Effects of Habitat Differences on Microbial Communities during Litter Decomposing in a Subtropical Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Foliar Litter Decomposition Experiment
2.3. Monitoring Environmental Conditions
2.4. PLFA Analysis
2.5. Data Analysis
3. Results
3.1. Microbial Total Biomass and Community Composition
3.2. Distribution Patterns of PLFA
3.3. Individual PLFA Analysis
3.4. PLFA Biomass and Litter Decomposition
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hatton, P.J.; Castanha, C.; Torn, M.S.; Bird, J.A. Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob. Change Biol. 2015, 21, 1358–1367. [Google Scholar] [CrossRef]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Zhao, B.; Xing, P.; Wu, Q.L. Interactions between bacteria and fungi in macrophyte leaf litter decomposition. Environ. Microbiol. 2021, 23, 1130–1144. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Palacios, P.; Shaw, E.A.; Wall, D.H.; Hattenschwiler, S. Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol. Lett. 2016, 19, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Graca, M.A.S.; Ferreira, V.; Canhoto, C.; Encalada, A.C.; Guerrero-Bolano, F.; Wantzen, K.M.; Boyero, L. A conceptual model of litter breakdown in low order streams. Int. Rev. Hydrobiol. 2015, 100, 1–12. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Yue, K.; Garcia-Palacios, P.; Parsons, S.A.; Yang, W.; Peng, Y.; Tan, B.; Huang, C.; Wu, F. Assessing the temporal dynamics of aquatic and terrestrial litter decomposition in an alpine forest. Funct. Ecol. 2018, 32, 2464–2475. [Google Scholar] [CrossRef]
- Joly, F.X.; Kurupas, K.L.; Throop, H.L. Pulse frequency and soil-litter mixing alter the control of cumulative precipitation over litter decomposition. Ecology 2017, 98, 2255–2260. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.A.; Warren, R.J.; Baldrian, P.; Crowther, T.W.; Maynard, D.S.; Oldfield, E.E.; Wieder, W.R.; Wood, S.A.; King, J.R. Climate fails to predict wood decomposition at regional scales. Nat. Clim. Change 2014, 4, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Shumilova, O.; Zak, D.; Datry, T.; von Schiller, D.; Corti, R.; Foulquier, A.; Obrador, B.; Tockner, K.; Allan, D.C.; Altermatt, F.; et al. Simulating rewetting events in intermittent rivers and ephemeral streams: A global analysis of leached nutrients and organic matter. Glob. Change Biol. 2019, 25, 1591–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenoy, E.; Pradhan, A.; Pascoal, C.; Rubio-Rios, J.; Batista, D.; Moyano-Lopez, F.J.; Cassio, F.; Casas, J.J. Elevated temperature may reduce functional but not taxonomic diversity of fungal assemblages on decomposing leaf litter in streams. Glob. Change Biol. 2022, 28, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, K.A. Lentic and lotic habitats as templets for fungal communities: Traits, adaptations, and their significance to litter decomposition within freshwater ecosystems. Fungal Ecol. 2016, 19, 135–154. [Google Scholar] [CrossRef] [Green Version]
- Larned, S.T.; Datry, T.; Arscott, D.B.; Tockner, K. Emerging concepts in temporary-river ecology. Freshw. Biol. 2010, 55, 717–738. [Google Scholar] [CrossRef]
- Febria, C.M.; Hosen, J.D.; Crump, B.C.; Palmer, M.A.; Williams, D.D. Microbial responses to changes in flow status in temporary headwater streams: A cross-system comparison. Front. Microbiol. 2015, 6, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Gomez, J.; Duarte, S.; Cassio, F.; Pascoal, C.; Romani, A.M. Microbial decomposition is highly sensitive to leaf litter emersion in a permanent temperate stream. Sci. Total Environ. 2018, 621, 486–496. [Google Scholar] [CrossRef]
- Schlief, J.; Mutz, M. Leaf Decay Processes during and after a Supra-Seasonal Hydrological Drought in a Temperate Lowland Stream. Int. Rev. Hydrobiol. 2011, 96, 633–655. [Google Scholar] [CrossRef]
- Willers, C.; van Rensburg, P.J.J.; Claassens, S. Phospholipid fatty acid profiling of microbial communities-a review of interpretations and recent applications. J. Appl. Microbiol. 2015, 119, 1207–1218. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Elliott, G.N.; Graham, K.J.; Scow, K.M. Comparison of phospholipid fatty acid (PLFA) and total soil fatty acid methyl esters (TSFAME) for characterizing soil microbial communities. Soil Biol. Biochem. 2004, 36, 1793–1800. [Google Scholar] [CrossRef]
- Yu, G.R.; Chen, Z.; Piao, S.L.; Peng, C.H.; Ciais, P.; Wang, Q.F.; Li, X.R.; Zhu, X.J. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardgett, R.D.; Walker, L.R. Impact of coloniser plant species on the development of decomposer microbial communities following deglaciation. Soil Biol. Biochem. 2004, 36, 555–559. [Google Scholar] [CrossRef]
- Tonin, A.M.; Goncalves, J.F., Jr.; Bambi, P.; Couceiro, S.R.M.; Feitoza, L.A.M.; Fontana, L.E.; Hamada, N.; Hepp, L.U.; Lezan-Kowalczuk, V.G.; Leite, G.F.M.; et al. Plant litter dynamics in the forest-stream interface: Precipitation is a major control across tropical biomes. Sci. Rep. 2017, 7, 10799. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wu, F.; Yang, W.; Tan, B.; He, W. Variations in bacterial communities during foliar litter decomposition in the winter and growing seasons in an alpine forest of the eastern Tibetan Plateau. Can. J. Microbiol. 2016, 62, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Zelles, L.; Bai, Q.Y. Fractionation of fatty acids derived from soil lipids by solid phase extraction and their quantitative analysis by GC-MS. Soil Biol. Biochem. 1993, 25, 495–507. [Google Scholar] [CrossRef]
- Otaki, M.; Takeuchi, F.; Tsuyuzaki, S. Changes in microbial community composition in the leaf litter of successional communities after volcanic eruptions of Mount Usu, northern Japan. J. Mt. Sci. 2016, 13, 1652–1662. [Google Scholar] [CrossRef] [Green Version]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hattenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.A.; Campany, C.E.; Classen, A.T. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Foulquier, A.; Artigas, J.; Pesce, S.; Datry, T. Drying responses of microbial litter decomposition and associated fungal and bacterial communities are not affected by emersion frequency. Freshw. Sci. 2015, 34, 1233–1244. [Google Scholar] [CrossRef]
- Arnstadt, T.; Hoppe, B.; Kahl, T.; Kellner, H.; Krüger, D.; Bauhus, J.; Hofrichter, M. Dynamics of fungal community composition, decomposition and resulting deadwood properties in logs of Fagus sylvatica, Picea abies and Pinus sylvestris. For. Ecol. Manag. 2016, 382, 129–142. [Google Scholar] [CrossRef]
- Marks, J.C. Revisiting the Fates of Dead Leaves That Fall into Streams. In Annual Review of Ecology, Evolution, and Systematics; Futuyma, D.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2019; Volume 50, pp. 547–568. [Google Scholar]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanova, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Bird, J.A.; Herman, D.J.; Firestone, M.K. Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil Biol. Biochem. 2011, 43, 718–725. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Hogberg, M.N.; Hogbom, L.; Kleja, D.B. Soil microbial community indices as predictors of soil solution chemistry and N leaching in Picea abies (L.) Karst. forests in S. Sweden. Plant Soil 2013, 372, 507–522. [Google Scholar] [CrossRef]
- Bragazza, L.; Bardgett, R.D.; Mitchell, E.; Buttler, A. Linking soil microbial communities to vascular plant abundance along a climate gradient. New Phytol. 2015, 205, 1175–1182. [Google Scholar] [CrossRef]
- Artigas, J.; Gaudes, A.; Munoz, I.; Romani, A.M.; Sabater, S. Fungal and Bacterial Colonization of Submerged Leaf Litter in a Mediterranean Stream. Int. Rev. Hydrobiol. 2011, 96, 221–234. [Google Scholar] [CrossRef]
- Duarte, S.; Pascoal, C.; Alves, A.; Correia, A.; Cassio, F. Assessing the dynamic of microbial communities during leaf decomposition in a low-order stream by microscopic and molecular techniques. Microbiol. Res. 2010, 165, 351–362. [Google Scholar] [CrossRef]
- Otaki, M.; Tsuyuzaki, S. Succession of litter-decomposing microbial organisms in deciduous birch and oak forests, northern Japan. Acta Oecologica 2019, 101, 103485. [Google Scholar] [CrossRef]
- Wilkinson, S.C.; Anderson, J.M.; Scardelis, S.P.; Tisiafouli, M.; Taylor, A.; Wolters, V. PLFA profiles of microbial communities in decomposing conifer litters subject to moisture stress. Soil Biol. Biochem. 2002, 34, 189–200. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Chen, Y.M.; Gao, J.T.; Xiong, D.C.; Yuan, P.; Zeng, X.M.; Xie, J.S.; Yang, Y.S. Effects of soil warming on soil microbial community structure and soil available nitrogen in subtropical young Chinese fir plantation. J. Subtropic. Resour. Environ. 2016, 11, 1–8. [Google Scholar]
- Penuelas, J.; Rico, L.; Ogaya, R.; Jump, A.S.; Terradas, J. Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biol. 2012, 14, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Huang, L.M.; Lin, Z.C.; Cheng, G.S. Controlling factors of litter decomposition rate in China’s forests. J. Subtropic. Resour. Environ. 2010, 5, 56–63. [Google Scholar]
- Herzog, C.; Hartmann, M.; Frey, B.; Stierli, B.; Rumpel, C.; Buchmann, N.; Brunner, I. Microbial succession on decomposing root litter in a drought-prone Scots pine forest. ISME J. 2019, 13, 2346–2362. [Google Scholar] [CrossRef] [Green Version]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. Fems Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.A.; Veen, G.F.; Bonis, A.; Bradford, E.M.; Classen, A.T.; Cornelissen, J.H.C.; Crowther, T.W.; De Long, J.R.; Freschet, G.T.; Kardol, P.; et al. A test of the hierarchical model of litter decomposition. Nat. Ecol. Evol. 2017, 1, 1836. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhou, W.; Hu, W.; Hu, J.; Hu, M. Changes in litter traits induced by vegetation restoration accelerate litter decomposition in Robinia pseudoacacia plantations. Land Degrad. Dev. 2022, 33, 179–192. [Google Scholar] [CrossRef]

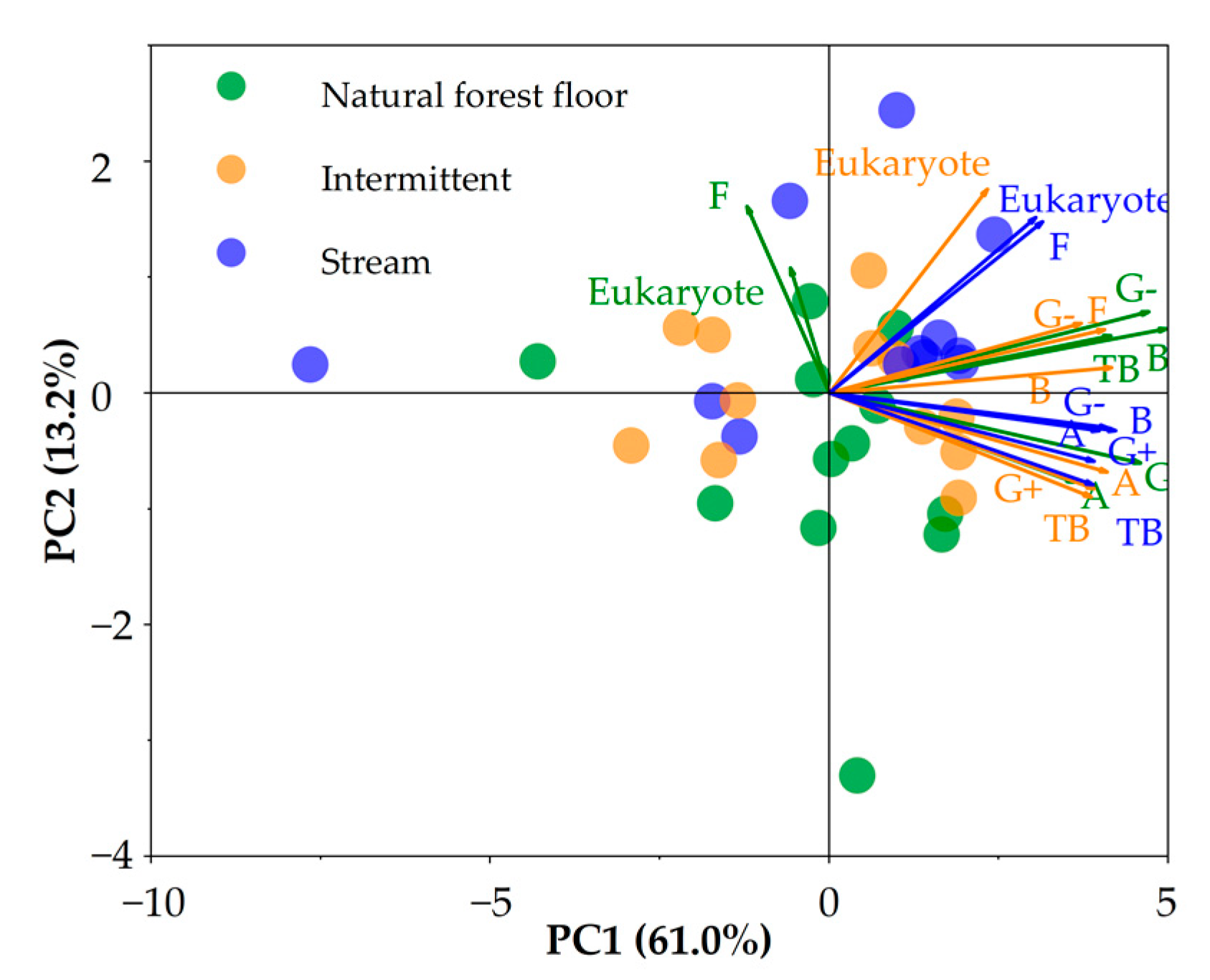
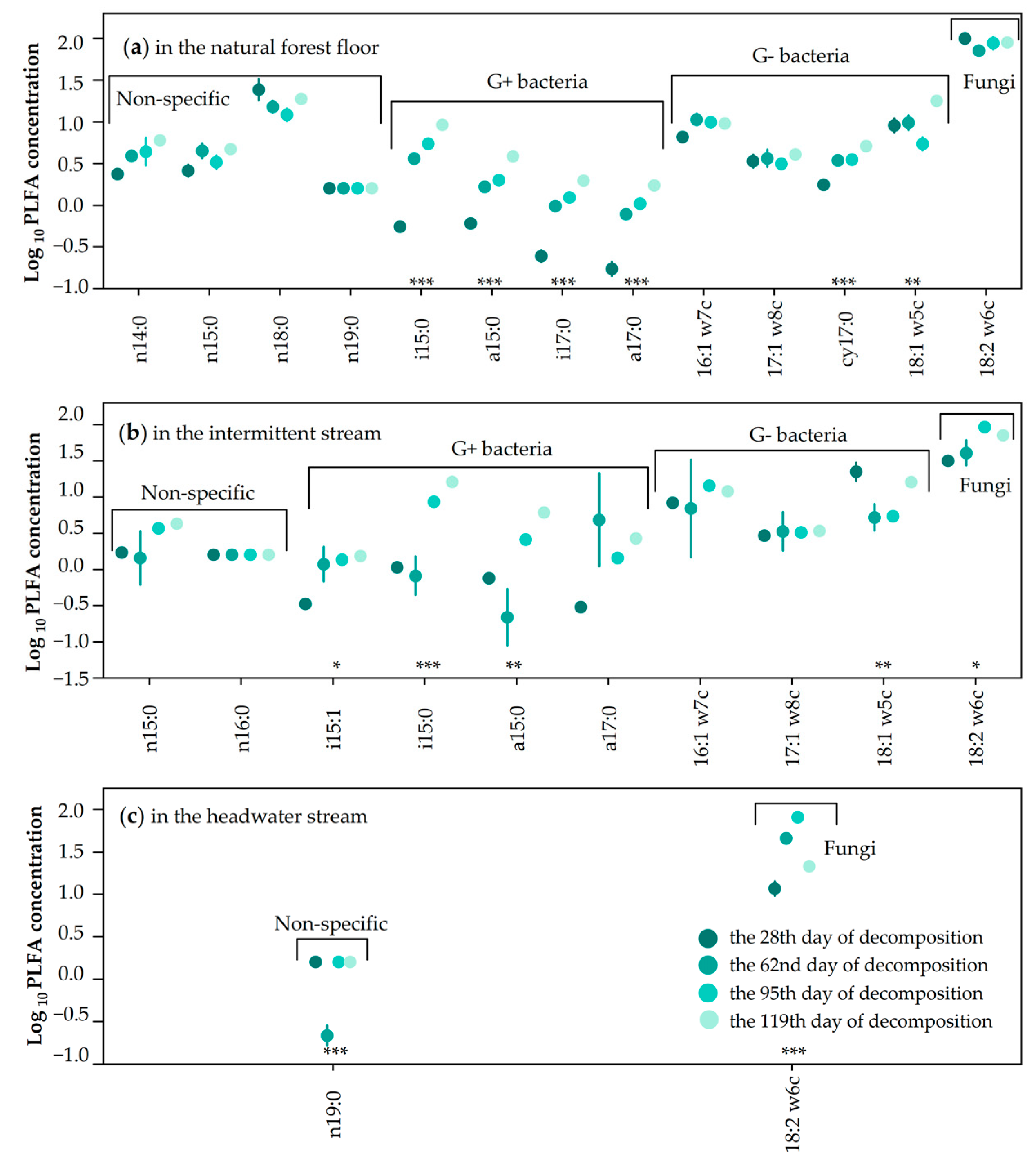

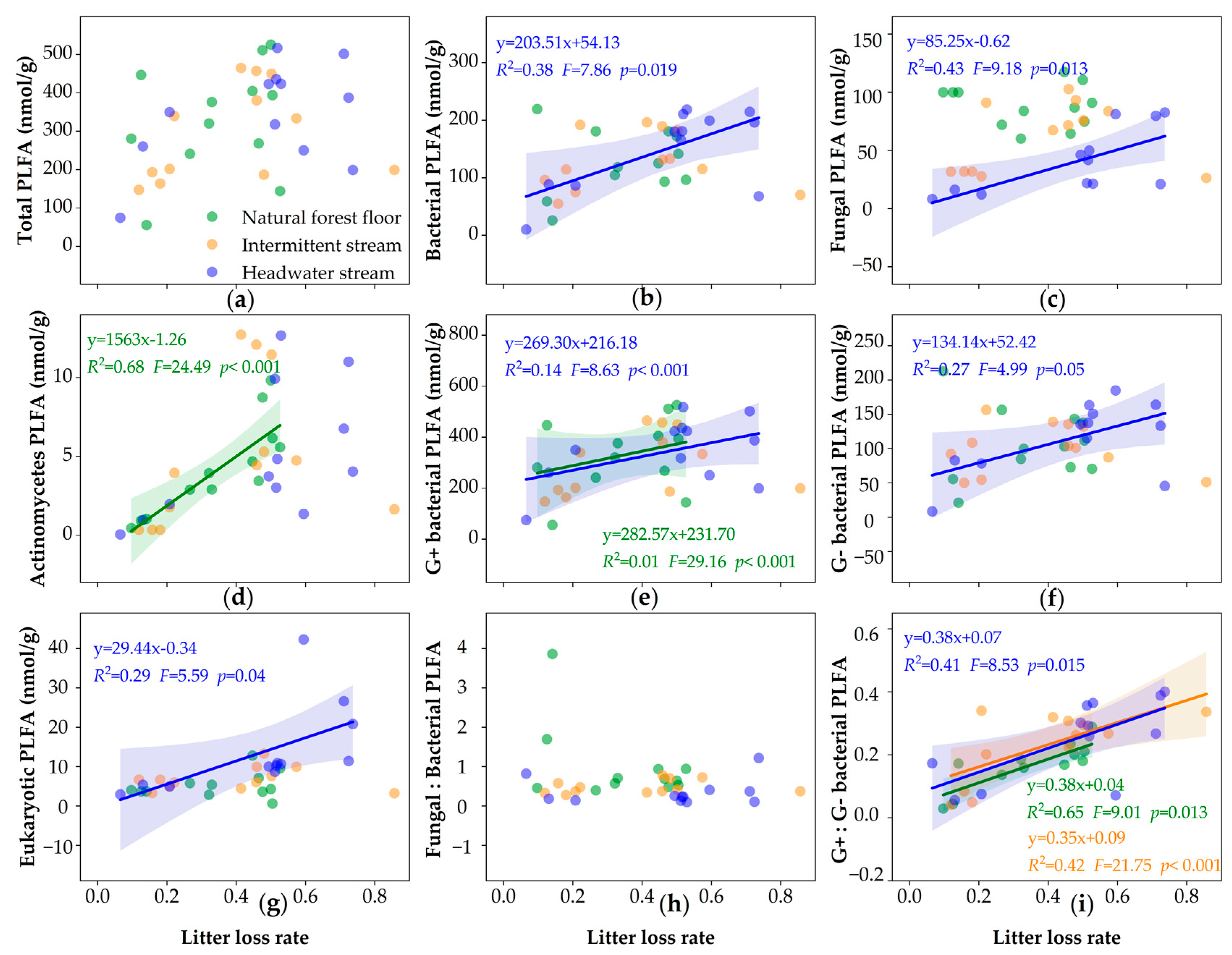
| Index | PCA-1 | PCA-2 | ||||
|---|---|---|---|---|---|---|
| Natural Forest Floor | Intermittent Stream | Headwater Stream | Natural Forest Floor | Intermittent Stream | Headwater Stream | |
| Total Biomass | 0.467 ** | 0.703 *** | 0.816 *** | 0.030 | −0.030 | −0.027 |
| Bacteria | 0.820 *** | 0.803 *** | 0.943 *** | 0.039 | −0.011 | −0.089 |
| Fungi | 0.002 | 0.851 *** | 0.337 * | 0.035 | 0.018 | 0.520 ** |
| G+ bacteria | 0.716 *** | 0.727 *** | 0.882 *** | 0.141 | −0.069 | −0.100 |
| G− bacteria | 0.723 *** | 0.612 ** | 0.866 *** | 0.143 | −0.029 | −0.085 |
| Actinomycete | 0.366 * | 0.816 *** | 0.851 *** | 0.378 | −0.084 | −0.079 |
| Eukaryote | −0.086 | 0.232 | 0.376 * | −0.098 | 0.322 * | 0.383 * |
| Microbial Composition | Environmental Factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | Rainfall | pH | |||||||
| Natural forest floor | R2 | F | p | R2 | F | p | R2 | F | p |
| Total Biomass | 0.16 | 3.11 | >0.05 | 0.01 | 1.16 | >0.05 | - | - | - |
| Bacteria | 0.03 | 1.29 | >0.05 | −0.09 | 0.12 | >0.05 | - | - | - |
| Fungi | −0.09 | 0.11 | >0.05 | 0.10 | 2.18 | >0.05 | - | - | - |
| G+ | 0.85 | 64.8 | <0.001 | 0.01 | 1.05 | >0.05 | - | - | - |
| G− | −0.07 | 0.27 | >0.05 | −0.10 | 0.01 | >0.05 | - | - | - |
| Actinomycetes | 0.84 | 56.7 | <0.001 | 0.25 | 4.66 | >0.05 | - | - | - |
| Eukaryote | −0.09 | 0.12 | >0.05 | −0.10 | 0.01 | >0.05 | - | - | - |
| Intermittent stream | R2 | F | p | R2 | F | p | R2 | F | p |
| Total Biomass | 0.71 | 27.3 | <0.001 | 0.26 | 4.71 | >0.05 | 0.44 | 9.48 | <0.05 |
| Bacteria | 0.46 | 10.5 | <0.01 | 0.17 | 3.18 | >0.05 | 0.14 | 2.75 | >0.05 |
| Fungi | 0.39 | 7.98 | <0.05 | 0.09 | 2.13 | >0.05 | −0.10 | 0.01 | >0.05 |
| G+ | 0.87 | 73.1 | <0.001 | 0.10 | 2.58 | >0.05 | 0.57 | 15.47 | <0.01 |
| G− | 0.20 | 3.68 | >0.05 | 0.11 | 2.34 | >0.05 | −0.02 | 0.83 | >0.05 |
| Actinomycetes | 0.83 | 53.6 | <0.001 | 0.41 | 8.75 | <0.05 | 0.28 | 5.28 | <0.05 |
| Eukaryote | 0.01 | 1.03 | >0.05 | 0.06 | 1.67 | >0.05 | 0.03 | 1.32 | >0.05 |
| Headwater stream | R2 | F | p | R2 | F | p | R2 | F | p |
| Total Biomass | 0.08 | 0.84 | >0.05 | 0.01 | 1.02 | >0.05 | −0.01 | 0.93 | >0.05 |
| Bacteria | 0.33 | 0.51 | <0.05 | −0.10 | 0.01 | >0.05 | −0.13 | 0.10 | >0.05 |
| Fungi | 0.06 | 1.64 | >0.05 | −0.09 | 0.12 | >0.05 | −0.01 | 1.10 | >0.05 |
| G+ | 0.43 | 9.43 | <0.05 | −0.10 | 0.02 | >0.05 | 0.46 | 7.69 | <0.05 |
| G− | 0.18 | 3.43 | >0.05 | −0.09 | 0.08 | >0.05 | −0.13 | 0.10 | >0.05 |
| Actinomycetes | 0.62 | 19.1 | <0.001 | 0.22 | 4.08 | >0.05 | 0.32 | 4.77 | >0.05 |
| Eukaryote | 0.15 | 2.93 | >0.05 | −0.06 | 0.43 | >0.05 | −0.04 | 0.73 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Wu, F.; Zhang, X.; Wei, W.; Zhu, L.; Wu, R.; Wang, D. Effects of Habitat Differences on Microbial Communities during Litter Decomposing in a Subtropical Forest. Forests 2022, 13, 919. https://doi.org/10.3390/f13060919
Guo H, Wu F, Zhang X, Wei W, Zhu L, Wu R, Wang D. Effects of Habitat Differences on Microbial Communities during Litter Decomposing in a Subtropical Forest. Forests. 2022; 13(6):919. https://doi.org/10.3390/f13060919
Chicago/Turabian StyleGuo, Hongrong, Fuzhong Wu, Xiaoyue Zhang, Wentao Wei, Ling Zhu, Ruobing Wu, and Dingyi Wang. 2022. "Effects of Habitat Differences on Microbial Communities during Litter Decomposing in a Subtropical Forest" Forests 13, no. 6: 919. https://doi.org/10.3390/f13060919
APA StyleGuo, H., Wu, F., Zhang, X., Wei, W., Zhu, L., Wu, R., & Wang, D. (2022). Effects of Habitat Differences on Microbial Communities during Litter Decomposing in a Subtropical Forest. Forests, 13(6), 919. https://doi.org/10.3390/f13060919







