Responses of Fungal Community Structure and Functional Composition to Short-Term Fertilization and Dry Season Irrigation in Eucalyptus urophylla × Eucalyptus grandis Plantation Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Soil Sampling
2.2. Soil Physicochemical Property Analyses
2.3. Soil Microbial Biomass
2.4. Soil Total DNA Extraction
2.5. PCR Amplification and Illumina Sequencing
2.6. Bioinformatics Analyses
2.7. Statistical Analysis
3. Results
3.1. Effects of Fertilization and Irrigation on Eucalyptus Growth
3.2. Effects of Fertilization and Irrigation on Soil Physicochemical Properties
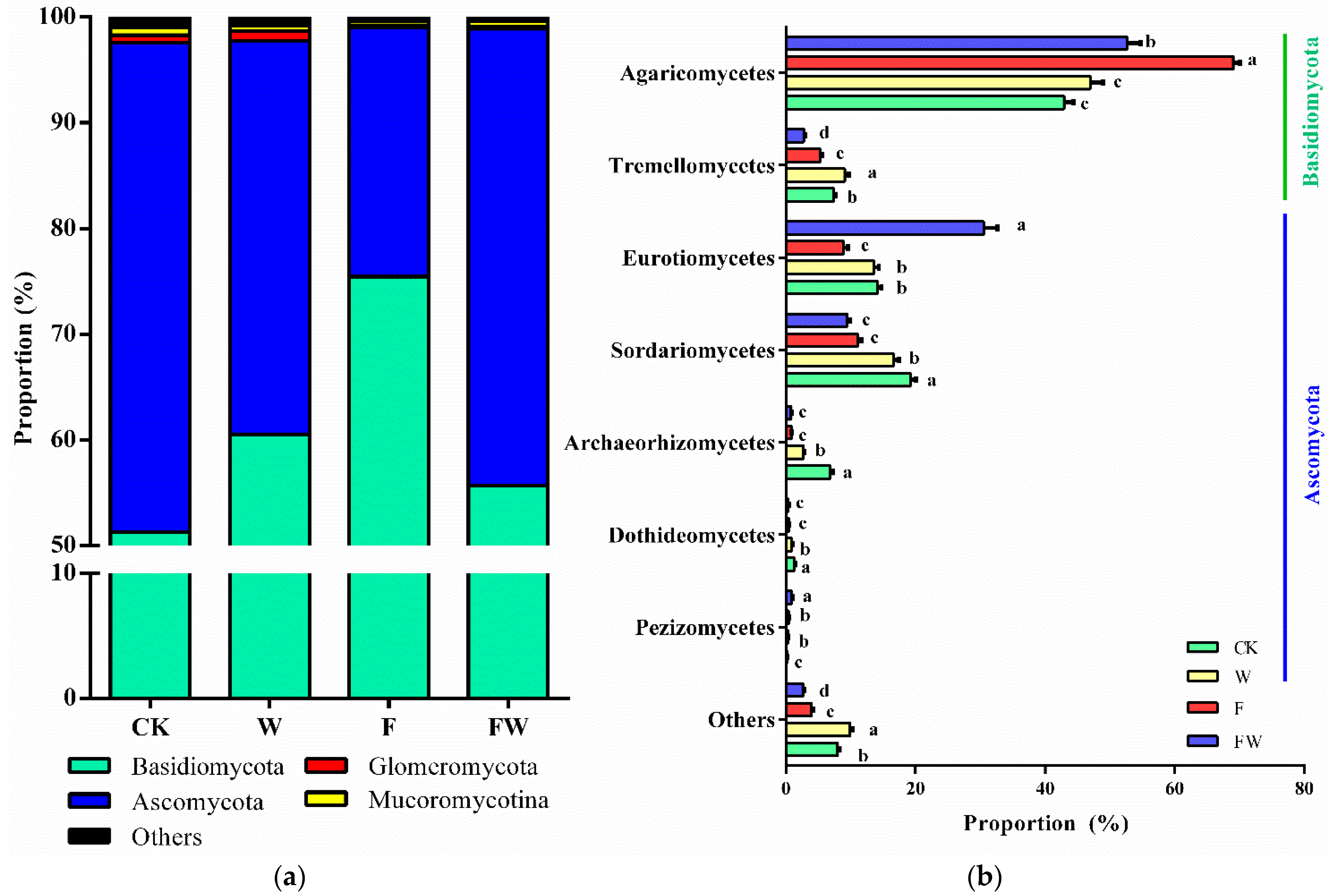
3.3. Effects of Fertilization and Irrigation on Soil Fungal Community Structure
3.4. Fungi Trophic Groups and Guild Assignment
3.5. Effects of Environmental Factors on Fungal Community Structure and Functional Groups
3.6. Correlation between Tree Growth and Partial Fungal Taxon or Physicochemical Properties in the Eucalyptus Plantation Soils
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Fungi | Height/cm | CBH/cm | Crown Diameter /m | GD/mm | DBH/mm |
|---|---|---|---|---|---|
| Symbiotroph | 0.883 | −0.697 | 0.895 | 0.9 | 0.89 |
| Saprotroph | −0.478 | 0.555 | −0.501 | −0.511 | −0.498 |
| Pathotroph | −0.986 * | 0.376 | −0.988 * | −0.988 * | −0.990 ** |
| unidentified fungi | −0.884 | 0.746 | −0.883 | −0.883 | −0.873 |
| Arbuscular Mycorrhizal | −0.956 * | 0.691 | −0.958 * | −0.959 * | −0.951 * |
| Ectomycorrhizal | 0.889 | −0.699 | 0.901 | 0.905 | 0.895 |
| Endophyte | −0.962 * | 0.656 | −0.963 * | −0.963 * | −0.957 * |
| Pisolithus | 0.91 | −0.689 | 0.921 | 0.925 | 0.916 |
| Glomeromycota | −0.93 | 0.564 | −0.939 | −0.943 | −0.938 |
| Phialophora verrucosa | −0.979 * | 0.615 | −0.983 * | −0.984 * | −0.979 * |
| Scleroderma | 0.422 | −0.21 | 0.443 | 0.451 | 0.448 |
| Ambispora | −0.73 | 0.936 | −0.738 | −0.742 | −0.723 |
| Physicochemical Property | Height/cm | CBH/cm | Crown Diameter/m | GD/mm | DBH/mm |
|---|---|---|---|---|---|
| SOC (g/Kg) | 0.986 * | −0.322 | 0.981 * | 0.979 * | 0.984 * |
| MBC (mg/Kg) | 0.997 ** | −0.4 | 0.995 ** | 0.994 ** | 0.996 ** |
| MBN (mg/Kg) | 0.815 | 0.038 | 0.816 | 0.816 | 0.828 |
| Moisture (%) | −0.388 | 0.983 * | −0.397 | −0.401 | −0.376 |
| Soil pH | 0.004 | 0.172 | −0.021 | −0.031 | −0.023 |
| TN (g kg−1) | 0.938 | −0.472 | 0.946 | 0.949 | 0.947 |
| NO3−-N (mg kg−1) | 0.992 ** | −0.422 | 0.988 * | 0.986 * | 0.988 * |
| NH4+-N (mg kg−1) | 0.712 | 0.228 | 0.71 | 0.709 | 0.726 |
| TP (g kg−1) | 0.939 | −0.12 | 0.932 | 0.93 | 0.94 |
| AP (mg kg−1) | 0.672 | 0.053 | 0.652 | 0.644 | 0.657 |
| AK (mg kg−1) | 0.909 | −0.271 | 0.915 | 0.918 | 0.921 |
| MBN (mg kg−1) | 0.857 | −0.022 | 0.858 | 0.858 | 0.869 |
| SOC (g/Kg) | 0.986 * | −0.322 | 0.981 * | 0.979 * | 0.984 * |
| MBC (mg/Kg) | 0.997 ** | −0.4 | 0.995 ** | 0.994 ** | 0.996 ** |
References
- Thormann, M.N. Diversity and function of fungi in peatlands: A carbon cycling perspective. Can. J. Soil Sci. 2006, 86, 281–293. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, H.; Guo, L.; Anderson, I.C.; Powell, J.R. Dryland forest management alters fungal community composition and decouples assembly of root- and soil-associated fungal communities. Soil Biol. Biochem. 2017, 109, 14–22. [Google Scholar] [CrossRef]
- Bai, H.; He, S.; Qin, T.; Yan, D.; Weng, B.; Zhao, X.; Li, X.; Bai, Y.; Ma, J. Influences of irrigation amount on the rhizospheric microorganism composition and carbon dioxide flux of maize crops. Geoderma 2019, 343, 1–9. [Google Scholar] [CrossRef]
- Moreno, J.L.; Bastida, F.; Ondoño, S.; García, C.; Andrés-Abellán, M.; López-Serrano, F.R. Agro-forestry management of Paulownia plantations and their impact on soil biological quality: The effects of fertilization and irrigation treatments. Appl. Soil Ecol. 2017, 117–118, 46–56. [Google Scholar] [CrossRef]
- Wang, J.C.; Geoff, R.; Huang, Q.W.; Shen, Q.R. Plant growth stages and fertilization regimes drive soil fungal community compositions in a wheat-rice rotation system. Biol. Fert. Soils 2018, 54, 731–742. [Google Scholar] [CrossRef]
- Nie, S.; Lei, X.; Zhao, L.; Brookes, P.C.; Wang, F.; Chen, C.; Yang, W.; Xing, S. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Ma, H.; Bai, G.; Sun, Y.; Kostenko, O.; Zhu, X.; Lin, S.; Ruan, W.; Zhao, N.; Bezemer, T.M. Opposing effects of nitrogen and water addition on soil bacterial and fungal communities in the Inner Mongolia steppe: A field experiment. Appl. Soil Ecol. 2016, 108, 128–135. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Liu, H.; Zhao, J.; Li, G.; Wang, H.; Lai, X.; Li, J.; Xiu, W.; Yang, D. Nitrogen deposition combined with elevated precipitation is conducive to maintaining the stability of the soil fungal diversity on the Stipa baicalensis steppe. Soil Biol. Biochem. 2018, 117, 135–138. [Google Scholar] [CrossRef]
- Li, X.; Zhu, T.; Peng, F.; Chen, Q.; Lin, S.; Christie, P.; Zhang, J. Inner Mongolian steppe arbuscular mycorrhizal fungal communities respond more strongly to water availability than to nitrogen fertilization. Environ. Microbiol. 2015, 17, 3051–3068. [Google Scholar] [CrossRef]
- Graham, E.B.; Wieder, W.R.; Leff, J.W.; Weintraub, S.R.; Townsend, A.R.; Cleveland, C.C.; Philippot, L.; Nemergut, D.R. Do we need to understand microbial communities to predict ecosystem function? A comparison of statistical models of nitrogen cycling processes. Soil Biol. Biochem. 2014, 68, 279–282. [Google Scholar] [CrossRef]
- Zhou, G.; Wei, X.; Wu, Y.; Liu, S.; Huang, Y.; Yan, J.; Zhang, D.; Zhang, Q.; Liu, J.; Meng, Z.; et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Change Biol. 2011, 17, 3736–3746. [Google Scholar] [CrossRef]
- Martiny, A.C.; Martiny, J.B.H.; Nelson, M.B. Global biogeography of microbial nitrogen-cycling traits in soil. Proc. Natl. Acad. Sci. USA 2016, 113, 8033–8040. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, S.M. Microbial community ecology: Function over phylogeny. Nat. Ecol. Evol. 2017, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Chen, G.; Chen, G.; Li, S.; Peng, T.; Qiu, X.; Luo, J.; Yang, S.; Hu, T.; Hu, H.; et al. Soil biochemical responses to nitrogen addition in a secondary evergreen broad-leaved forest ecosystem. Sci. Rep. 2017, 7, 2783. [Google Scholar] [CrossRef]
- Turner, B.L.; Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Fanin, N.; Hättenschwiler, S.; Schimann, H.; Fromin, N. Interactive effects of C, N and P fertilization on soil microbial community structure and function in an Amazonian rain forest. Funct. Ecol. 2014, 29, 140–150. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Liu, M.; Jiang, C.; Chen, X.; Kuzyakov, Y.; Rinklebe, J.; Li, Z. Responses of Soil Enzyme Activities and Microbial Community Composition to Moisture Regimes in Paddy Soils under Long-Term Fertilization Practices. Pedosphere 2018, 28, 323–331. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.Z.; Yan, R.R.; Wei, Z.J.; Bai, Y.T.; Zhang, S.; Wang, T.L.; Sun, S.X. Effects of short-term fertilization on soil microorganisms in a mown Leymus chinensis meadow. Chin. J. Ecol. 2017, 36, 2431–2437. [Google Scholar] [CrossRef]
- Gleeson, D.B.; Müller, C.; Banerjee, S.; Ma, W.; Siciliano, S.D.; Murphy, D.V. Response of ammonia oxidizing archaea and bacteria to changing water filled pore space. Soil Biol. Biochem. 2010, 42, 1888–1891. [Google Scholar] [CrossRef]
- Banerjee, S.; Helgason, B.; Wang, L.; Winsley, T.; Ferrari, B.; Siciliano, S. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016, 95, 40–50. [Google Scholar] [CrossRef]
- Zeng, S.; Jacobs, D.F.; Sloan, J.L.; Xue, L.; Li, Y.; Chu, S. Split fertilizer application affects growth, biomass allocation, and fertilizer uptake efficiency of hybrid Eucalyptus. New For. 2013, 44, 703–718. [Google Scholar] [CrossRef]
- Yu, F.; Van Truong, T.; He, Q.; Hua, L.; Su, Y.; Li, J. Dry Season Irrigation Promotes Leaf Growth in Eucalyptus urophylla × E. grandis under Fertilization. Forests 2019, 10, 67. [Google Scholar] [CrossRef]
- Fu, W.B.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Du, H.; Wen, Y.G.; Xu, H.F. Biomass and its allocation characteristics of Eucalyptus urophylla × E. grandis plantations at different stand ages. Acta Ecol. Sin. 2014, 34, 5234–5241. [Google Scholar]
- Hua, L.; Yu, F.; Qiu, Q.; He, Q.; Su, Y.; Liu, X.; Li, J. Relationships between diurnal and seasonal variation of photosynthetic characteristics of Eucalyptus plantation and environmental factors under dry-season irrigation with fertilization. Agric. Water Manag. 2021, 248, 106737. [Google Scholar] [CrossRef]
- Madejón, P.; Alaejos, J.; García-Álbala, J.; Fernández, M.; Madejón, E. Three-year study of fast-growing trees in degraded soils amended with composts: Effects on soil fertility and productivity. J. Environ. Manag. 2016, 169, 18–26. [Google Scholar] [CrossRef]
- Finzi, A.C.; Berthrong, S.T. The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology 2005, 86, 3345–3353. [Google Scholar] [CrossRef] [Green Version]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2010; pp. 39–114. [Google Scholar]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W. Response of soil microbial communities to continuously mono-cropped cucumber under greenhouse conditions in a calcareous soil of north China. J. Soils Sediments 2020, 20, 2446–2459. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Bolger, A.M.; Marc, L.; Bjoern, U. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, H.; Liu, J.; Wang, Q.; Shen, T.; Guo, W.; Wang, R. Shifts in microbial community function and structure along the successional gradient of coastal wetlands in Yellow River Estuary. Eur. J. Soil Biol. 2012, 49, 12–21. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Warton, D.I.; Wright, S.T.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Cao, B.; Sun, Q.; Song, L. Response of grass interplanting on bacterial and fungal communities in a jujube orchard in Ningxia, northwest China. Heliyon 2020, 6, e03489. [Google Scholar] [CrossRef]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems—A meta-analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Chen, R.; Zhong, L.; Jing, Z.; Guo, Z.; Li, Z.; Lin, X.; Feng, Y. Fertilization decreases compositional variation of paddy bacterial community across geographical gradient. Soil Biol. Biochem. 2017, 114, 181–188. [Google Scholar] [CrossRef]
- Carney, K.M.; Matson, P.A. Plant communities, soil microorganisms, and soil carbon cycling: Does altering the world belowground matter to ecosystem functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Waring, B.G.; Hawkes, C.V. Short-Term precipitation exclusion alters microbial responses to soil moisture in a wet tropical forest. Microb. Ecol. 2015, 69, 843–854. [Google Scholar] [CrossRef]
- McHugh, T.A.; Schwartz, E. A watering manipulation in a semiarid grassland induced changes in fungal but not bacterial community composition. Pedobiologia 2016, 59, 121–127. [Google Scholar] [CrossRef]
- Kramer, S.; Marhan, S.; Ruess, L.; Armbruster, W.; Butenschoen, O.; Haslwimmer, H.; Kuzyakov, Y.; Pausch, J.; Scheunemann, N.; Schoene, J.; et al. Carbon flow into microbial and fungal biomass as a basis for the belowground food web of agroecosystems. Pedobiologia 2012, 55, 111–119. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avolio, M.L.; Tuininga, A.R.; Lewis, J.; Marchese, M. Ectomycorrhizal responses to organic and inorganic nitrogen sources when associating with two host species. Mycol. Res. 2009, 113, 897–907. [Google Scholar] [CrossRef]
- Jones, M.D.; Durall, D.M.; Tinker, P.B. A comparison of arbuscular and ectomycorrhizal Eucalyptus coccifera: Growth response, phosphorus uptake efficiency and external hyphal production. New Phytol. 1998, 140, 125–134. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]




| Treatment | Number | Height/cm | CBH/cm | Crown Diameter/m | GD/mm | DBH/mm |
|---|---|---|---|---|---|---|
| CK | 168 | 163.02 ± 84.03 d | 24.35 ± 8.32 b | 1.20 ± 0.46 d | 25.85 ± 14.68 c | 17.23 ± 8.04 c |
| W | 194 | 191.39 ± 85.02 c | 29.61 ± 9.67 a | 1.30 ± 0.40 c | 29.54 ± 14.56 b | 20.57 ± 8.24 b |
| F | 195 | 451.55 ± 106.67 b | 22.18 ± 8.85 c | 2.18 ± 0.29 b | 63.41 ± 14.59 a | 43.39 ± 13.56 a |
| FW | 186 | 488.94 ± 91.98 a | 25.86 ± 9.36 b | 2.27 ± 0.20 a | 66.14 ± 12.80 a | 45.99 ± 11.85 a 1 |
| Treatment | CK | W | F | FW |
|---|---|---|---|---|
| Moisture (%) | 10.09 ± 0.45 c | 15.40 ± 0.29 a | 9.36 ± 0.30 d | 11.68 ± 0.36 b |
| Soil pH | 4.94 ± 0.18 a | 4.76 ± 0.03 b | 4.64 ± 0.04 b | 5.03 ± 0.04 a |
| TN (g kg−1) | 0.18 ± 0.00 b | 0.19 ± 0.01 b | 0.22 ± 0.02 a | 0.21 ± 0.00 a |
| NO3−-N (mg kg−1) | 0.93 ± 0.17 b | 0.96 ± 0.13 b | 1.85 ± 0.83 a | 2.17 ± 0.24 a |
| NH4+-N (mg kg−1) | 4.80 ± 0.63 b | 6.63 ± 0.40 a | 6.66 ± 0.57 a | 7.04 ± 0.95 a |
| TP (g kg−1) | 0.13 ± 0.00 c | 0.14 ± 0.01 b | 0.15 ± 0.00 a | 0.16 ± 0.00 a |
| AP (mg kg−1) | 0.20 ± 0.00 b | 0.23 ± 0.02 b | 0.42 ± 0.03 b | 6.02 ± 0.78 a |
| AK (mg kg−1) | 9.49 ± 0.25 c | 10.52 ± 0.23 b | 11.75 ± 0.58 a | 11.41 ± 0.41 a |
| SOC (g kg−1) | 7.53 ± 0.19 b | 6.64 ± 0.55 c | 7.81 ± 0.50 b | 8.40 ± 0.33 a |
| DOC (mg kg−1) | 342.325 ± 17.48 b | 376.44 ± 33.02 a | 407.88 ± 22.29 a | 408.67 ± 4.90 a |
| MBC (mg kg−1) | 49.22 ± 13.40 b | 52.83 ± 3.63 b | 75.61 ± 9.13 a | 81.93 ± 8.73 a |
| MBN (mg kg−1) | 22.60 ± 4.81 b | 29.00 ± 3.44 ab | 31.90 ± 13.72 a | 32.63 ± 8.08 a 1 |
| Treatment | Shannon Index | Simpson Index | Chao1 Index |
|---|---|---|---|
| CK | 4.46 ± 0.08 a | 0.91 ± 0.01 a | 423.24 ± 19.84 a |
| W | 4.41 ± 0.09 a | 0.91 ± 0.01 a | 424.82 ± 22.67 a |
| F | 3.44 ± 0.12 b | 0.81 ± 0.02 b | 353.36 ± 28.10 b |
| FW | 3.33 ± 0.25 b | 0.81 ± 0.04 b | 342.83 ± 16.36 b 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; He, Q.; Huang, D.; Wang, Z.; Mao, J.; Xie, X.; Su, Y.; Qiu, Q.; Li, J.; Chen, Z. Responses of Fungal Community Structure and Functional Composition to Short-Term Fertilization and Dry Season Irrigation in Eucalyptus urophylla × Eucalyptus grandis Plantation Soils. Forests 2022, 13, 854. https://doi.org/10.3390/f13060854
Gao S, He Q, Huang D, Wang Z, Mao J, Xie X, Su Y, Qiu Q, Li J, Chen Z. Responses of Fungal Community Structure and Functional Composition to Short-Term Fertilization and Dry Season Irrigation in Eucalyptus urophylla × Eucalyptus grandis Plantation Soils. Forests. 2022; 13(6):854. https://doi.org/10.3390/f13060854
Chicago/Turabian StyleGao, Shangkun, Qian He, Di Huang, Zhengmu Wang, Jianhui Mao, Xianan Xie, Yan Su, Quan Qiu, Jiyue Li, and Zujing Chen. 2022. "Responses of Fungal Community Structure and Functional Composition to Short-Term Fertilization and Dry Season Irrigation in Eucalyptus urophylla × Eucalyptus grandis Plantation Soils" Forests 13, no. 6: 854. https://doi.org/10.3390/f13060854






