Water Uptake and Hormone Modulation Responses to Nitrogen Supply in Populus simonii under PEG-Induced Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of Gas Exchange, Photosynthetic Pigments, and Root Growth
2.3. RWC, MDA Content, and Rate of Superoxide Anion Radical (O2−) Production
2.4. Determination of Soluble Sugars, Soluble Phenolics, and Amino Acid Compounds
2.5. Analysis of Total C, Total N, 13C, and 15N
2.6. Antioxidant Enzymes Activities
2.7. IAA and ABA Contents
2.8. Analysis of Transcript Levels
2.9. Data Processing and Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. RWC, Photosynthesis, MDA, and the Rate of O2− Production
3.3. Soluble Sugars, Soluble Phenolics, and Amino Acid Compounds
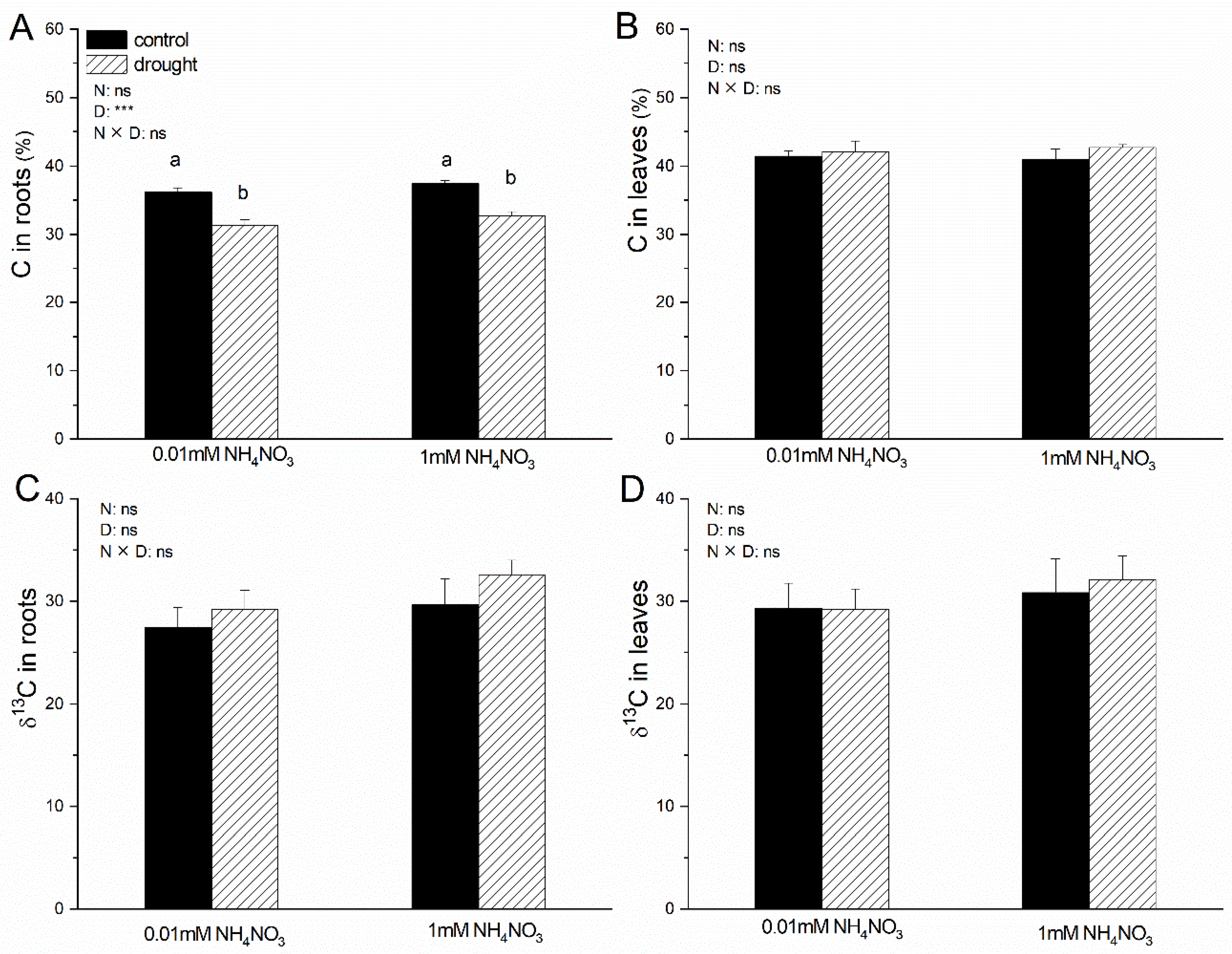
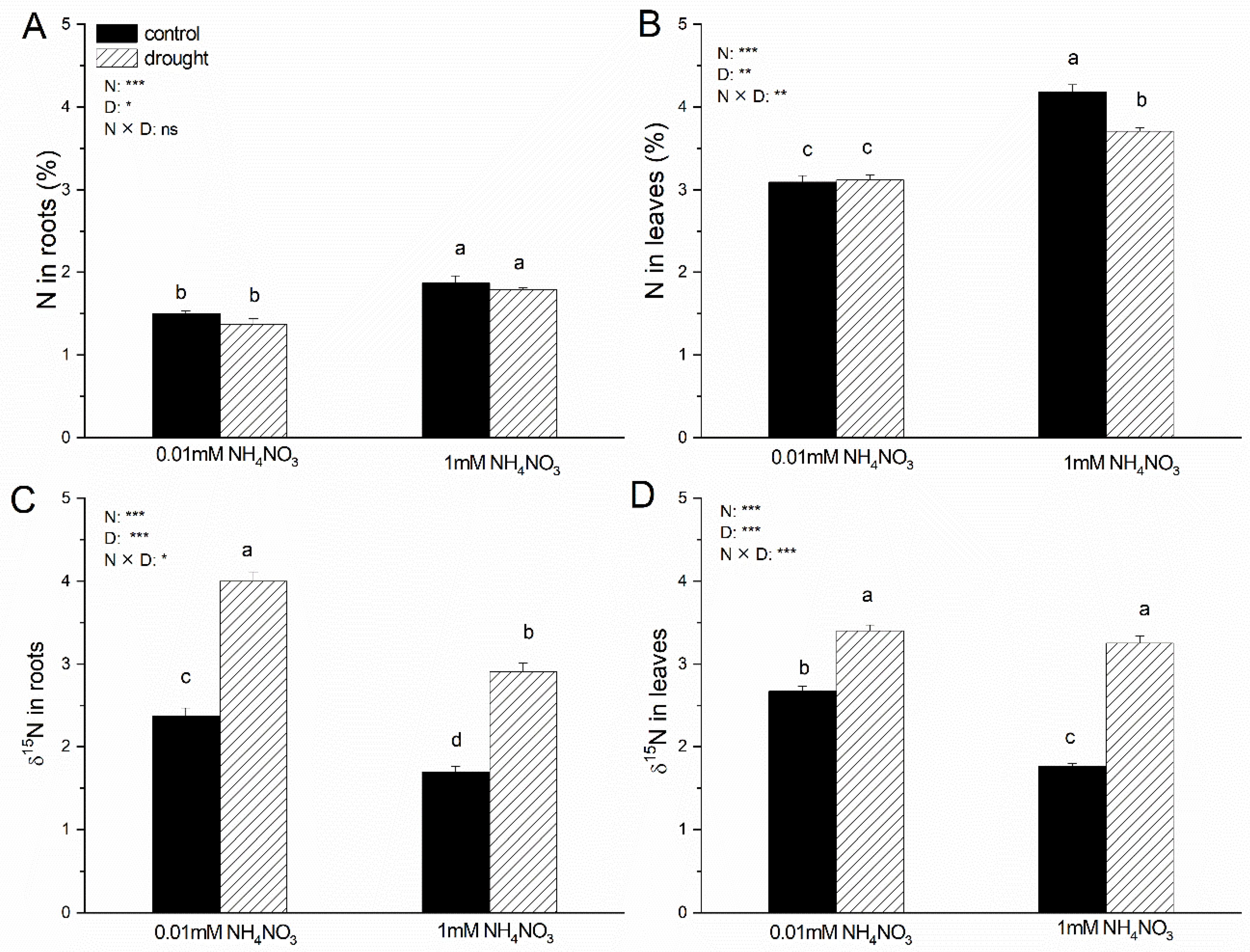
3.4. Total C, Total N, 13C, and 15N
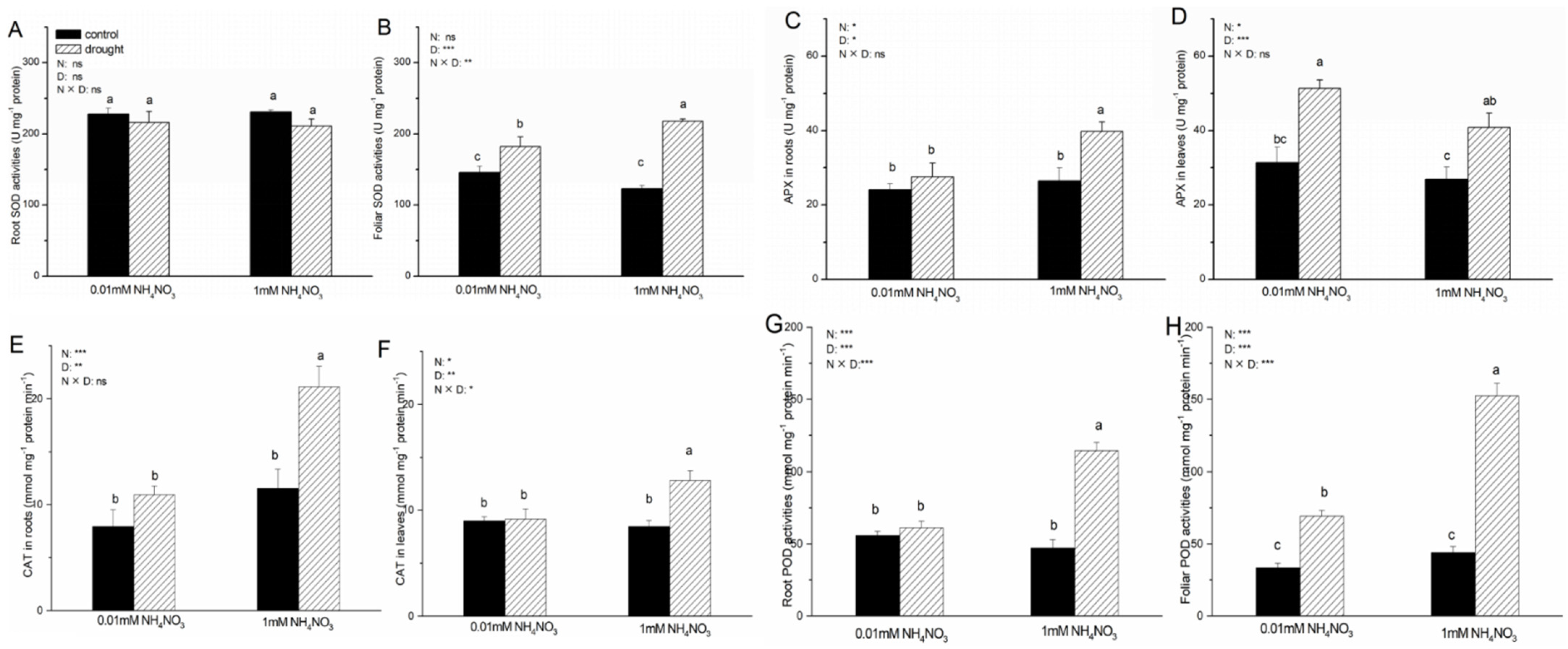
3.5. Antioxidant Enzymes Activities
3.6. IAA and ABA Concentrations
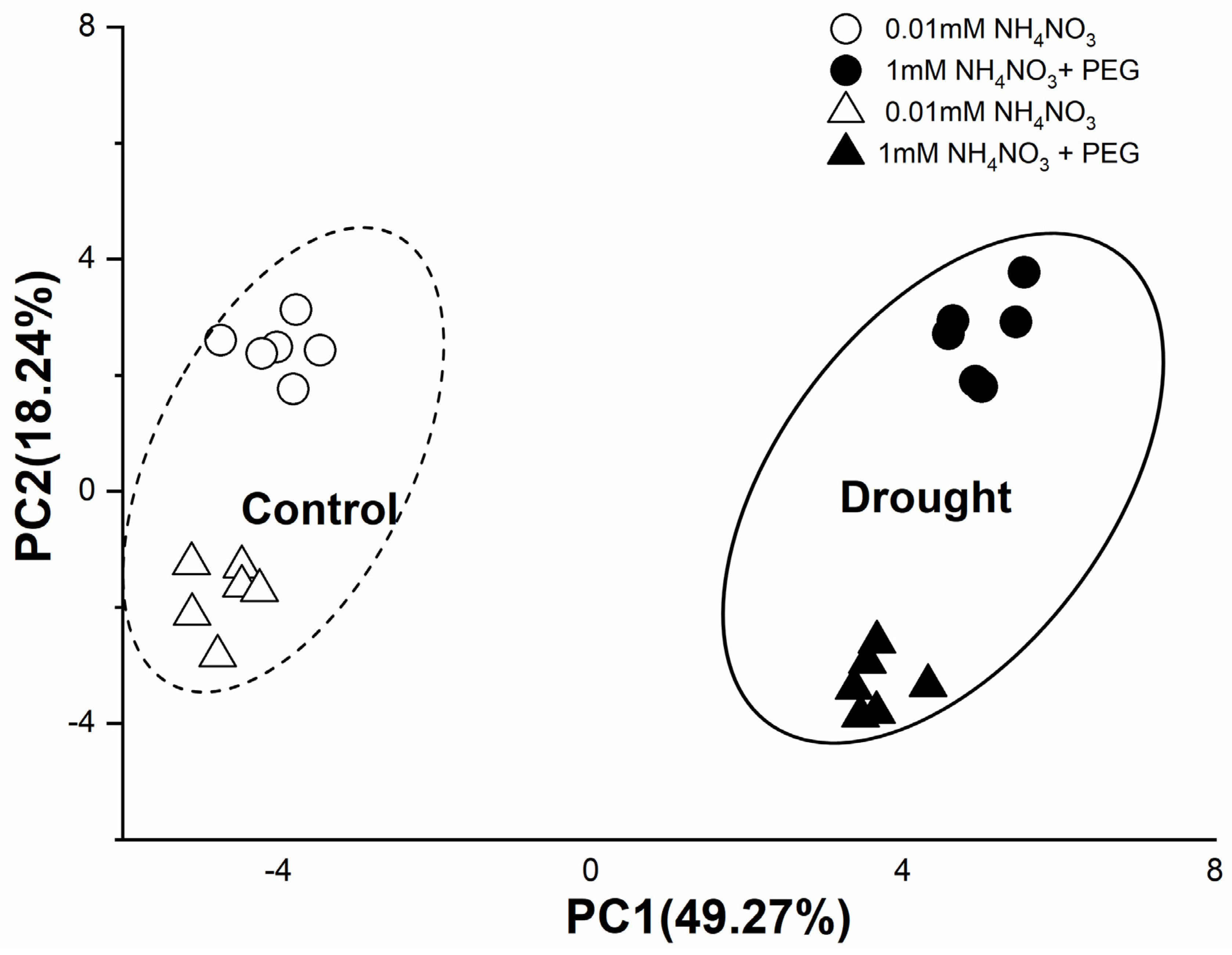
3.7. PCA of Morphological and Physiological Responses
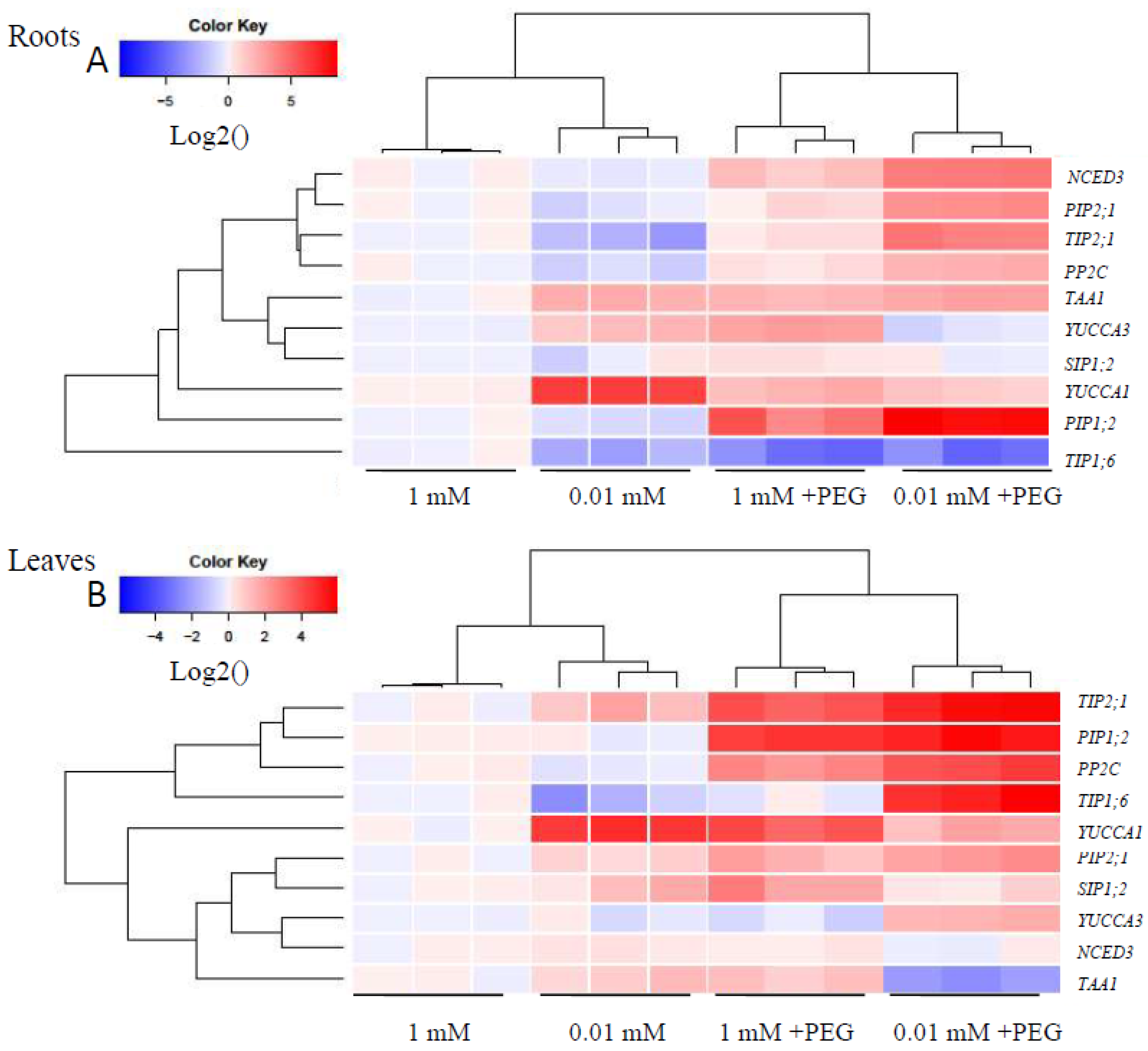
3.8. Transcript Levels of AQPs and Representative Genes Involved in IAA and ABA Signaling Pathways
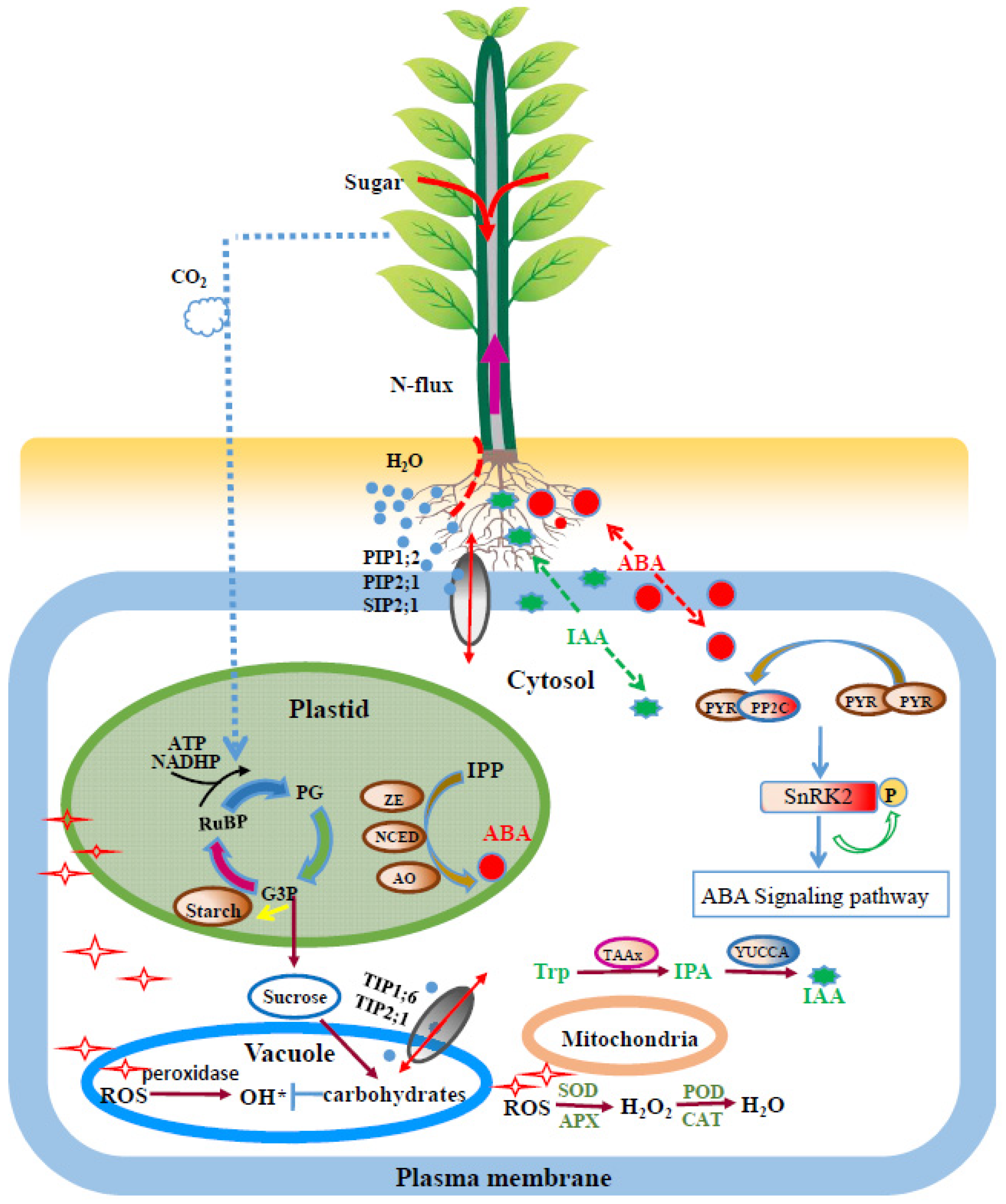
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, J.; Fu, B.; Lu, N.; Zhang, L. Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid Loess Plateau. Sci. Total Environ. 2017, 609, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, C.; Su, L.; Li, Y.; Zhao, Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ. Exp. Bot. 2016, 123, 78–87. [Google Scholar] [CrossRef]
- Keen, R.M.; Voelker, S.L.; Wang, S.S.; Bentz, B.J.; Goulden, M.L.; Dangerfield, C.R.; Reed, C.C.; Hood, S.M.; Csank, A.Z.; Dawson, T.E.; et al. Changes in tree drought sensitivity provided early warning signals to the California drought and forest mortality event. Glob. Chang. Biol. 2022, 28, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, D.; Liu, Y.; Xi, Y.; Wang, X.; Meng, S. Analysis of Populus glycosyl hydrolase family I members and their potential role in the ABA treatment and drought stress response. Plant Physiol. Biochem. 2021, 163, 178–188. [Google Scholar] [CrossRef]
- Soon, F.F.; Ng, L.M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Jia, J.; Zhang, C.; Li, H.; Liu, T.; Jiang, X.; Polle, A.; Peng, C.; Luo, Z.B. Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2012, 151, 480–494. [Google Scholar] [CrossRef]
- Lu, M.; Chen, M.; Song, J.; Wang, Y.; Pan, Y.; Wang, C. Anatomy and transcriptome analysis in leaves revealed how nitrogen (N) availability influence drought acclimation of populus. Trees 2019, 33, 1003–1014. [Google Scholar] [CrossRef]
- Meng, S.; Cao, Y.; Li, H.; Bian, Z.; Wang, D.; Lian, C.; Yin, W.; Xia, X. PeSHN1 regulates water-use efficiency and drought tolerance by modulating wax biosynthesis in poplar. Tree Physiol. 2019, 39, 1371–1386. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, X.; Lu, J.; Wang, D.; Zhou, Y.; Liu, Y.; Wang, S.; Yu, Z.; Xu, D.; Meng, S. The yellowhorn AGL transcription factor gene XsAGL22 contributes to ABA biosynthesis and drought tolerance in poplar. Tree Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 2013, 64, 4207–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.S.M.A.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Popko, J.; Hänsch, R.; Mendel, R.R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef]
- Pawłowicz, I.; Masajada, K. Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants. Gene 2019, 687, 166–172. [Google Scholar] [CrossRef]
- Danielson, J.Å.; Johanson, U. Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol. 2008, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Wang, G.; Yang, L.; Sun, X. Ecophysiological responses of Abies fabri seedlings to drought stress and nitrogen supply. Physiol. Plant. 2010, 139, 335–347. [Google Scholar] [PubMed]
- Meng, S.; Su, L.; Li, Y.; Wang, Y.; Zhang, C.; Zhao, Z. Nitrate and ammonium contribute to the distinct nitrogen metabolism of Populus simonii during moderate salt stress. PLoS ONE 2016, 11, e0150354. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, S.; Peterson, C.; Runion, G.B.; Prior, S.; Rogers, H. Atmospheric CO2 concentration, N availability, and water status affect patterns of ergastic substance deposition in longleaf pine (Pinus palustris Mill.) foliage. Trees 1997, 11, 494–503. [Google Scholar] [CrossRef]
- Ehlting, B.; Dluzniewska, P.; Dietrich, H.; Selle, A.; Teuber, M.; Hänsch, R.; Nehls, U.; Polle, A.; Schnitzler, J.P.; Rennenberg, H.; et al. Interaction of nitrogen nutrition and salinity in Grey poplar (Populus tremula× alba). Plant Cell Environ. 2007, 30, 796–811. [Google Scholar] [CrossRef]
- De Diego, N.; Rodríguez, J.L.; Dodd, I.C.; Pérez-Alfocea, F.; Moncaleán, P.; Lacuesta, M. Immunolocalization of IAA and ABA in roots and needles of radiata pine (Pinus radiata) during drought and rewatering. Tree Physiol. 2013, 33, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Brunner, A.M.; Yakovlev, I.A.; Strauss, S.H. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana Lamb. seedlings. Forests 2019, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Ma, H.B.; Li, Z.S.; Yang, F.C.; Wang, S.K.; Lu, J.K. Impacts of nitrogen on physiological interactions of the hemiparasitic Santalum album and its N2-fixing host Dalbergia odorifera. Trees 2021, 35, 1039–1051. [Google Scholar] [CrossRef]
- Álvarez, S.; Navarro, A.; Bañón, S.; Sánchez-Blanco, M.J. Regulated deficit irrigation in potted Dianthus plants: Effects of severe and moderate water stress on growth and physiological responses. Sci. Hortic. 2009, 122, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Yin, C.; Li, C. Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol. Plant. 2006, 127, 182–191. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C.; Liu, Q.; Lin, B.; Wang, J. Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol. Plant. 2008, 30, 433–440. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.D.; Harish Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent Developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Ottow, E.A.; Brinker, M.; Teichmann, T.; Fritz, E.; Kaiser, W.; Brosché, M.; Kangasjärvi, J.; Jiang, X.; Polle, A. Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol. 2005, 139, 1762–1772. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shao, L.; Sun, Z.; Huang, Y.; Liu, N. An atmospheric pollutant (inorganic nitrogen) alters the response of evergreen broad-leaved tree species to extreme drought. Ecotoxicol. Environ. Saf. 2020, 187, 109750. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Addo-Danso, S.D.; Ding, G.; Sun, M.; Wu, S.; Lin, S. Nitrogen supply enhances the physiological resistance of Chinese fir plantlets under polyethylene glycol (PEG)-induced drought stress. Sci. Rep. 2020, 10, 7509. [Google Scholar] [CrossRef]
- Saneoka, H.; Moghaieb RE, A.; Premachandra, G.S.; Fujita, K. Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ. Exp. Bot. 2004, 52, 131–138. [Google Scholar] [CrossRef]
- Gou, W.; Zheng, P.; Tian, L.; Gao, M.; Zhang, L.; Akram, N.A.; Ashraf, M. Exogenous application of urea and a urease inhibitor improves drought stress tolerance in maize (Zea mays L.). J. Plant Res. 2017, 130, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Nabi RB, S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Indoliya, Y.; Agrawal, L.; Awasthi, S.; Deeba, F.; Dwivedi, S.; Chakrabarty DShirke, P.; Pandey, V.; Singh, N.; Dhankher Om, P.; et al. Genomic and proteomic responses to drought stress and biotechnological interventions for enhanced drought tolerance in plants. Curr. Plant Biol. 2022, 29, 100239. [Google Scholar] [CrossRef]
- Warren, C.R.; Aranda, I.; Cano, F.J. Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant Cell Environ. 2011, 34, 1609–1629. [Google Scholar] [CrossRef]
- Guimarães, G.F.; Gorni, P.H.; Vitolo, H.F.; Carvalho ME, A.; Pacheco, A.C. Sweetpotato tolerance to drought is associated to leaf concentration of total chlorophylls and polyphenols. Theor. Exp. Plant Physiol. 2021, 33, 385–396. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Amitrano, C.; Arena, C.; Rouphael, Y.; De Pascale, S.; De Micco, V. Vapour pressure deficit: The hidden driver behind plant morphofunctional traits in controlled environments. Ann. Appl. Biol. 2019, 175, 313–325. [Google Scholar] [CrossRef]
- Fichot, R.; Laurans, F.; Monclus, R.; Moreau, A.; Pilate, G.; Brignolas, F. Xylem anatomy correlates with gas exchange, water-use efficiency and growth performance under contrasting water regimes: Evidence from Populus deltoides × Populus nigra hybrids. Tree Physiol. 2009, 29, 1537–1549. [Google Scholar] [CrossRef]
- Meng, S.; Wang, S.; Quan, J.; Su, W.; Lian, C.; Wang, D.; Xia, X.; Yin, W. Distinct carbon and nitrogen metabolism of two contrasting poplar species in response to different N supply levels. Int. J. Mol. Sci. 2018, 19, 2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, D.; Bogeat-Triboulot, M.B.; Vialet-Chabrand, S.; Merret, R.; Courty, P.E.; Moretti, S.; Bizet, F.; Guilliot, A.; Hummel, I. Developmental and environmental regulation of Aquaporin gene expression across Populus species: Divergence or redundancy? PLoS ONE 2013, 8, e55506. [Google Scholar] [CrossRef] [PubMed]
- Muries, B.; Mom, R.; Benoit, P.; Brunel-Michac, N.; Cochard, H.; Drevet, P.; Petel, G.; Badel, E.; Fumanal, B.; Gousset-dupont, A.; et al. Aquaporins and water control in drought-stressed poplar leaves: A glimpse into the extraxylem vascular territories. Environ. Exp. Bot. 2019, 162, 25–37. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Huang, Y.; Li, Y.; Su, C.; Zeng, X. EuPIP1;2, a plasma membrane aquaporin gene from Eucommia ulmoides, enhances drought and salt tolerance in transgenic tobacco. Agronomy 2022, 12, 615. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, H.; Lee, J.S.; Noh, E.W. Drought, salt and wounding stress induce the expression of the plasma membrane intrinsic protein 1 gene in poplar (Populus alba × P. tremula var. glandulosa). Gene 2011, 483, 43–48. [Google Scholar] [CrossRef]
- Grabov, A.; Blatt, M.R. Co-ordination of signalling elements in guard cell ion channel control. J. Exp. Bot. 1998, 49, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Lohse, G.; Hedrich, R. Characterization of the plasma-membrane H+-ATPase from Vicia faba guard cells. Planta 1992, 188, 206–214. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Pan, Y.; Pang, J.; Zhang, X.; Fan, J.; Zhang, Y. The influence of nitrogen availability on anatomical and physiological responses of Populus alba× P. glandulosa to drought stress. BMC Plant Biol. 2019, 19, 63. [Google Scholar] [CrossRef] [Green Version]
- Mubarik, M.S.; Khan, S.H.; Sajjad, M.; Raza, A.; Hafeez, M.B.; Yasmeen, T.; Rizwan, M.; Ali, S.; Arif, M.S. A manipulative interplay between positive and negative regulators of phytohormones: A way forward for improving drought tolerance in plants. Physiol. Plant. 2021, 172, 1269–1290. [Google Scholar] [CrossRef]
- Uc-Chuc, M.A.; Pérez-Hernández, C.; Galaz-Ávalos, R.M.; Brito-Argaez, L.; Aguilar-Hernández, V.; Loyola-Vargas, V.M. YUCCA-mediated biosynthesis of the auxin IAA is required during the somatic embryogenic induction process in Coffea canephora. Int. J. Mol. Sci. 2020, 21, 4751. [Google Scholar] [CrossRef]
- Brunetti, C.; Savi, T.; Nardini, A.; Loreto, F.; Gori, A.; Centritto, M. Changes in abscisic acid content during and after drought are related to carbohydrate mobilization and hydraulic recovery in poplar stems. Tree Physiol. 2020, 40, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Nong, Q.; Lin, L.; Xie, J.; Mo, Z.; Huang, X.; Song, X.; Malviya, M.K.; Solanki, M.K.; et al. Physiological changes and transcriptome profiling in Saccharum spontaneum L. leaf under water stress and re-watering conditions. Sci. Rep. 2021, 11, 5525. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Resco de Dios, V.; Wang, D.; Zhao, N.; Huang, G.; Liu, W.; Wu, J.; Zhou, S.; Choat, B.; Tissue, D.T. Testing the limits of plant drought stress and subsequent recovery in four provenances of a widely distributed subtropical tree species. Plant Cell Environ. 2022, 45, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986, 82, 1169–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekmekci, Y.; Terzioglu, S. Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic. Biochem. Physiol. 2005, 83, 69–81. [Google Scholar] [CrossRef]
- He, J.L.; Qin, J.J.; Long, L.Y.; Ma, Y.L.; Li, H.; Li, K.; Jiang, X.N.; Liu, T.X.; Polle, A.; Liang, Z.S.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus x canescens. Physiol. Plant. 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]



| N Supply (mM) | Drought Treatment | RWC (%) | A (μmol CO2 m−2 s−1) | gs (mmol m−2 s−1) | E (mmol H2O m−2 s−1) | WUEi (μmol CO2 mol−1 H2O) | MDA in Roots (μmol g−1 FW) | MDA in Leaves (μmol g−1 FW) | O2− in Roots (nmol g−1 FW min−1) | O2− in Leaves (nmol g−1 FW min−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | Control | 88.65 ± 0.95a | 11.9 ± 0.4a | 221.67 ± 9.10a | 4.85 ± 0.15a | 53.87 ± 2.51b | 12.18 ± 1.90b | 11.22 ± 1.47c | 0.11 ± 0.02a | 0.04 ± 0.01b |
| Drought | 77.57 ± 1.34b | 2.7 ± 0.2c | 33.33 ± 4.22b | 1.28 ± 0.16b | 83.54 ± 6.44a | 35.75 ± 3.65a | 28.62 ± 2.12a | 0.12 ± 0.01a | 0.14 ± 0.02a | |
| 1 | Control | 90.41 ± 1.29a | 9.8 ± 0.2b | 255.00 ± 9.92a | 4.97 ± 0.19a | 38.48 ± 1.63bc | 15.23 ± 3.09b | 15.57 ± 1.07bc | 0.14 ± 0.02a | 0.04 ± 0.01b |
| Drought | 80.01 ± 1.10b | 2.4 ± 0.4c | 71.67 ± 7.49b | 0.26 ± 0.02c | 33.60 ± 4.77c | 30.32 ± 2.08a | 20.63 ± 1.65b | 0.12 ± 0.01a | 0.06 ± 0.00b | |
| p-values | N | ns | * | *** | ** | *** | ns | ns | ns | ** |
| D | *** | *** | *** | *** | ** | *** | *** | ns | *** | |
| N × D | ns | ns | ns | ** | *** | ns | ** | ns | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, X.; Liu, Y.; Zhou, Y.; Qian, Z.; Yu, Z.; Wu, N.; Bian, Z. Water Uptake and Hormone Modulation Responses to Nitrogen Supply in Populus simonii under PEG-Induced Drought Stress. Forests 2022, 13, 907. https://doi.org/10.3390/f13060907
Li Z, Wang X, Liu Y, Zhou Y, Qian Z, Yu Z, Wu N, Bian Z. Water Uptake and Hormone Modulation Responses to Nitrogen Supply in Populus simonii under PEG-Induced Drought Stress. Forests. 2022; 13(6):907. https://doi.org/10.3390/f13060907
Chicago/Turabian StyleLi, Zhen, Xiaoling Wang, Yunshan Liu, Yangyan Zhou, Zhiliang Qian, Zequn Yu, Na Wu, and Zhan Bian. 2022. "Water Uptake and Hormone Modulation Responses to Nitrogen Supply in Populus simonii under PEG-Induced Drought Stress" Forests 13, no. 6: 907. https://doi.org/10.3390/f13060907






