Influence of Climate and Coastal Flooding on Eastern Red Cedar Growth along a Marsh-Forest Ecotone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Site
2.3. Field Methods
2.4. Laboratory Methods
2.5. Data Analysis
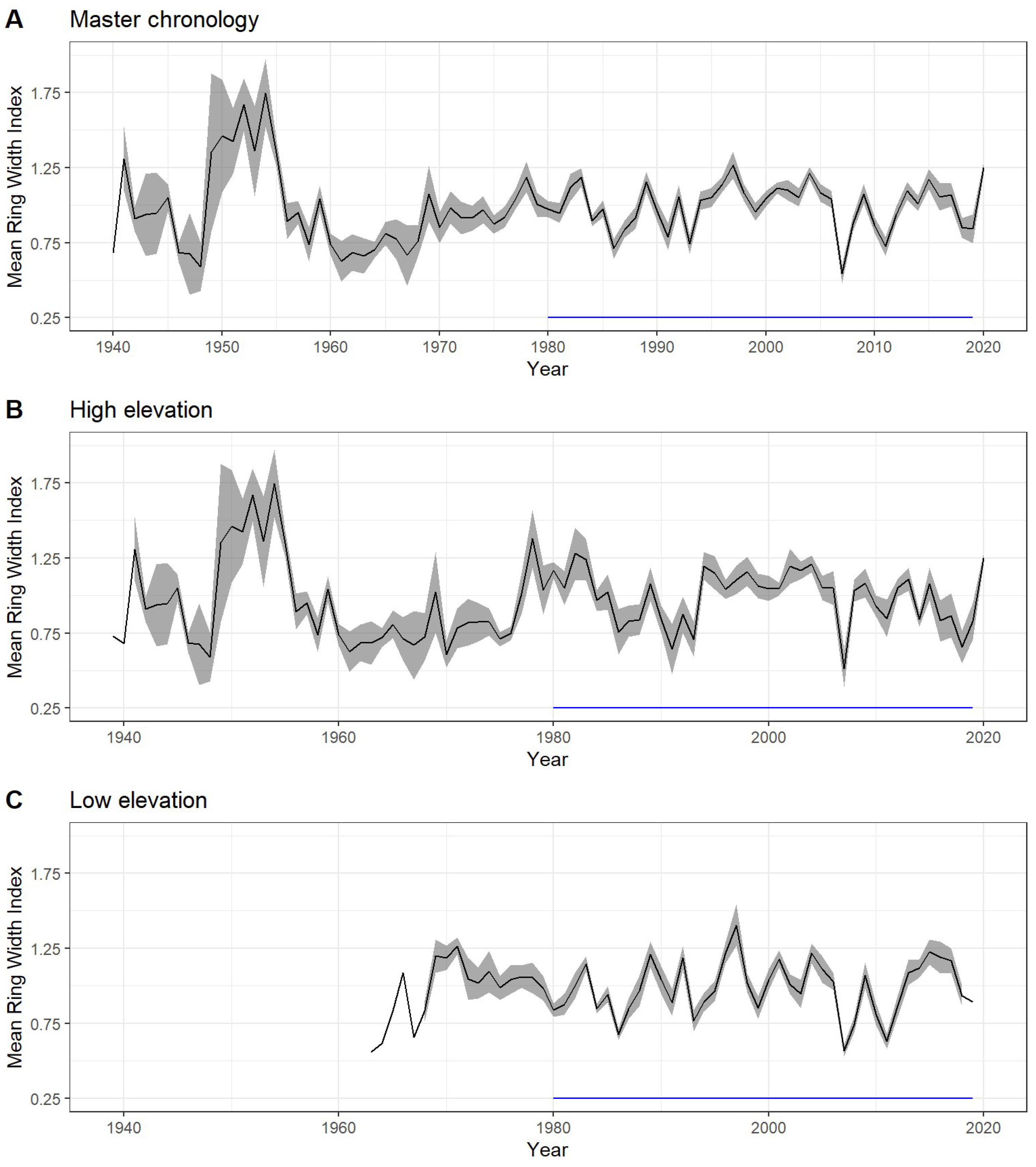
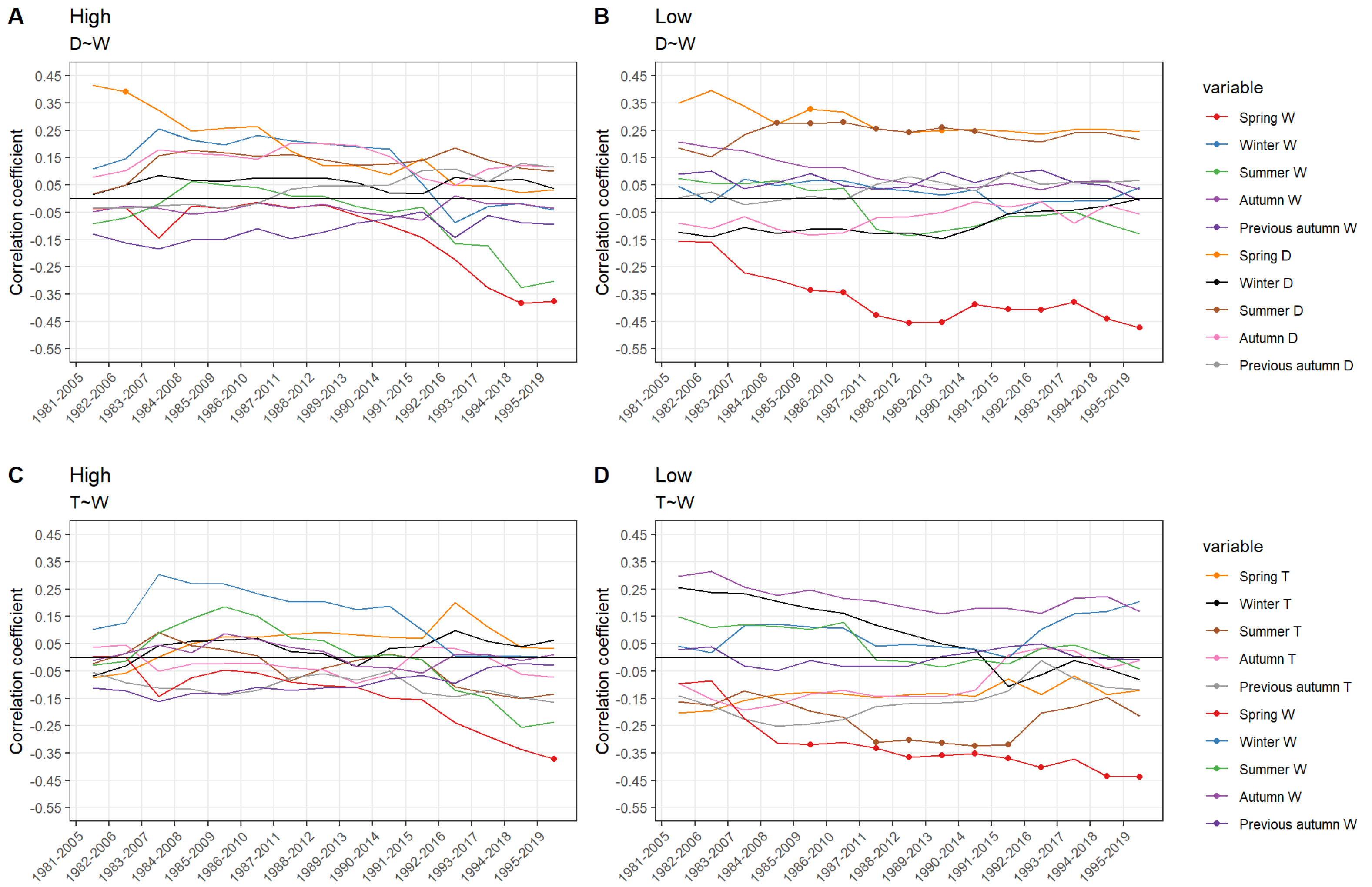
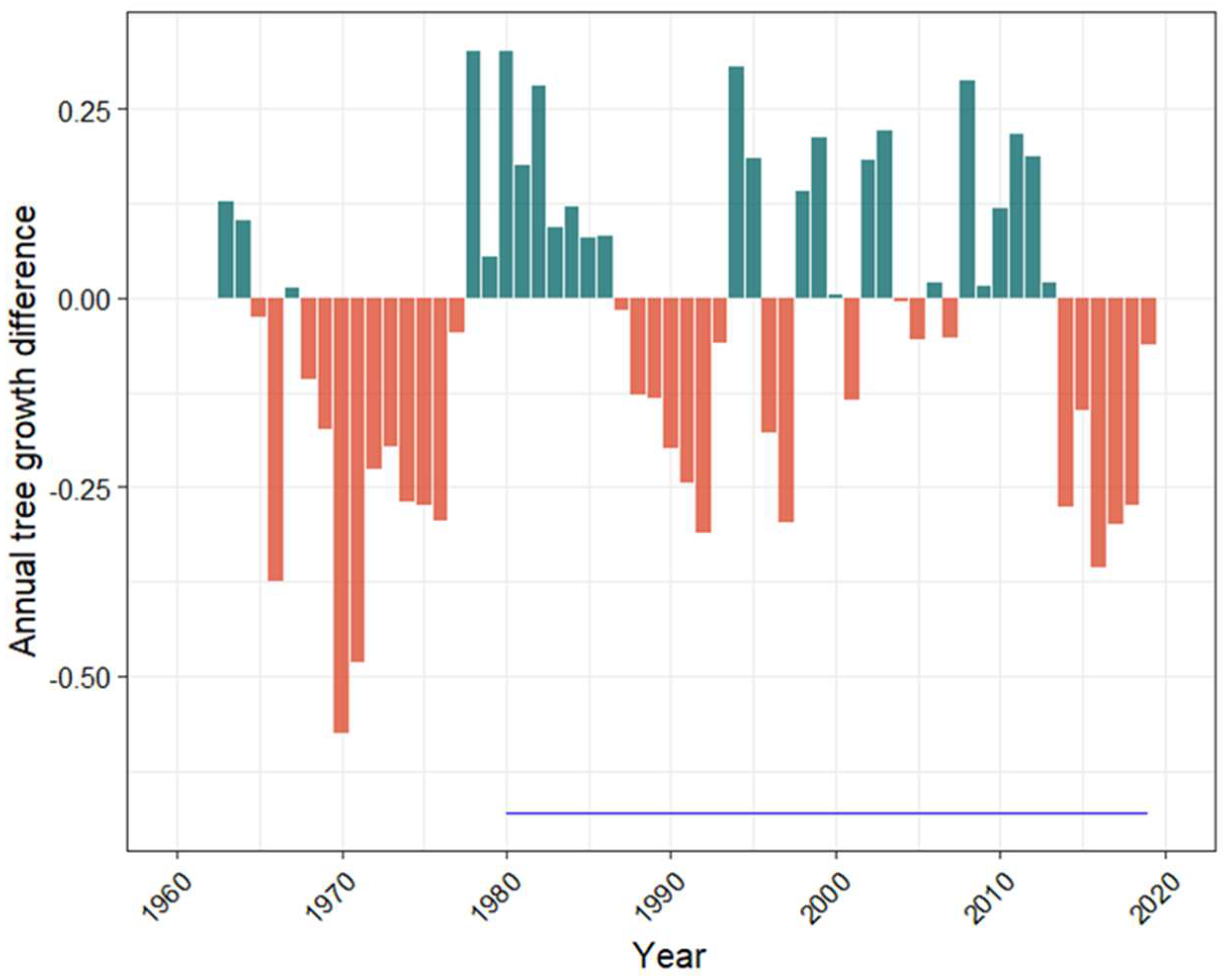
3. Results
4. Discussion
4.1. Flooding and Subsequent Stress along an Elevation Gradient
4.2. Growth Response of Eastern Red Cedar along an Elevation Gradient
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The Value of Estuarine and Coastal Ecosystem Services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Smart, L.S.; Taillie, P.J.; Poulter, B.; Vukomanovic, J.; Singh, K.K.; Swenson, J.J.; Mitasova, H.; Smith, J.W.; Meentemeyer, R.K. Aboveground Carbon Loss Associated with the Spread of Ghost Forests as Sea Levels Rise. Environ. Res. Lett. 2020, 15, 104028. [Google Scholar] [CrossRef]
- Kopp, R.E.; Andrews, C.J.; Garner, A.; Miller, J.A.; Broccoli, A.; Kreeger, D.; Miller, J.K.; Leichenko, R.; Lin, N.; Little, C.M.; et al. New Jersey’s Rising Seas and Changing Coastal Storms: Report of the 2019 Science and Technical Advisory Panel; Rutgers: New Brunswick, NJ, USA, 2019. [Google Scholar]
- Callahan, J.A.; Horton, B.P.; Nikitina, D.L.; Sommerfield, C.K.; McKenna, T.E.; Swallow, D. Recommendation of Sea-Level Rise Planning Scenarios for Delaware: Technical Report, Prepared for Delaware Department of Natural Resources and Environmental Control (DNREC) Delaware Coastal Programs; Delaware Geological Survey: Newark, DE, USA, 2017. [Google Scholar]
- Kirwan, M.L.; Kirwan, J.L.; Copenheaver, C.A. Dynamics of an Estuarine Forest and Its Response to Rising Sea Level. J. Coast. Res. 2007, 232, 457–463. [Google Scholar] [CrossRef]
- Schieder, N.W.; Walters, D.C.; Kirwan, M.L. Massive Upland to Wetland Conversion Compensated for Historical Marsh Loss in Chesapeake Bay, USA. Estuaries Coasts 2018, 41, 940–951. [Google Scholar] [CrossRef]
- Taillie, P.J.; Moorman, C.E.; Poulter, B.; Ardón, M.; Emanuel, R.E. Decadal-Scale Vegetation Change Driven by Salinity at Leading Edge of Rising Sea Level. Ecosystems 2019, 22, 1918–1930. [Google Scholar] [CrossRef]
- Schieder, N.W.; Kirwan, M.L. Sea-Level Driven Acceleration in Coastal Forest Retreat. Geology 2019, 47, 1151–1155. [Google Scholar] [CrossRef]
- Smith, J.A.M. The Role of Phragmites Australis in Mediating Inland Salt Marsh Migration in a Mid-Atlantic Estuary. PLoS ONE 2013, 8, e65091. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.H. Modeling of Sea-Level Rise and Deforestation in Submerging Coastal Ultisols of Chesapeake Bay. Soil Sci. Soc. Am. J. 2009, 73, 185–196. [Google Scholar] [CrossRef]
- Haaf, L.; Dymond, S.F.; Kreeger, D.A. Principal Factors Influencing Tree Growth in Low-Lying Mid Atlantic Coastal Forests. Forests 2021, 12, 1351. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Gedan, K.B. Sea-Level Driven Land Conversion and the Formation of Ghost Forests. Nat. Clim. Chang. 2019, 9, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Desantis, L.R.G.; Bhotika, S.; Williams, K.; Putz, F.E. Sea-Level Rise and Drought Interactions Accelerate Forest Decline on the Gulf Coast of Florida, USA. Glob. Change Biol. 2007, 13, 2349–2360. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Best, G.R.; Coutant, C.C.; Hornberger, G.M.; Meyer, J.L.; Robinson, P.J.; Stenberg, J.R.; Turner, R.E.; Vera-Herrera, F.; Wetzel, R.G. Effects of Climate Change on Freshwater Ecosystems of the South-Eastern United States and the Gulf Coast of Mexico. Hydrol. Processes 1997, 11, 949–970. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A Global Perspective on Wetland Salinization: Ecological Consequences of a Growing Threat to Freshwater Wetlands. Ecosphere 2015, 6, art206. [Google Scholar] [CrossRef]
- Chapman, E.L.; Chambers, J.Q.; Ribbeck, K.F.; Baker, D.B.; Tobler, M.A.; Zeng, H.; White, D.A. Hurricane Katrina Impacts on Forest Trees of Louisiana’s Pearl River Basin. For. Ecol. Manag. 2008, 256, 883–889. [Google Scholar] [CrossRef]
- Middleton, B.A. Differences in Impacts of Hurricane Sandy on Freshwater Swamps on the Delmarva Peninsula, Mid-Atlantic Coast, USA. Ecol. Eng. 2016, 87, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Fagherazzi, S.; Anisfeld, S.C.; Blum, L.K.; Long, E.V.; Feagin, R.A.; Fernandes, A.; Kearney, W.S.; Williams, K. Sea Level Rise and the Dynamics of the Marsh-Upland Boundary. Front. Environ. Sci. 2019, 7, 25. [Google Scholar] [CrossRef]
- Brinson, M.M.; Christian, R.R.; Blum, L.K. Multiple States in the Sea-Level Induced Transition from Terrestrial Forest to Estuary. Estuaries 1995, 18, 648–659. [Google Scholar] [CrossRef]
- Williams, K.; Meads, M.V.; Sauerbrey, D.A. The Roles of Seedling Salt Tolerance and Resprouting in Forest Zonation on Thewest Coast of Florida, USA. Am. J. Bot. 1998, 85, 1745–1752. [Google Scholar] [CrossRef]
- Wendelberger, K.S.; Richards, J.H. Halophytes Can Salinize Soil When Competing with Glycophytes, Intensifying Effects of Sea Level Rise in Coastal Communities. Oecologia 2017, 184, 729–737. [Google Scholar] [CrossRef]
- Williams, K.; Ewel, K.C.; Stumpf, R.P.; Putz, F.E.; Workman, T.W. Sea-Level Rise and Coastal Forest Retreat on the West Coast of Florida, Usa. Ecology 1999, 80, 2045–2063. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbágy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic Regulation of Plant Rooting Depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messerschmidt, T.C.; Langston, A.K.; Kirwan, M.L. Asymmetric Root Distributions Reveal Press–Pulse Responses in Retreating Coastal Forests. Ecology 2021, 102, e03468. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.R.; Michener, W.K.; Williams, T.M.; Blood, E.R.; Kjerve, B.; Smock, L.A.; Lipscomb, D.J.; Gresham, C. Disturbance Effects of Hurricane Hugo on a Pristine Coastal Landscape: North Inlet, South Carolina, USA. Neth. J. Sea Res. 1992, 30, 249–263. [Google Scholar] [CrossRef]
- Sklar, F.H.; Browder, J.A. Coastal Environmental Impacts Brought About by Alterations to Freshwater Flow in the Gulf of Mexico. Environ. Manag. 1998, 22, 547–562. [Google Scholar] [CrossRef]
- Poulter, B.; Goodall, J.L.; Halpin, P.N. Applications of Network Analysis for Adaptive Management of Artificial Drainage Systems in Landscapes Vulnerable to Sea Level Rise. J. Hydrol. 2008, 357, 207–217. [Google Scholar] [CrossRef]
- Stotts, S.; Callahan, J.; Gulledge, O. Impact of Channel Dredging and Straightening in an Atlantic White Cedar (Chamaecyparis Thyoides L. (B.S.P.)) Freshwater Tidal Wetland. J. Coast. Res. 2021, 37, 973–986. [Google Scholar] [CrossRef]
- Krauss, K.W.; Duberstein, J.A.; Doyle, T.W.; Conner, W.H.; Day, R.H.; Inabinette, L.W.; Whitbeck, J.L. Site Condition, Structure, and Growth of Baldcypress along Tidal/Non-Tidal Salinity Gradients. Wetlands 2009, 29, 505–519. [Google Scholar] [CrossRef]
- Martin, D.W.; Young, D.R. Small-Scale Distribution and Salinity Response of Juniperus Virginiana on an Atlantic Coast Barrier Island. Can. J. Bot. 1997, 75, 77–85. [Google Scholar] [CrossRef]
- Little, E.L., Jr. Atlas of United States Trees-Conifers and Important Hardwoods; Miscellaneous Publication 1; United States Department of Agriculture: Washington, DC, USA, 1971.
- Ferguson, E.R.; Lawson, E.R.; Maple, W.R.; Mesavage, C. Managing Eastern Redcedar; US Department of Agriculture Forest Service: New Orleans, LA, USA, 1968.
- Burns, R.M. Silvics of North America: Conifers; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990.
- Johnson, S.R.; Young, D.R. Factors Contributing to the Decline of Pinus Taeda on a Virginia Barrier Island. Bull. Torrey Bot. Club 1993, 120, 431–438. [Google Scholar] [CrossRef]
- Tolliver, K.S.; Martin, D.W.; Young, D.R. Freshwater and Saltwater Flooding Response for Woody Species Common to Barrier Island Swales. Wetlands 1997, 17, 10–18. [Google Scholar] [CrossRef]
- Harper, R.M. The Diverse Habits of the Eastern Red Cedar and Their Interpretation. Torreya 1912, 12, 145–154. [Google Scholar]
- NOAA NCEI Climate at a Glance|National Centers for Environmental Information (NCEI). Available online: https://www.ncdc.noaa.gov/cag/ (accessed on 15 March 2022).
- Delaware Department of Natural Resources and Environmental Control Delaware National Estuarine Research Reserve Estuarine Profile. 1999. Available online: https://coast.noaa.gov/data/docs/nerrs/Reserves_DEL_SiteProfile.pdf (accessed on 11 April 2022).
- The Delaware Geological Survey Elevation Contours of Delaware. 2019. Available online: https://www.dgs.udel.edu/datasets/elevation-contours-delaware (accessed on 11 April 2022).
- Ciecko, L.; Kimmett, D.; Saunders, J.; Katz, R.; Wolf, K.L.; Bazinet, O.; Richardson, J.; Brinkley, W.; Blahna, D.J. Forest Landscape Assessment Tool (FLAT): Rapid Assessment for Land Management; U.S. Department of Agriculture, Forest Service: Portland, OR, USA; Pacific Northwest Research Station: Portland, OR, USA, 2016; p. PNW-GTR-941.
- Hiziroglu, S.; Zhang, D. Impact Assessment and Utilization of Eastern Redcedar. Am. J. Appl. Sci. 2010, 7, 1032–1037. [Google Scholar] [CrossRef]
- Douglas, S. Update on the Eastern Red Cedar Problem. Available online: https://portal.ct.gov/CAES/Fact-Sheets/Plant-Pathology/Update-on-the-Eastern-Red-Cedar-Problem (accessed on 23 March 2022).
- Gilman, E.F.; Watson, D.G. Juniperus Virginiana: Eastern Redcedar. 1993. Available online: https://edis.ifas.ufl.edu/pdf/ST/ST32700.pdf (accessed on 11 April 2022).
- Parker, D.R. How to Take a Soil Sample for Home Lawns and Gardens. 2007. Available online: https://www.udel.edu/content/dam/udelImages/canr/pdfs/extension/environmental-stewardship/Circular-19-How-to-Take-a-Soil-Sample-for-Home-Lawns-and-Gardens.pdf (accessed on 11 April 2022).
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2011; ISBN 978-0-8165-2685-7. [Google Scholar]
- Holmes, Richard Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78.
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C.; Buras, A.; Cecile, J.; Mudelsee, M.; et al. DplR: Dendrochronology Program Library in R. 2021. Available online: https://cran.r-project.org/web/packages/dplR/index.html (accessed on 11 April 2022).
- Cook, E.; Briffa, K.; Shiyatov, S.; Mazepa, V.; Jones, P.D. Methods of Dendrochronology; Springer: Dordrecht, The Netherlands, 1990; pp. 97–162. ISBN 978-0792305866. [Google Scholar]
- Huntington, J.L.; Hegewisch, K.C.; Daudert, B.; Morton, C.G.; Abatzoglou, J.T.; McEvoy, D.J.; Erickson, T. Cloud Computing and Visualization of Climate and Remote Sensing Data for Advanced Natural Resource Monitoring and Process Understanding. Bull. Am. Meteorol. Soc. 2017, 98, 2397–2410. [Google Scholar] [CrossRef]
- NOAA CO-OPS Map—NOAA Tides & Currents. Available online: https://tidesandcurrents.noaa.gov/map/index.html?region=%20NewJersey (accessed on 15 March 2022).
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C.; Buras, A.; Cecile, J.; Mudelsee, M.; et al. DplR: Dendrochronology Program Library in R. 2016. Available online: https://cran.r-project.org/web/packages/dplR/index.html (accessed on 11 April 2022).
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; ISBN 978-0122684500. [Google Scholar]
- Kratsch, H. Boron- and Salt-Tolerant Trees and Shrubs for Northern Nevada; UNCE Special Publication 12-04; University of Nevada, Cooperative Extention: Reno, NV, USA, 2012; Available online: https://www.fs.fed.us/rm/pubs_other/rmrs_2012_kratsch_h001.pdf (accessed on 11 April 2022).
- Edmondson, J.R. The Meteorological Significance of False Rings in Eastern Redcedar (Juniperus Virginiana L.) from the Southern Great Plains, U.S.A. Trre 2010, 66, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Weakly, H.E. A Tree-Ring Record of Precipitation in Western Nebraska. J. For. 1943, 41, 816–819. [Google Scholar] [CrossRef]
- Guyette, R.; McGinnes, E.A.; Probasco, G.E.; Evans, K.E. A Climate History of Boone County, Missouri, From Tree-Ring Analysis of Eastern Redcedar. Wood Fiber Sci. 1980, 12, 17–28. [Google Scholar]
- Butler, D.; Butler, W.S.; David, R.; Walsh, S.J. The Use of Eastern Redcedar in a Tree-Ring Study in Oklahoma. Prairie Nat. 1988, 20, 47–56. [Google Scholar]
- Copenheaver, C.A.; Kyle, K.H.; Stevens, G.N.; Kamp, M.H. Comparing Juniperus Virginiana Tree-Ring Chronologies from Forest Edge vs. Forest Interior Positions in the Cedars Natural Area Preserve in Virginia, USA. Dendrochronologia 2005, 23, 39–45. [Google Scholar] [CrossRef]
- Larson, D.W. Dendroecological Potential of Juniperus Virginiana L. Growing on Cliffs in Western Virginia. Banisteria 1997, 10, 6. [Google Scholar]
- Noe, G.B.; Bourg, N.A.; Krauss, K.W.; Duberstein, J.A.; Hupp, C.R. Watershed and Estuarine Controls Both Influence Plant Community and Tree Growth Changes in Tidal Freshwater Forested Wetlands along Two U.S. Mid-Atlantic Rivers. Forests 2021, 12, 1182. [Google Scholar] [CrossRef]
- Yanosky, T.M.; Hupp, C.R.; Hackney, C.T. Chloride Concentrations in Growth Rings of Taxodium Distichum in a Saltwater-Intruded Estuary. Ecol. Appl. 1995, 5, 785–792. [Google Scholar] [CrossRef]
- Thomas, B.L.; Doyle, T.; Krauss, K. Annual Growth Patterns of Baldcypress (Taxodium Distichum) Along Salinity Gradients. Wetlands 2015, 35, 831–839. [Google Scholar] [CrossRef]
- Rheinhardt, R.D. Tidal Freshwater Swamps of a Lower Chesapeake Bay Subestuary. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 161–182. ISBN 978-1-4020-5095-4. [Google Scholar]
- Anderson, C.J.; Lockaby, B.G. Foliar Nutrient Dynamics in Tidal and Non-Tidal Freshwater Forested Wetlands. Aquat. Bot. 2011, 95, 153–160. [Google Scholar] [CrossRef]
- Noe, G.B.; Krauss, K.W.; Lockaby, B.G.; Conner, W.H.; Hupp, C.R. The Effect of Increasing Salinity and Forest Mortality on Soil Nitrogen and Phosphorus Mineralization in Tidal Freshwater Forested Wetlands. Biogeochemistry 2013, 114, 225–244. [Google Scholar] [CrossRef] [Green Version]
- Weston, N.B.; Dixon, R.E.; Joye, S.B. Ramifications of Increased Salinity in Tidal Freshwater Sediments: Geochemistry and Microbial Pathways of Organic Matter Mineralization. J. Geophys. Res. Biogeosci. 2006, 111, 1–14. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, S.; Stotts, S.; Haaf, L. Influence of Climate and Coastal Flooding on Eastern Red Cedar Growth along a Marsh-Forest Ecotone. Forests 2022, 13, 862. https://doi.org/10.3390/f13060862
Hall S, Stotts S, Haaf L. Influence of Climate and Coastal Flooding on Eastern Red Cedar Growth along a Marsh-Forest Ecotone. Forests. 2022; 13(6):862. https://doi.org/10.3390/f13060862
Chicago/Turabian StyleHall, Sydney, Stephanie Stotts, and LeeAnn Haaf. 2022. "Influence of Climate and Coastal Flooding on Eastern Red Cedar Growth along a Marsh-Forest Ecotone" Forests 13, no. 6: 862. https://doi.org/10.3390/f13060862
APA StyleHall, S., Stotts, S., & Haaf, L. (2022). Influence of Climate and Coastal Flooding on Eastern Red Cedar Growth along a Marsh-Forest Ecotone. Forests, 13(6), 862. https://doi.org/10.3390/f13060862






