Modeling Optimal Forest Rotation Age for Carbon Sequestration in the Great Khingan Mountains of Northeast China
Abstract
:1. Introduction
2. Decision Model
2.1. Assumptions and Justifications
2.2. The Establishment of the CS-H-P Model
2.3. EEE Model: Ecological–Economic–Environmental Model
3. Empirical Analysis
3.1. Initial Conditions of the Great Khingan Forest
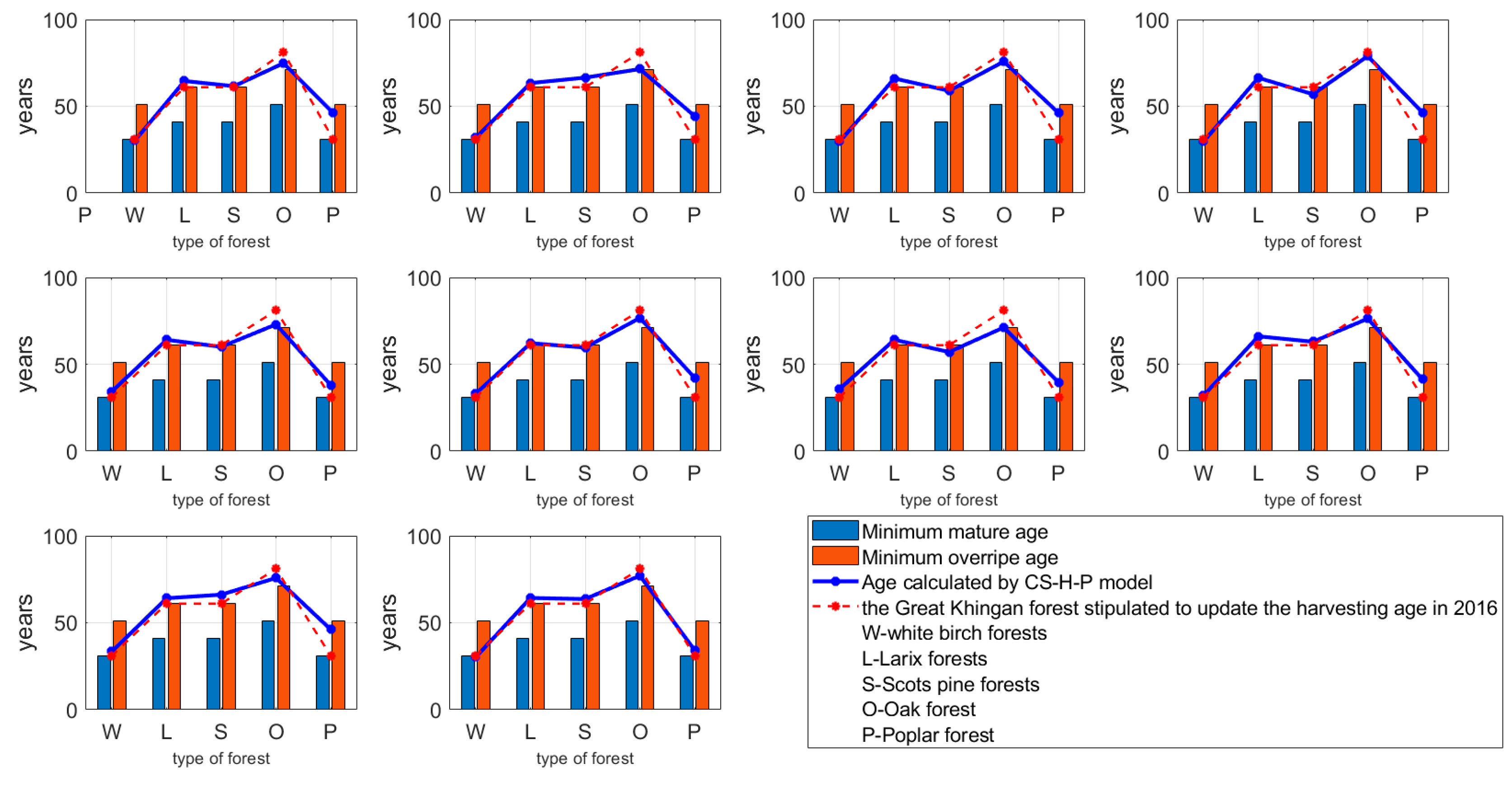
3.2. Data of Eight Forests Used to Calculate the Weights
4. Results
4.1. Regeneration Cutting Ages of Five Tree Species in the Great Khingan Forests by the CS-H-P Model
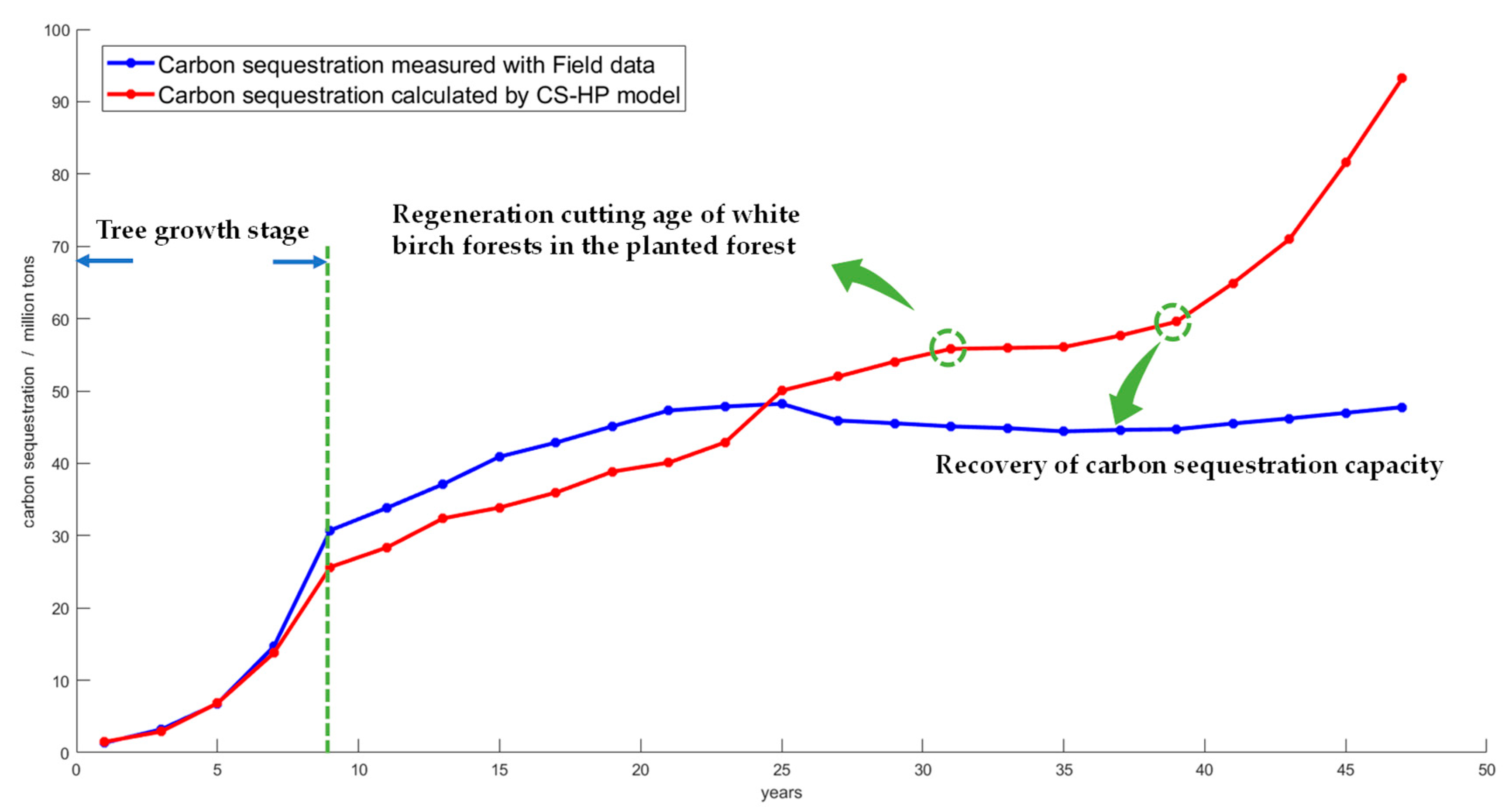
4.2. Analysis and Prediction of Carbon Dioxide Absorption
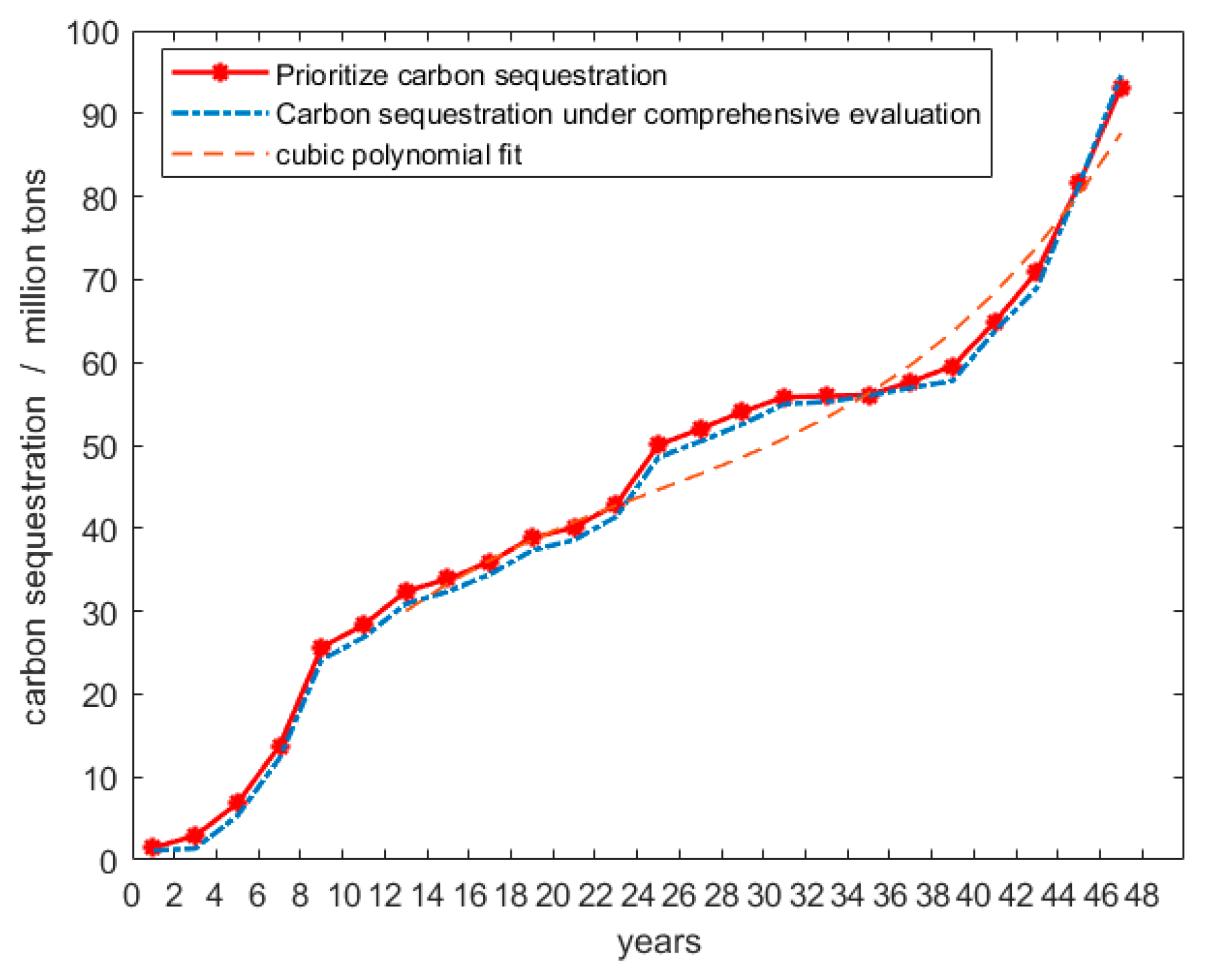
4.3. Regeneration Cutting Ages of Five Tree Species in the Great Khingan Region by the EEE Model
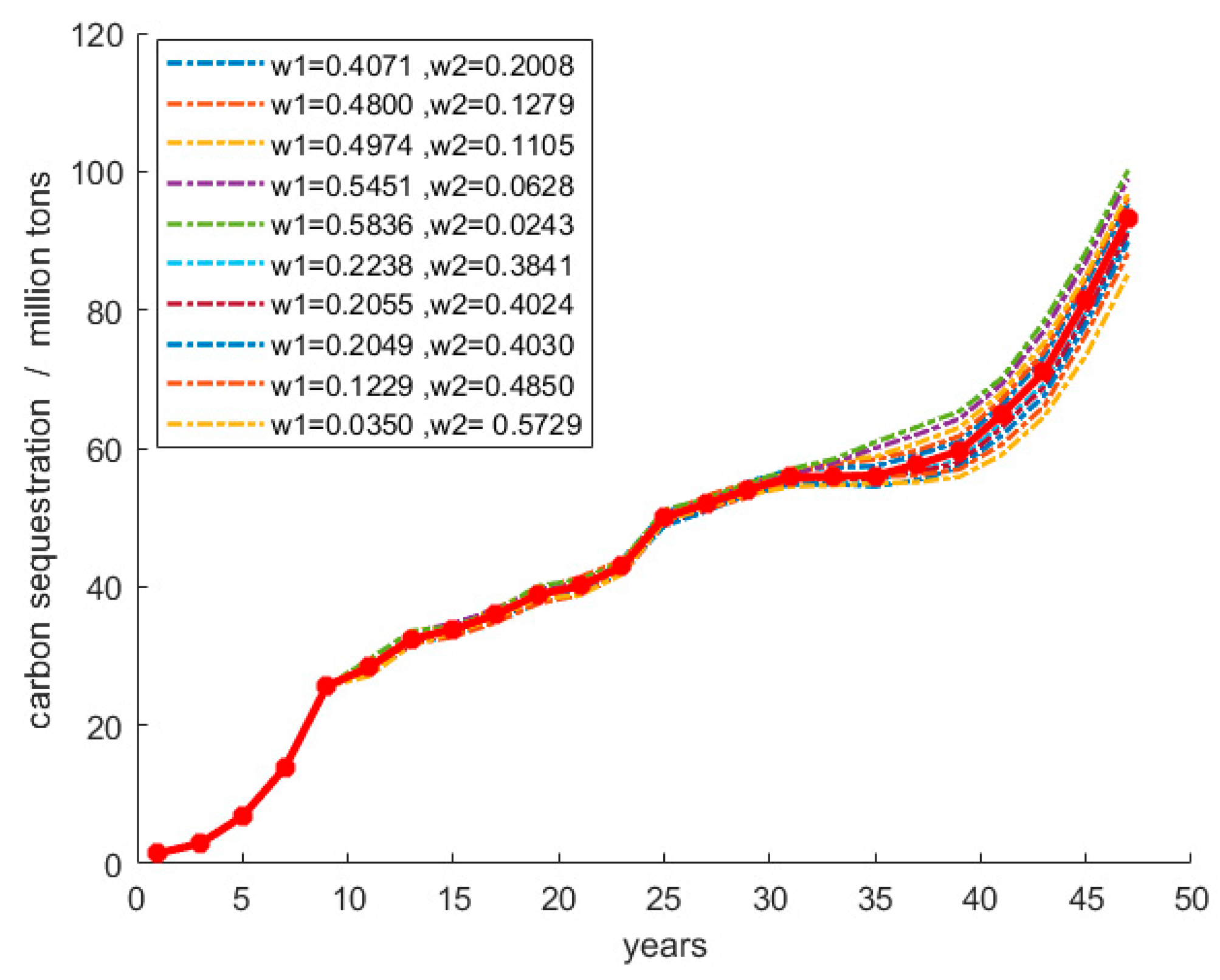
4.4. Sensitivity Analysis
4.4.1. The CS-H-P Model
4.4.2. The EEE Model
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Basic Model of the Biomass Expansion Factor
Appendix B. Description of the Ten Indicators and Their Interpretation
- (1)
- Ecological functions
- (a)
- Carbon sequestration (tons per 100 m2). Forest carbon sequestration refers to the process and mechanism by which forest plants absorb CO2 in the atmosphere and fix it in trees or soil, thereby reducing the concentration of this gas in the atmosphere [54], which can bring certain ecological benefits in the future. Therefore, we introduced carbon sequestration to assess forests.
- (b)
- Living wood growing stock (cubic meters per 100 m2). Living wood growing stock represents the timber productivity under the site ecological conditions of the local forest land, and it is an important factor reflecting the ecological value of forests.
- (c)
- Mean annual temperature (Celsius). Temperature can affect plant photosynthesis and respiration, resulting in changes in forest vegetation productivity and biomass [55]. Therefore, we chose the annual mean temperature as one of the eco-logical function indicators.
- (d)
- Mean annual precipitation (millimeters). Precipitation is an important way for plants to obtain water, which can affect the growth and development of plants, community characteristics and ecosystem structure, and then affect the distribution of forest biomass [56]. Thus, we introduced annual mean precipitation as an indicator for forest value assessment.
- (2)
- Economic benefits
- (a)
- Profits (100 million Chinese Yuan). Forests can be divided into commercial forests and ecological public welfare forests according to their resources [57], among which commercial forests can reflect their economic value in the form of currency, so it is necessary to use economic profit to make a comprehensive and objective assessment of the value of forests.
- (b)
- Forest harvesting (hectares). Timber and other forest products are essential raw materials in the construction of various industries, and forest harvesting is one of the ways to obtain raw materials. Appropriate forest harvesting can promote the economic development of society. Therefore, we introduced forest harvesting as an indicator for forest value assessment.
- (3)
- Environmental qualities
- (a)
- Number of tree species (number). Different types of trees have different abilities to absorb and fix carbon dioxide. Generally, the community structure is complex, and the species of higher plants that make up the community are abundant in places with favorable environmental conditions, whereas in places with harsh environmental conditions, only a few higher plants can adapt and the community structure is relatively simple. Therefore, the number of tree species is also a factor to be considered for the forest value.
- (b)
- Forest cover area (10,000 ha). Forest cover area is an important indicator reflecting the abundance of forest resources and ecological balance [58]. If the important natural factors affecting the forest system are considered, the forest cover area can also be used as an indicator for evaluating the value of forests.
- (c)
- Average number of fires per year (number). Forests have a long growth cycle and management cycle and are easily affected by natural disasters. After each disaster, the recovery of forests is very slow [59]. Among natural disasters, abiotic factors, including fire, are one of the main reasons that affect forest restoration and increase carbon sequestration. We chose the average number of fires per year as one of the environmental quality indicators.
- (d)
- Average annual pests and diseases area (10,000 ha). Biological factors, including pests and diseases, are the main natural risks faced when operating carbon sequestration forests [60]. we introduced average annual pests and diseases area as an indicator for forest value assessment.
Appendix C
| (a) | |||||
| Forest | Carbon Sequestration (t/hm2) | Living Wood Growing Stock (m3/hm2) | Mean Annual Temperature (°C) | Mean Annual Precipitation (mm) | Profit (100 MillionCNY) |
| Great Khingan | 0.1583 [38] | 80.370 [38] | –1.42 [61] | 471.0 [61] | 8000 |
| Xishuangbanna Tropical Rainforest | 8.9374 [62] | 124.621 [63] | 22.5 [64] | 1309.5 [64] | 1406 |
| Saihanba Forest | 0.1120 [65] | 138.199 [66] | –1.3 | 460.3 | 136 [66] |
| Changbai Mountain Forest | 2.5390 [67] | 108.264 | 3.6 [68] | 632.8 [68] | 8899 [69] |
| Xiaoxing’an Mountain | 2.9519 | 94.000 [70] | –1 [71] | 629.6 [71] | 74 [70] |
| Shennongjia Forestry District | 0.2451 [72] | 73.019 | 12.6 [73] | 913.7 [73] | 286 [74] |
| Mordaoga National Forest | 0.9597 [75] | 100.435 [76] | –5.3 [77] | 414.0 [78] | 6 |
| Jianfengling Tropical Rainforest | 2.8789 | 108.177 [79] | 24.5 [80] | 2100.0 [80] | 2045 |
| (b) | |||||
| Forest | Forest Harvesting (10,000 m3) | Number of Tree Species | Forest Cover Area (10,000 hm2) | Average Number of Fires per Year | Average Annual Pests andDiseases Area (10,000 hm2) |
| Great Khingan | 270.0 | 10 [38] | 997.883 [38] | 26 [81] | 13.33 [82] |
| Xishuangbanna Tropical Rainforest | 3259.6 | 20 [83] | 151.660 [63] | 0 [84] | 29.13 [85] |
| Saihanba Forest | 20.4 | 19 [86] | 7.333 [66] | 0 [87] | 0.5008 [88] |
| Changbai Mountain Forest | 56.3 [69] | 5 | 19.647 [69] | 2.46 [89] | 1.33 [90] |
| Xiaoxing’an Mountain | 25.0 | 15 [70] | 500.000 [70] | 30 [91] | 0 |
| Shennongjia Forestry District | 20.0 [92] | 10 [73] | 27.650 [74] | 3.27 [93] | 0.3709 [94] |
| Mordaoga National Forest | 21.1 [76] | 6 [78] | 45.500 | 3.2 [95] | 0 |
| Jianfengling Tropical Rainforest | 10.9 [95] | 20 [79] | 8.597 | 1 | 0.039 |
Appendix D
| Decision Model | Fitting Equation | Goodness-of-Fit R2 |
|---|---|---|
| CS-H-P Model | f(t)= 0.002t3 − 0.1505t2 + 4.702t − 8.582 | 0.9896 |
| EEE Model | f(t)= 0.002017t3 − 0.1498t2 + 4.654t − 9.598 | 0.9917 |
Appendix E
| Species | Origin | Regeneration Cutting Age |
|---|---|---|
| Korean pine forests Spruce forests | Natural forests | 161 |
| Planted forests | 121 | |
| Larch forests Fir forests Scots pine forests | Natural forests | 121 |
| Planted forests | 61 | |
| Sweet poplar forests Willow forests White birch forests Oak tree Soft broadleaf tree | Natural forests | 61 |
| Planted forests | 31 | |
| Elm Black birch Oak tree Hard broadleaf tree | Natural forests | 121 |
| Planted forests | 81 |
References
- Lin, D. Research on the Marketization of Forest Carbon Sequestration Services. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2005. [Google Scholar]
- Xu, L. The effect of forest management on forest carbon sequestration and its improvement measures. Mod. Agric. Technol. 2013, 179, 188. [Google Scholar]
- Zhao, H. Analysis on the importance of perfecting the forest harvesting management system. Biotech World 2012, 10, 59. [Google Scholar]
- Zhou, J. Research on Sustainable Forest Management and Management Mechanism in Southern Collective Forest Areas. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2010. [Google Scholar]
- Zhang, S.; Tang, X.; Cui, W.; Zhang, Y.; Wang, H.; Chen, Y.; Xie, S.; Wang, H.; Zhang, H.; Wang, Z. Compiled by the Forest Resources Management Department of the State Forestry Administration. Forest Harvesting Operation Regulations; China Forestry Publishing House: Beijing, China, 2007; Volume 6. [Google Scholar]
- Jiang, J.; Chen, A.; Han, J. Demonstration on the renewal and cutting age of farmland shelterbelts in aohan banner. Inn. Mong. For. Surv. Des. 2011, 34, 16–17. [Google Scholar] [CrossRef]
- Zhang, N.; Ning, Z.; Yang, H. The fursman model and its generalized improvement: Evolution of methodology based on woodland expectation. For. Econ. 2020, 42, 3–15. [Google Scholar] [CrossRef]
- Dai, Q. Simulation Research on Forest Harvesting Scheme Based on FSOS Model; Zhejiang Agriculture and Forestry University: Hangzhou China, 2020. [Google Scholar] [CrossRef]
- Faustmann, M. Calculation of the value which forest land and immature stands possess for forestry. In Economics of Forestry; Sedjo, R.A., Ed.; Ashgate: Burlington, MI, USA, 2003; pp. 1–40. [Google Scholar]
- Cui, W.; Wang, H.; Wang, S.; Zhang, J. A study on the renewal cutting age of farmland shelter forest in Nong’an county, Jilin province. For. Sci. Res. 2000, 13, 519–523. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, Z.; Yuan, C. Discussion on the harvesting age of farmland shelterbelts. Jiangsu For. Technol. 1999, 1, 58–62. [Google Scholar]
- Li, H.; Fan, H.; Ma, H. Talking about the determination of cutting age from the view of forest benefit. Agric. Syst. Sci. Integr. Res. 1992, 1, 60–63. [Google Scholar]
- Hu, Y.; Su, Z.; Li, P.; Li, W. A comparative study of stand biomass carbon measurement models. J. Cent. South Univ. For. Technol. 2015, 35, 84–88. [Google Scholar] [CrossRef]
- Xu, S.; Yao, S. Analysis of regional differences in forest carbon storage in my country based on biomass conversion factor method. J. Beijing For. Univ. 2009, 8, 109–114. [Google Scholar]
- Xu, S. A review of forest, carbon storage estimation methods. For. Inventory Plan. 2014, 39, 28–33. [Google Scholar] [CrossRef]
- Hu, H.; Luo, B.; Wei, S.; Wen, Z.; Sun, L.; Luo, S.; Wang, L.; Ma, H. Forest biological carbon storage of five typical forest types in the Great Khingan Mountains. J. Ecol. 2015, 35, 16. [Google Scholar]
- Yang, H.; Ji, C.; Yang, H.; Nie, Y.; Yang, X. The emission reduction contribution of China’s forest products under global climate change: Accounting based on the carbon sequestration function of wood forest products. J. Nat. Resour. 2013, 28, 11. [Google Scholar]
- Gao, Y. Evaluation of Forest Resources in Beijing Mountains. Ph.D. Thesis, China Agricultural University, Beijing, China, 2005. [Google Scholar] [CrossRef]
- van Kooten, C.G.; Binkley, C.S.; Delcourt, G. Effect of carbon taxes and subsidies on optimal forest rotation age and supply of carbon services. Am. J. Agric. Econ. 1995, 77, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Hou, G.; Delang, C.O.; Lu, X.; Olschewski, R. Optimizing rotation periods of forest plantations: The effects of carbon accounting regimes. For. Pol. Econ. 2020, 118, 102263. [Google Scholar] [CrossRef]
- Cambero, C.; Alexandre, M.H.; Sowlati, T. Life cycle greenhouse gas analysis of bioenergy generation alternatives using forest and wood residues in remote locations: A case study in British Columbia, Canada. Resour. Conserv. Recycl. 2015, 105, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Kolo, H.; Kindu, M.; Knoke, T. Optimizing forest management for timber production, carbon sequestration and groundwater recharge. Ecosyst. Serv. 2020, 44, 101147. [Google Scholar] [CrossRef]
- Du, Z.; Su, T.; Ge, J.; Wang, X. Forest carbon sequestration and their spatial spillover effects in the context of carbon neutrality. Econ. Res. 2021, 56, 187–202. [Google Scholar]
- Wang, D.; Gu, J.; Holmes, T.P. Stochastic dynamic programming model for deforestation strategy optimization. Inf. Control. 1992, 21, 180–183. [Google Scholar]
- Fang, J.; Guo, Z.; Piao, S.; Chen, A. Estimation of China’s terrestrial tree carbon sequestration from 1981 to 2000. Chin. Sci. Ser. D Earth Sci. 2007, 37, 804–812. [Google Scholar]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Isaev, A.S.; Korovin, G.N.; Bartalev, S.A.; Ershov, D.V.; Janetos, A.; Kasischke, E.S.; Shugart, H.H.; French, N.H.F.; Orlick, B.E.; Murphy, T.L. Using Remote Sensing to Assess Russian Forest Fire Carbon Emissions. Clim. Change 2002, 55, 235–249. [Google Scholar] [CrossRef]
- Dixon, R.K.; Krankina, O.N. Forest fires in Russia: Carbon dioxide emissions to the atmosphere. Can. J. For. Res. 1993, 23, 700–705. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Zavadskas, E.K.; Mardani, A.; Turskis, Z.; Jusoh, A.; Nor, K.M. Development of TOPSIS method to solve complicated decision-making problems:an overview on developments from 2000 to 2015. Int. J. Inf. Technol. Decis. Mak. 2016, 15, 645–682. [Google Scholar] [CrossRef]
- Cheng, Q. Structural Entropy Weight Method for Determining the Weight of Evaluation Index. Syst. Eng. Theory Pract. 2010, 4, 1225–1228. [Google Scholar]
- Yu, M.; Liu, X.; Xue, L. Research progress on the effects of temperature and precipitation on forest biomass allocation. Ecol. Sci. 2021, 40, 204–209. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Peñuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Change Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z. On the selection of index forwarding and dimensionless methods in multi-index comprehensive evaluation. Stat. Sci. Pract. 2003, 4, 24–25. [Google Scholar]
- Qin, X.; Si, Y.; Deng, L. A Study on the Maintenance and Management Fee Standards of Forest Parks in Big Cities: Taking Beijing Forest Park as an Example. J. Cent. South Univ. For. Technol. Soc. Sci. Ed. 2017, 11, 49–54. [Google Scholar] [CrossRef]
- Tong, B.; Yang, Y. Research on the application of main garden tree resources in the Great Khingan Mountains. Res. Land Nat. Resour. 2008, 4, 93–94. [Google Scholar] [CrossRef]
- State Forestry and Grassland Administration. Guidelines for Accounting of Carbon Pools in Forest Ecosystems: LY/T 2988-2018[S]; State Forestry and Grassland Administration: Beijing, China, 2018.
- Zhao, B.; Zhang, J. Evaluation of the status quo of forest resources in the Daxinganling forest area of Inner Mongolia. Inn. Mong. For. Surv. Des. 2014, 37, 29–30, 37. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Wang, J.; Song, W.; Qin, J.; Hu, X.; Zhang, W.; Zhou, J. Carbon stock dynamics and sequestration potential of forest tree in Xishuangbanna. J. Ecol. Environ. 2011, 20, 397–402. [Google Scholar] [CrossRef]
- Zhu, Q. Design and Realization of Forest Resources Data Visualization System in Saihanba Forest Farm. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Y.; Wu, B.; Li, X.; Wu, W. Extraction and analysis of tree coverage in Three-North Shelter Forest Project area based on MODIS data. J. Ecol. 2009, 28, 1712–1718. [Google Scholar]
- Zhang, Z.; Xu, Y. MATLAB Tutorial (Version 7.0); Beihang University Press: Beijing, China, 2005. [Google Scholar]
- Wei, C.; Zhao, X.; Li, H. Estimation of forest carbon storage and carbon sequestration in Daxing’an Mountains, Heilongjiang. Shelter. For. Sci. Technol. 2016, 6, 51–53. [Google Scholar]
- Measures for the Administration of Regeneration and Cutting of Public Welfare Forests. Available online: http://lcj.nmg.gov.cn/xxgkzl/fdzdgknr/gzxzgfxwj/flfg/202111/t20211118_1954103.html (accessed on 18 November 2021).
- Wu, S.; Li, Y.; Yu, D.; Zhou, L.; Zhou, W.; Guo, Y.; Wang, X.; Dai, L. Analysis of factors affecting carbon storage in forest vegetation based on VAR model—taking Shaanxi Province as an example. Acta Ecol. 2015, 35, 196–203. [Google Scholar]
- Fu, Y.; He, H.S.; Zhao, J.; Larsen, D.R.; Zhang, H.; Sunde, M.G.; Duan, S. Climate and Spring Phenology Effects on Autumn Phenology in the Greater Khingan Mountains, Northeastern China. Remote Sens. 2018, 10, 449. [Google Scholar] [CrossRef] [Green Version]
- Ecology Research—Forest Ecology; Researchers at Public University of Navarre Release New Data on Forest Ecology (Incorporating Climate Effects In Larix Gmelinii Improves Stem Taper Models In the Greater Khingan Mountains of Inner Mongolia, Northeast China). Ecol. Environ. Conserv. 2020, 464, 118065.
- Jiang, Y.; Zhang, J.; Han, S.; Chen, Z.; Setälä, H.; Yu, J.; Zheng, X.; Guo, Y.; Gu, Y. Radial Growth Response of Larix gmelinii to Climate along a Latitudinal Gradient in the Greater Khingan Mountains, Northeastern China. Forests 2016, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Trancoso, R.; Ma, Q.; Yue, C.; Wei, X.; Blanco, J.A. Incorporating climate effects in Larix gmelinii improves stem taper models in the Greater Khingan Mountains of Inner Mongolia, northeast China. For. Ecol. Manag. 2020, 464, 118065. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, C.; Wei, X.; Blanco, J.A.; Trancoso, R. Tree profile equations are significantly improved when adding tree age and stocking degree: An example for Larix gmelinii in the Greater Khingan Mountains of Inner Mongolia, northeast China. Eur. J. For. Res. 2020, 139, 443–458. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, P.; Peng, J. Discussion on the development advantages of bamboo plants in forest carbon sequestration. Green Sci. Technol. 2012, 2, 45–46. [Google Scholar]
- Liu, B.; Tian, X. Estimation of carbon release from Huzhong forest fire in the Daxingan Mountains. For. Resour. Manag. 2011, 3, 47–51. [Google Scholar] [CrossRef]
- Jiang, R.; Wei, C.; Zhou, Y. Functional orientation and management strategies of forest resources around central cities. Econ. Res. Guide 2015, 6, 156–158. [Google Scholar]
- Ma, W.; Liu, Z.; Wang, Z.; Wang, W.; Liang, C.; Tang, Y.; He, J.; Fang, J. Climate change alters interannual variation of grassland aboveground productivity: Evidence from a 22-year measurement series in the Inner Mongolian grassland. J. Plant Res. 2010, 123, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Knapp Alan, K.; Ciais, P.; Smith Melinda, D. Reconciling inconsistencies in precipitation-productivity re-lationships: Implications for climate change. New Phytol. 2017, 214, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Kong, F.; Jiang, D. Current situation, problems and countermeasures of forest classification management in China. J. Northeast. For. Univ. 2003, 31, 3. [Google Scholar]
- Forestry and Grassland Bureau of Gansu Province, China. Statistical Yearbook Main Statistical Indicators Explained; Forestry and Grassland Bureau of Gansu Province: Gansu, China, 2020.
- Qin, J.; Gao, L.; Zhou, W. Risk factors analysis of carbon sink forest management in Guangdong Province. Guangdong Agric. Sci. 2014, 41, 200–205. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, L. An Empirical Analysis of the Impact of Forest Disasters on Carbon Sequestration: Taking Guangdong Province as an Example. J. Agric. For. Econ. Manag. 2017, 16, 224–229, 239. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H. Climate change in the Greater Khingan Mountains and its impact on forest fires. J. Northeast. For. Univ. 2008, 7, 29–31, 36. [Google Scholar]
- Zhang, Y.; Zhao, S.; Yu, G.; Dou, J.; Liu, W.; Sun, X. Observation of microclimate characteristics and CO2 flux on the canopy of tropical seasonal rain forest in Xishuangbanna in dry and hot season. Acta Ecol. 2005, 25, 2540–2549. [Google Scholar]
- State Forestry and Grassland Administration. Biodiversity Conservation in China—Yunnan Forestry Chapter 1_Forest and Grass Science_State Forestry and Grassland Administration Government Network; State Forestry and Grassland Administration: Beijing, China, 2020.
- Yu, Y.; Meng, G.; Zhang, L. Characteristics of climate change in Xishuangbanna in the past 45 years. Meteorol. Sci. Technol. 2008, 4, 410–413. [Google Scholar] [CrossRef]
- Zhang, P. Research measures on carbon sinks for afforestation management in Saihanba Forest Farm. Jiangxi Agric. 2018, 6, 107. [Google Scholar] [CrossRef]
- Fu, L.; Yu, S.; Cheng, S.; Xu, Z.; Li, Y. Evaluation of service value of forest ecosystem in Saihanba Mechanical Forest Farm in Hebei Province. For. Ecol. Sci. 2022, 34, 386–392. [Google Scholar]
- Yu, J.; Luo, C.; Xu, Q.; Meng, S.; Li, J.; Liu, Q. Radial growth and carbon sequestration potential of Pinus koraiensis in primeval forest in Changbai Mountains. J. Ecol. 2016, 36, 2626–2636. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, L.; Chen, J.; Wang, Z.; Dai, H.; Li, X. Study on natural regeneration conditions of mixed forest of Korean pine and broad-leaved forest in Changbai Mountain. Acta Ecol. 2001, 21, 1413–1420. [Google Scholar]
- Shi, Y.; Liu, S. Dynamic analysis of forest resources in key state-owned forest areas in Changbai Mountain: Taking Linjiang Forestry Bureau as an example. J. Beihua Univ. Nat. Sci. Ed. 2016, 17, 469–472. [Google Scholar]
- Ren, P.; Chen, W. Assessment of Forest Ecosystem Service Value in Xiaoxing’an Mountains. For. Sci. Technol. Inf. 2020, 52, 1–4. [Google Scholar]
- Zhou, W.; Mu, C.; Liu, X.; Gu, H. Carbon sink function of natural forest swamp ecosystem in Xiaoxing’an Mountains. J. Northeast. For. Univ. 2012, 40, 71–75, 127. [Google Scholar] [CrossRef]
- Kong, S. Research on low-carbon economic development in ecological functional areas: Taking Shennongjia forest area as an example. Mark. Wkly. Theor. Res. 2016, 8, 62–64. [Google Scholar]
- Chen, L.; Wang, Z.; Lei, Y.; Lin, L.; Liao, M.; Yang, J. Analysis on the characteristics of climate change in Shennongjia forest area from 1990 to 2010. J. Hubei Univ. Nat. Sci. Ed. 2013, 35, 11–16. [Google Scholar]
- Xiao, Y.; He, P.; Zhang, L.; Bao, L. Shennongjia: Making Forest Carbon Sink Sustainable. Hubei Daily, 3 August 2010. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.; Meng, S.; Luo, C.; Chen, X. Forest carbon storage and its changes in Moerdaoga forest area. J. For. Environ. 2016, 36, 319–324. [Google Scholar]
- Wang, H.; Lu, D. Analysis of forest resources change in Moerdaoga Forestry Bureau. Inn. Mong. For. Surv. Des. 2012, 35, 56–58. [Google Scholar] [CrossRef]
- Bian, K. A brief discussion on the status of natural forest resources protection project of Mordaoga Forestry Bureau and the sustainable development of forest resources. Inn. Mong. For. Surv. Des. 2012, 35, 3–5, 127. [Google Scholar] [CrossRef]
- Ce, R.; Xie, Z.; Li, B. Shallow light on the landscape resources and tourism development of Moerdaoga National Forest Park in the Great Khingan Mountains. J. Inn. Mong. For. Surv. Des. 2001, 24, 32–34, 43. [Google Scholar]
- Guo, N.; Jiang, Z.; Li, Y.; Xie, M. Sustainable management of tropical forest ecosystem in Jianfengling, Hainan. Trop. For. 2002, 30, 16–19. [Google Scholar]
- Luo, W.; Zhong, Y.; Chen, J.; Chen, H.; Mo, S. Analysis of Geographical Flora of Ancient Trees in Jianfengling National Forest Park, Hainan. Trop. Agric. Sci. 2020, 40, 62–67. [Google Scholar]
- Yu, C.; Zhang, H.; Yang, X. Analysis on the characteristics of forest fires in the Daxing’an Mountains. Heilongjiang Meteorol. 2007, 4, 28–29, 34. [Google Scholar]
- Mei, M.; Liu, Z.; Cao, J. Analysis on the Causes and Control Countermeasures of Forest Diseases and Insect Pests in the Daxinganling Forest Region of Inner Mongolia. Inn. Mong. For. 2013, 4, 14–15. [Google Scholar] [CrossRef]
- Zhu, H. On the classification of forest vegetation in Xishuangbanna, southern Yunnan. Yunnan Plant Res. 2007, 4, 377–387. [Google Scholar]
- Wu, C. Xishuangbanna Nature Reserve has no forest fires for 10 consecutive years. Yunnan For. 1999, 4, 26. [Google Scholar]
- Li, Y. The occurrence and trend forecast of forest pests in Yunnan. Yunnan For. 2009, 30, 52. [Google Scholar]
- Ma, C. Study on Soil Species and Suitable Tree Species in Saihanba Forest. Jiangxi Agric. 2017, 99, 102. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.; Ma, J. Thoughts on forest fire prevention and fire fighting in state-owned forest farms: Taking Hebei Saihanba Mechanical Forest Farm as an example. China Emerg. Manag. 2022, 1, 58–61. [Google Scholar]
- Liu, X. Occurrence characteristics and forecast analysis of major leaf-eating pests in Saihanba National Forest Park. Anhui Agric. Sci. Bull. 2019, 25, 62–64, 81. [Google Scholar] [CrossRef]
- Corps, J. Research on forest fire prevention and fighting strategies in Changbai Mountain Nature Reserve. In Proceedings of the 2016 Modern Forest Fire Development Forum, Xiamen, China, 1 November 2016. [Google Scholar]
- Zhang, Z. Research on the characteristics and control measures of forest pests in Changbai Mountain. China Agric. Mater. 2013, 40, 60. [Google Scholar]
- He, J.; Zhang, G.; Wang, G. The regularity of forest fire occurrence and the establishment of forest fire weather grade model in Xiaoxing’an Mountains. Heilongjiang Meteorol. 2011, 28, 21–22. [Google Scholar] [CrossRef]
- Editorial Department of This Journal. The leap from “wood finance” to “ecological tourism finance”—Financial support for ecological and tourism economic development in Shennongjia Forest Region. Hubei Financ. Tax. 2002, 21, 12–13. [Google Scholar]
- Li, C. Characteristics of forest fires in Shennongjia forest area and fire fighting countermeasures. For. Fire Prev. 1994, 4, 21–22. [Google Scholar]
- Li, W.; Zhou, J.; Tan, J. Strengthening the prevention and control of forest diseases and insect pests in Shennongjia and promoting regional social development. Hubei Agric. Sci. 2009, 48, 239–242. [Google Scholar]
- The Ministry of Civil Affairs of the People’s Republic of China. Inner Mongolia Autonomous Region of the People’s Republic of China Political Ceremony; Huang, S., Lan, E., Eds.; China Society Press: Beijing, China, 2018; Volume 1, pp. 0426–0428.
- Zeng, Q.; Qiu, J. Forest harvesting and climate in Jianfengling forest area of Hainan Island. Trop. For. Sci. Technol. 1980, 3, 26–34. [Google Scholar]




| Tree Species | Mean BEF of Different Ages | SVD | Aij/hm2 | Average DBII/cm | Average Tree Height/m | q1j/% | q2j/% |
|---|---|---|---|---|---|---|---|
| White birch forests (W) | 1.314 | 0.541 | 2,422,232.1 | 5.60 | 8.17 | 0.093 | 0.090 |
| Larch forests (L) | 1.651 | 0.413 | 5,067,690.3 | 6.72 | 7.53 | 0.151 | 0.139 |
| Scots pine forests (S) | 1.651 | 0.413 | 111,714.4 | 9.98 | 10.06 | 0.205 | 0.172 |
| Oak forests (O) | 1.414 | 0.676 | 329,047.0 | 5.14 | 5.25 | 0.072 | 0.071 |
| Poplar forests (P) | 1.515 | 0.478 | 304,164.4 | 5.87 | 6.27 | 0.107 | 0.104 |
| Species | Regeneration Cutting Age | |
|---|---|---|
| Natural Forests | Planted Forests | |
| White birch forests | 64 | 32 |
| Larch forests | 116 | 65 |
| Scots pine forests | 136 | 61 |
| Oak forests | 110 | 75 |
| Poplar forests | 71 | 42 |
| Species | Regeneration Cutting Age | |
|---|---|---|
| Natural Forests | Planted Forests | |
| White birch forests | 65 | 34 |
| Larch forests | 134 | 66 |
| Scots pine forests | 123 | 64 |
| Oak forests | 111 | 77 |
| Poplar forests | 73 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Luo, T.; Li, S.; Liu, B. Modeling Optimal Forest Rotation Age for Carbon Sequestration in the Great Khingan Mountains of Northeast China. Forests 2022, 13, 838. https://doi.org/10.3390/f13060838
Li Y, Luo T, Li S, Liu B. Modeling Optimal Forest Rotation Age for Carbon Sequestration in the Great Khingan Mountains of Northeast China. Forests. 2022; 13(6):838. https://doi.org/10.3390/f13060838
Chicago/Turabian StyleLi, Yuzhe, Tao Luo, Shuzhen Li, and Bin Liu. 2022. "Modeling Optimal Forest Rotation Age for Carbon Sequestration in the Great Khingan Mountains of Northeast China" Forests 13, no. 6: 838. https://doi.org/10.3390/f13060838






