossRef]
- Dodet, M.; Petit, R.J.; Gasquez, J. Local spread of the invasive Cyperus esculentus (Cyperaceae) inferred using molecular genetic markers. Weed Res. 2008, 48, 19–27. [Google Scholar] [CrossRef]
- Jones, T.H.; Vaillancourt, R.E.; Potts, B.M. Detection and visualization of spatial genetic structure in continuous Eucalyptus globulus forest. Mol. Ecol. 2007, 16, 697–707. [Google Scholar] [CrossRef]
- Freeman, J.S.; Marques, C.M.P.; Carocha, V.; Borralho, N.; Potts, B.M.; Vaillancourt, R.E. Origins and diversity of the Portuguese Landrace of Eucalyptus globulus. Ann. For. Sci. 2007, 64, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.M.; Sanchez, L.; Ribeiro, C.; Cunha, F.; Araújo, J.; Borralho, N.M.G.; Marques, C. A case study of Eucalyptus globulus fingerprinting for breeding. Ann. For. Sci. 2011, 68, 701–744. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.; Vaillancourt, R.E.; Steane, D.A.; Jones, R.C.; Marques, C. Microsatellite analysis of population structure in Eucalyptus globulus. Genome 2017, 60, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.C.; Steane, D.A.; Potts, B.M.; Vaillancourt, R.E. Microsatellite and morphological analysis of Eucalyptus globulus populations. Can. J. For. Res. 2002, 32, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Steane, D.A.; Conod, N.; Jones, R.C.; Vaillancourt, R.E.; Potts, B.M. A comparative analysis of population structure of a forest tree, Eucalyptus globulus (Myrtaceae), using microsatellite markers and quantitative traits. Tree Genet. Genomes 2006, 2, 30–38. [Google Scholar] [CrossRef]
- Jones, M.E.; Shepherd, M.; Henry, R.; Delves, A. Pollen flow in Eucalyptus grandis determined by paternity analysis using microsatellite markers. Tree Genet. Genomes 2008, 4, 37–47. [Google Scholar] [CrossRef]
- Rei, M.A. Ensecamento do Pantano do Juncal Gordo e Causas Que o Determinaram; Typ. La Bécarre de Francisco J. Carneiro: Lisboa, Portugal, 1914. [Google Scholar]
- Doyle, J.; Doyle, J. A rapid DNA isolation for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Steane, D.A.; Vaillancourt, R.E.; Russell, J.; Powell, W.; Marshall, D.; Potts, B.M. Development and characterisation of microsatellite loci in Eucalyptus globulus (Myrtaceae). Silvae Genet. 2001, 50, 89–91. [Google Scholar]
- Brondani, R.P.V.; Brondani, C.; Tarchini, R.; Grattapaglia, D. Development, characterization and mapping of microsatellite markers in Eucalyptus grandis and E. urophylla. Theor. Appl. Genet. 1998, 97, 816–827. [Google Scholar] [CrossRef]
- Brondani, R.; Brondani, C.; Grattapaglia, D. Towards a genus-wide reference linkage map for Eucalyptus based exclusively on highly informative microsatellite markers. Mol. Genet. Genom. 2002, 267, 338–347. [Google Scholar] [CrossRef]
- Byrne, M.; Marquez-Garcia, M.I.; Uren, T.; Smith, D.S.; Moran, G.F. Conservation and genetic diversity of microsatellite loci in the genus Eucalyptus. Aust. J. Bot. 1996, 44, 331–341. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-statistics. J. Hered. 1995, 8, 485–486. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices (Vers. 2.9.3). 2001, Volume 149. Available online: www.Unil.Ch/Izea/Sofwares/Fstat.Html (accessed on 17 March 2022).
- Rousset F Genepop’007: A complete reimplementation of the Genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [CrossRef] [PubMed]
- Van Oosterhout, C.; Weetman, D.; Hutchinson, W.F. Estimation and adjustment of microsatellite null alleles in nonequilibrium populations. Mol. Ecol. Notes 2006, 6, 155–156. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [Green Version]
- Dow, B.D.; Ashley, M.V. Microsatellite analysis of seed dispersal and parentage of saplings in bur oak, Quercus macrocarpa. Mol. Ecol. 1996, 5, 615–627. [Google Scholar] [CrossRef]
- Bacles, C.F.E.; Lowe, A.J.; Ennos, R.A. Effective seed dispersal across a fragmented landscape. Science 2006, 311, 628. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.H.M.D.; Sebbenn, A.M.; Grattapaglia, D.; Conti, J.L.F. Realized pollen flow and wildling establishment from a genetically modified eucalypt field trial in Southeastern Brazil. For. Ecol. Manag. 2017, 385, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Cockerham, C.C. Group inbreeding and coancestry. Genetics 1967, 56, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, D.; Gea, L.; Jefferson, P. Status number for measuring genetic diversity. For. Genet. 1997, 4, 69–76. [Google Scholar]
- Cremer, K.W. Distance of seed dispersal in Eucalypts estimated from seed weights. Aust. For. Res. 1977, 7, 225–228. [Google Scholar]
- Cremer, K.W. How eucalypt fruits release their seed. Aust. J. Bot. 1965, 13, 11–16. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 31 December 2021).
- Sumathi, M.; Yasodha, R. Microsatellite resources of eucalyptus: Current status and future perspectives. Bot. Stud. 2014, 55, 136–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, T.H.; Steane, D.A.; Jones, R.C.; Pilbeam, D.; Vaillancourt, R.E.; Potts, B.M. Effects of domestication on genetic diversity in Eucalyptus globulus. For. Ecol. Manag. 2006, 234, 78–84. [Google Scholar] [CrossRef]
- Mimura, M.; Barbour, R.C.; Potts, B.M.; Vaillancourt, R.E.; Watanabe, K.N. Comparison of contemporary mating patterns in continuous and fragmented Eucalyptus globulus native forests. Mol. Ecol. 2009, 18, 4180–4192. [Google Scholar] [CrossRef]
- Foster, S.A.; McKinnon, G.E.; Steane, D.A.; Potts, B.M.; Vaillancourt, R.E. Parallel evolution of dwarf ecotypes in the forest tree Eucalyptus globulus. New Phytol. 2007, 175, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Amaro, A.; Reed, D.; Tomé, M.; Themido, I. Modeling dominant height growth: Eucalyptus plantations in Portugal. For. Sci. 1998, 44, 37–46. [Google Scholar]
- Booth, T.H. Going nowhere fast: A review of seed dispersal in eucalypts. Aust. J. Bot. 2017, 65, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Deus, E.; Silva, J.S.; Larcombe, M.J.; Catry, F.X.; Queirós, L.; dos Santos, P.; Matias, H.; Águas, A.; Rego, F.C. Investigating the invasiveness of Eucalyptus globulus in Portugal: Site-scale drivers, reproductive capacity and dispersal potential. Biol. Invasions 2019, 21, 2027–2044. [Google Scholar] [CrossRef]
- Hingston, A.; Potts, B. Floral visitors of Eucalyptus globulus subsp. globulus in eastern Tasmania. Tasforests 1998, 10, 125–139. [Google Scholar]
- Hingston, A.B.; Potts, B.M.; McQuillan, P.B. The swift parrot, Lathamus discolor (Psittacidae), social bees (Apidae) and native insects as pollinators of Eucalyptus globulus ssp. globulus (Myrtaceae). Aust. J. Bot. 2004, 52, 371–379. [Google Scholar] [CrossRef]
- Lopez, G.A.; Potts, B.M.; Vaillancourt, R.E.; Apiolaza, L.A. Maternal and carryover effects on early growth of Eucalyptus globulus. Can. J. For. Res. 2003, 33, 2108–2115. [Google Scholar] [CrossRef] [Green Version]
- da Silva, P.H.M.; Sebbenn, A.M.; Grattapaglia, D. Pollen-mediated gene flow across fragmented clonal stands of hybrid eucalypts in an exotic environment. For. Ecol. Manag. 2015, 356, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.; Elliott, C.P.; Yates, C.J.; Coates, D.J. Maintenance of high pollen dispersal in Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conserv. Genet. 2008, 9, 97–105. [Google Scholar] [CrossRef]
- Barbour, R.C.; Potts, B.M.; Vaillancourt, R.E. Pollen dispersal from exotic eucalypt plantations. Conserv. Genet. 2005, 6, 253–257. [Google Scholar] [CrossRef]
- Sampson, J.F.; Byrne, M. Outcrossing between an agroforestry plantation and remnant native populations of Eucalyptus loxophleba. Mol. Ecol. 2008, 17, 2769–2781. [Google Scholar] [CrossRef]
- McGowen, M.H.; Vaillancourt, R.E.; Pilbeam, D.J.; Potts, B.M. Sources of variation in self-incompatibility in the Australian forest tree, Eucalyptus globulus. Ann. Bot. 2010, 105, 737–745. [Google Scholar] [CrossRef] [Green Version]
- HARDNER, C.; POTTS, B. Inbreeding depression and changes in variation after selfing in Eucalyptus globulus ssp. globulus. Silvae Genet. 1995, 44, 46–54. [Google Scholar]
- Moncur, M.W.; Mitchell, A.; Fripp, Y.; Kleinschmidt, G.J. The role of honey bees (Apis mellifera) in eucalypt and acacia seed production areas. Commonw. For. Rev. 1995, 74, 350–354. [Google Scholar]
- Patterson, B.; Vaillancourt, R.E.; Pilbeam, D.J.; Potts, B.M. Factors affecting variation in outcrossing rate in Eucalyptus globulus. Aust. J. Bot. 2004, 52, 773–780. [Google Scholar] [CrossRef]
- Russell, J.; Marshall, D.; Griffin, D.; Harbard, J.; Powell, W. Gene flow in South American Eucalyptus grandis and E. globulus seed orchards. In Proceedings of the IUFRO International Symposium: Developing the Eucalypt of the Future; Valdivia, Chile, 10–15 September 2001, pp. 149–150.
- Nickolas, H.; Harrison, P.A.; Tilyard, P.; Vaillancourt, R.E.; Potts, B.M. Inbreeding depression and differential maladaptation shape the fitness trajectory of two co-occurring Eucalyptus species. Ann. For. Sci. 2019, 76, 10. [Google Scholar] [CrossRef] [Green Version]
- Seidler, T.G.; Plotkin, J.B. Seed dispersal and spatial pattern in tropical trees. PLoS Biol. 2006, 4, e344. [Google Scholar] [CrossRef]
- Ellstrand, N.C. Is gene flow the most important evolutionary force in plants? Am. J. Bot. 2014, 101, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Loveless, M.D.; Hamrick, J.L. Ecological determinants of genetic structure in plant populations. Annu. Rev. Ecol. Syst. 1984, 15, 65–95. [Google Scholar] [CrossRef]
- Duminil, J.; Hardy, O.J.; Petit, R.J. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evol. Biol. 2009, 9, 177. [Google Scholar] [CrossRef]
| Cohort | Count (n) | DBH (cm) | Height (m) | Resprout (%) | Reproductive (%) | |
|---|---|---|---|---|---|---|
| Pre-fire | Roadside (adult) | 10 | 44.2 (4.6) (14.0–60.0) | 25.9 (0.9) (16.8–32.2) | 0.0 | 100 |
| North (adult) | 8 | 17.4 (4.0) (8–40) | 7.4 (2.8) (5–12) | 100 | 12.5 | |
| Post-fire | South (seedlings) | 60 | 1.6 (0.1) (0.5–4.0) | 1.4 (0.1) (0.2–4.0) | 0.0 | 0.0 |
| Total | 78 | 14.5 ± 19.4 (0.5–60.0) | 5.0 ± 8.4 (0.2–32.2) | 10 | 13.8 | |
| Locus | A | Ae | Ho | He | Fis |
|---|---|---|---|---|---|
| Adult cohort (n = 18) | |||||
| Embra11 | 8 | 6.113 | 0.889 | 0.836 | −0.034 |
| Embra23 | 10 | 8.640 | 0.944 | 0.884 | −0.040 |
| Embra37 | 10 | 6.231 | 0.889 | 0.840 | −0.030 |
| Embra41 | 12 | 7.448 | 0.944 | 0.866 | −0.063 |
| Embra119 | 9 | 6.113 | 0.778 | 0.836 | 0.098 |
| Embra227 | 8 | 5.023 | 0.778 | 0.801 | 0.057 |
| Emcrc7 | 8 | 4.836 | 0.722 | 0.793 | 0.118 |
| Emcrc8 | 11 | 7.448 | 0.944 | 0.866 | −0.063 |
| Eg65 | 10 | 7.624 | 0.944 | 0.869 | −0.059 |
| En12 | 12 | 7.714 | 0.778 | 0.870 | 0.135 |
| En15 | 8 | 4.101 | 0.944 | 0.756 | −0.222 |
| Mean (SE) | 9.636 (0.472) | 6.481 (0.428) | 0.869 (0.026) | 0.838 (0.012) | −0.008 |
| Post-fire seedlings cohort (n = 60) | |||||
| Embra11 | 9 | 6.492 | 0.800 | 0.846 | 0.063 *** |
| Embra23 | 9 | 7.852 | 0.950 | 0.873 | −0.108 * |
| Embra37 | 9 | 5.337 | 0.817 | 0.813 | 0.003 * |
| Embra41 | 10 | 7.938 | 0.900 | 0.874 | −0.021 * |
| Embra119 | 6 | 3.900 | 0.817 | 0.744 | −0.090 |
| Embra227 | 7 | 5.007 | 0.750 | 0.800 | 0.071 ** |
| Emcrc7 | 9 | 5.161 | 0.900 | 0.806 | −0.108 * |
| Emcrc8 | 10 | 5.995 | 0.833 | 0.833 | 0.008 * |
| Eg65 | 10 | 5.975 | 0.733 | 0.833 | 0.128 ** |
| En12 | 10 | 6.238 | 0.712 | 0.840 | 0.0161 * |
| En15 | 7 | 3.976 | 0.833 | 0.748 | −0.105 * |
| Mean (SE) | 8.727 (0.428) | 5.807 (0.401) | 0.822 (0.022) | 0.819 (0.013) | 0.004 *** |
| Variable | Coefficients | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | −2.79 | 2.03 | −1.38 | ns |
| DBH | 0.10 | 0.04 | 2.85 | 0.03 |
| Class | θxy | # Pairs | % |
|---|---|---|---|
| Unrelated | 0 | 989 | 55.9 |
| Half siblings | 0.125 | 608 | 34.4 |
| Full siblings | 0.25 | 167 | 9.4 |
| Full siblings selfs | 0.5 | 6 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Silva, J.S.; Deus, E.; Pinho, S.; Pinto, J.F.; Borralho, N. The Genetics and Ecology of Post-Fire Eucalyptus globulus Recruitment in an Isolated Stand in Central Portugal. Forests 2022, 13, 680. https://doi.org/10.3390/f13050680
Costa J, Silva JS, Deus E, Pinho S, Pinto JF, Borralho N. The Genetics and Ecology of Post-Fire Eucalyptus globulus Recruitment in an Isolated Stand in Central Portugal. Forests. 2022; 13(5):680. https://doi.org/10.3390/f13050680
Chicago/Turabian StyleCosta, Joana, Joaquim S. Silva, Ernesto Deus, Simão Pinho, Joaquim F. Pinto, and Nuno Borralho. 2022. "The Genetics and Ecology of Post-Fire Eucalyptus globulus Recruitment in an Isolated Stand in Central Portugal" Forests 13, no. 5: 680. https://doi.org/10.3390/f13050680







