Review of Wood Modification and Wood Functionalization Technologies
Abstract
:1. Introduction: Wood as a Renewable Building Material
2. Nomenclature
3. Acetylation
3.1. Process for Producing Acetylated Wood
3.2. How Acetylatation Affects the Wood Cell Wall
3.3. Experimental Evidence on the Changes Caused by Acetylation
3.3.1. Hygroscopicity and Liquid Water Absorption
3.3.2. Dimensional Stability
3.4. Theoretical Basis for Wood Protection by Acetylation
The Role of Moisture in the Decay of Acetylated Wood
3.5. Future Perspectives for Acetylation
4. Furfurylation
4.1. Furfuryl Alcohol and Its Cationic Polymerization
4.2. Process for Producing Furfurylated Wood
4.3. How Furfurylation Affects the Wood Cell Wall
4.4. Experimental Evidence on Changes Caused by Furfurylation
4.4.1. Dimensional Stability
4.4.2. Hygroscopicity/Liquid Water Absorption
4.4.3. Biodeterioration
4.4.4. Hardness and Brittleness
4.5. Theoretical Basis for Wood Protection by Furfurylation
4.6. Gaps in Our Understanding of Furfurylation
4.6.1. Furan Polymerization and Molecular Structure
4.6.2. Mechanisms behind Prevention of Biodeterioration
4.6.3. Role of Wood Properties for Furfurylation Success
4.7. Future Perspectives for Furfurylation
5. Thermal Modification
5.1. Process for Producing Thermally Modified Wood
5.2. How Thermal Modification Affects the Wood Cell Wall
5.3. Experimental Evidence of Changes Caused by Thermal Modification
5.3.1. Hygroscopicity and Liquid Water Absorption
5.3.2. Dimensional Stability
5.3.3. Biodeterioration
5.3.4. Mechanical Properties
5.4. Theoretical Basis for Wood Protection by Thermal Modification
5.5. Gaps in Our Understanding of Thermal Modification
5.6. Future Perspectives for Thermal Modification
6. Surface Charring as a Wood Modification Method
6.1. Background: Process for Producing Charred Wood
6.2. How Charring Affects the Wood Structure
6.3. Dimensional Stability, Hygroscopicity, and Absorption of Surface Charred Wood
6.4. Biodeterioration and Weathering
6.5. Current Knowledge on Charring as a Form of Wood Modification
6.6. Gaps in Our Understanding of Charring as a Wood Modification and Future Perspectives
7. Other Polymerization Methods for Wood Modification
7.1. Process for Producing Resin-Treated Wood
7.2. Chemical Reagents and Its Modes of Reaction

7.3. Experimental Evidence on the Changes in Material Properties by Resin Treatments
7.4. Studying the Mode of Action of Thermosetting Resins
7.5. Future Perspectives for Resin Treated Wood
8. Wood-Based Functional Materials
8.1. Transparent and Multifunctional Transparent Wood

8.2. Energy Harvesting Wood-Based Materials—Triboelectric Wood Nanogenerators
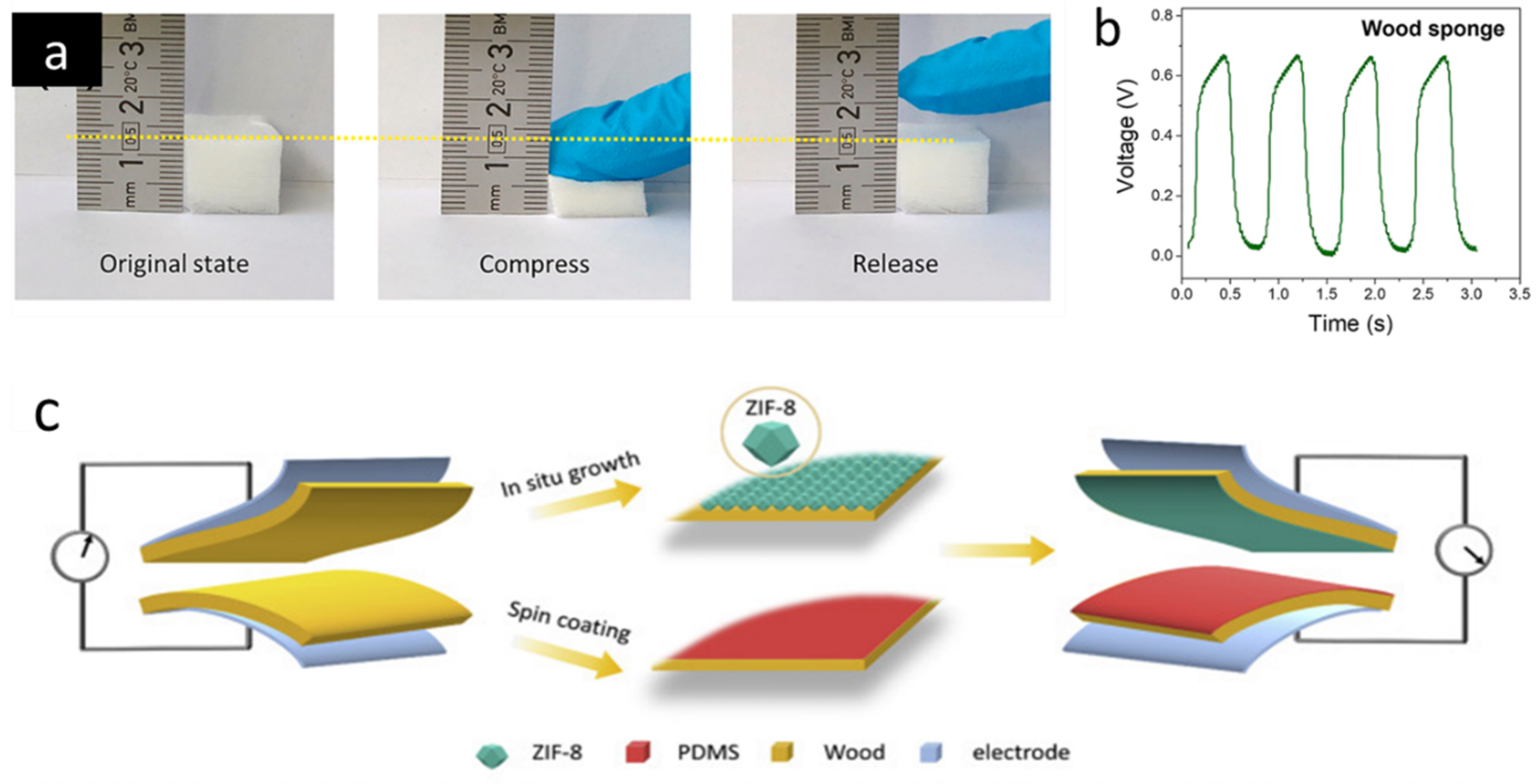
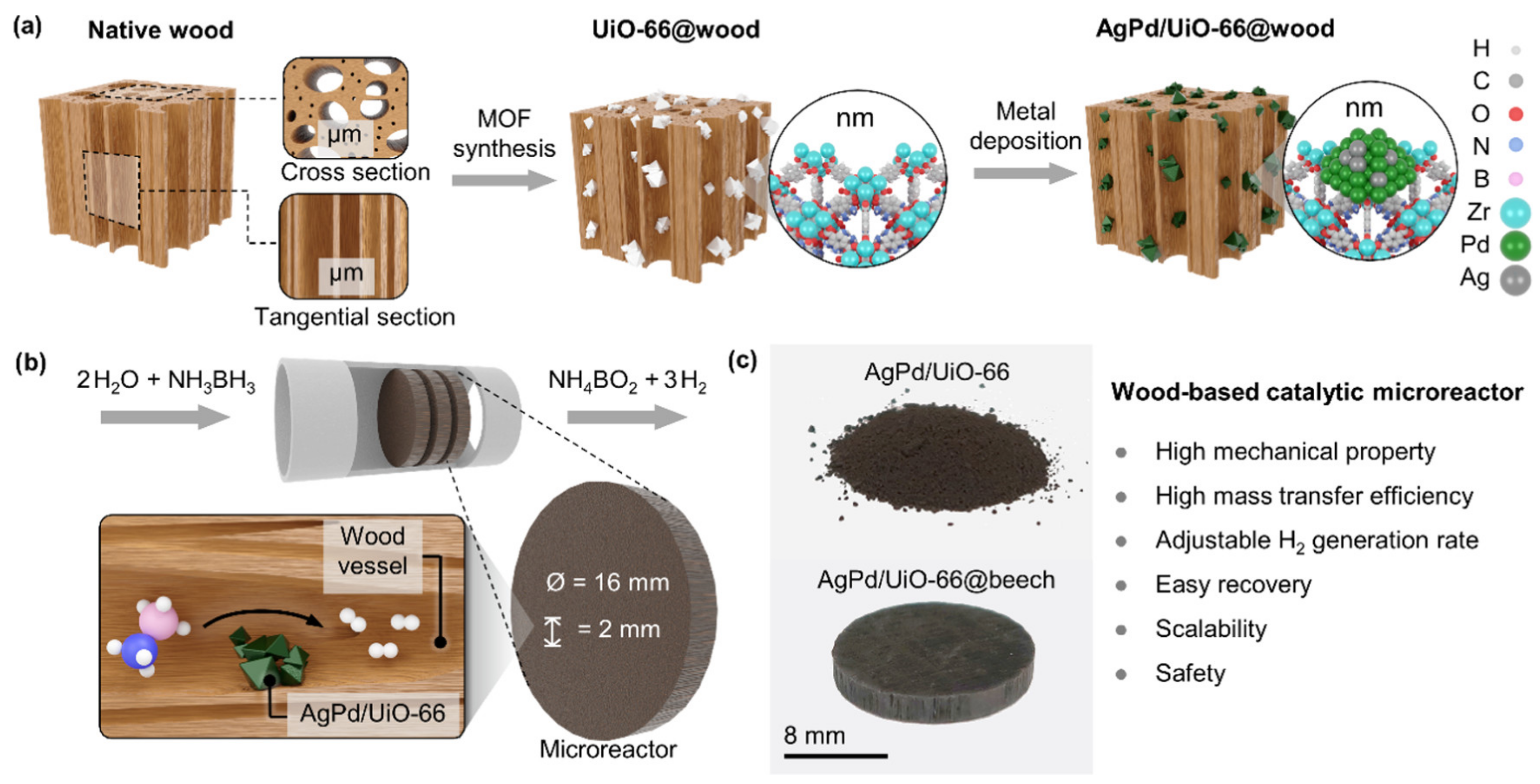
8.3. Wood as Filtration Membrane or Flow through Microreactor
8.4. Fire Protection of Wood by Modification
8.5. Conclusion and Outlook Regarding Wood-Based Functional Materials
9. The Future of Wood Modification
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dominguez-Rodrigo, M.; Serrallonga, J.; Juan-Tresserras, J.; Alcala, L.; Luque, L. Woodworking activities by early humans: A plant residue analysis on Acheulian stone tools from Peninj (Tanzania). J. Hum. Evol. 2001, 40, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Thedmar, A. Liber de Antiqus Legibus. (Chronicles of the Mayors and Sheriffs of London, A.D. 1188 to A.D. 1274); Corporation of the City of London: London, UK, 1274. [Google Scholar]
- Knowles, C.C.; Pitt, P.H. The History of Building Regulation in London, 1189–1972: With an Account of the District Surveyors’ Association; Architectural Press: New York, NY, USA, 1972. [Google Scholar]
- Brandeis, C.; Taylor, M.; Abt, K.L.; Alderman, D.; Buehlmann, U. Status and Trends for the U.S. Forest Products Sector. General Technical Report SRS-258; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2021. [Google Scholar] [CrossRef]
- Pugh, T.A.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, B.H.; Espinoza, O. A Decade of Improved Lumber Drying Technology. Curr. For. Rep. 2016, 2, 106–118. [Google Scholar] [CrossRef]
- Morrell, J.J. Protection of wood-based materials. In Handbook of Environmental Degrataion of Materials; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 299–317. [Google Scholar]
- Winandy, J.E.; Morrell, J.J. Relationship between incipient decay, strength, and chemical composition of Douglas-fir heartwood. Wood Fiber Sci. 1993, 25, 278–288. [Google Scholar]
- Imken, A.A.P.; Brischke, C.; Kögel, S.; Krause, K.C.; Mai, C. Resistance of different wood-based materials against mould fungi: A comparison of methods. Eur. J. Wood Wood Prod. 2020, 78, 661–671. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Blomquist, G.; Gyntelberg, F.; Järvholm, B.; Malmberg, P.; Nordvall, L.; Nielsen, A.; Pershagen, G.; Sundell, J. Dampness in buildings and health. Nordic interdisciplinary review of the scientific evidence on associations between exposure to “dampness” in buildings and health effects (NORDDAMP). Indoor Air 2001, 11, 72–86. [Google Scholar] [CrossRef]
- Zelinka, S.L. Chapter 23. Corrosion of Metals in Wood Products. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: Rijeka, Croatia, 2014; pp. 568–592. [Google Scholar]
- Terberger, T.; Zhilin, M.; Savchenko, S. The Shigir idol in the context of early art in Eurasia. Quat. Int. 2021, 573, 14–29. [Google Scholar] [CrossRef]
- Savchenko, S.; Lillie, M.C.; Zhilin, M.G.; Budd, C.E. New AMS dating of bone and antler weapons from the Shigir collections housed in the Sverdlovsk Regional Museum, Urals, Russia. In Proceedings of the Prehistoric Society; Cambridge University Press: Cambridge, UK, 2015; pp. 265–281. [Google Scholar]
- Rifai, M.M.; El Hadidi, N.M. Investigation and analysis of three gilded wood samples from the tomb of Tutankhamun. In Decorated Surfaces on Ancient Egyptian Objects, Technology, Deterioration and Conservation; Dawson, J., Rozeik, C., Wright, M.M., Eds.; Archetype Publications: London, UK, 2010; pp. 16–24. [Google Scholar]
- Clausen, C.; Glass, S.V. Build Green. Your Wood Can Last for Centuries; General Technical Report FPL-GTR-215; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2012.
- Christie, H.; Olsen, O.; Taylor, H. The Wooden Church of St. Andrew at Greensted, Essex. Antiqu. J. 1979, 59, 92–112. [Google Scholar] [CrossRef]
- Matsuo, M.; Yokoyama, M.; Umemura, K.; Sugiyama, J.; Kawai, S.; Gril, J.; Kubodera, S.; Mitsutani, T.; Ozaki, H.; Sakamoto, M. Aging of Wood: Analysis of Color Changes during Natural Aging and Heat Treatment; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Thun, T. Norwegian conifer chronologies constructed to date historical timber. Dendrochronologia 2005, 23, 63–74. [Google Scholar] [CrossRef]
- Freeman, M.H.; Shupe, T.F.; Vlosky, R.P.; Barnes, H. Past, present, and future of the wood preservation industry. For. Prod. J. 2003, 53, 8. [Google Scholar]
- Kirker, G.T.; Lebow, S. Chapter 15. Wood Preservation. In Wood Handbook—Wood as an Engineering Material; Ross, R.J., Ed.; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2021; p. 508. [Google Scholar]
- Anonymous. AWPA Standard U1: Use Category System. User Specifications for Treated Wood; American Wood Protection Association: Birmingham, AL, USA, 2019. [Google Scholar]
- Anonymous. EN 335: Durability of Wood and Wood-Based Products-Use Classes: Definitions, Application to Solid Wood and Wood-Based Products; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- Hill, C.A. Wood modification: An update. BioResources 2011, 6, 918–919. [Google Scholar]
- Hill, C.A. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: West Sussex, UK, 2006; Volume 5. [Google Scholar]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Ogilvie, S. Trading Update: Accys Technologies PLC. Number 6627V, Released 16 April, 2021; Accys Technologies: London, UK, 2021; Available online: https://www.accsysplc.com/app/uploads/2021/04/20210416-AXS-April-2021-Trading-Update.pdf (accessed on 7 May 2021).
- Jones, D.; Sandberg, D.; Goli, G.; Todaro, L. Wood Modification in Europe: A State-of-the-Art about Processes, Products, and Applications; Firenze University Press: Firenze, Italy, 2019. [Google Scholar]
- Vlosky, R.P. Statistical Overview of the U.S. Wood Preserving Industry: 2007; North American Wood Pole Council: Vancouver, WA, USA, 2009; p. 81. [Google Scholar]
- Salminen, E.; Valo, R.; Korhonen, M.; Jernlås, R. Wood Preservation with Chemicals: Best Available Techniques (BAT); Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Jakes, J.E.; Arzola, X.; Bergman, R.; Ciesielski, P.; Hunt, C.G.; Rahbar, N.; Tshabalala, M.; Wiedenhoeft, A.C.; Zelinka, S.L. Not Just Lumber—Using Wood in the Sustainable Future of Materials, Chemicals, and Fuels. JOM 2016, 68, 2395–2404. [Google Scholar] [CrossRef] [Green Version]
- Plaza, N.; Zelinka, S.L.; Stone, D.S.; Jakes, J.E. Plant-based torsional actuator with memory. Smart Mater. Struct. 2013, 22, 072001. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies—A review. iFor.-Biogeosci. For. 2017, 10, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Wang, K.; Li, J.; Zhang, S.; Shi, S.Q. Environmentally Benign Wood Modifications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 3532–3540. [Google Scholar] [CrossRef]
- Emmerich, L.; Bollmus, S.; Militz, H. Wood modification with DMDHEU (1.3-dimethylol-4.5-dihydroxyethyleneurea)—State of the art, recent research activities and future perspectives. Wood Mater. Sci. Eng. 2019, 14, 3–18. [Google Scholar] [CrossRef]
- Mantanis, G.I. Chemical modification of wood by acetylation or furfurylation: A review of the present scaled-up technologies. BioResources 2017, 12, 4478–4489. [Google Scholar] [CrossRef] [Green Version]
- Thybring, E.E.; Fredriksson, M. Wood Modification as a Tool to Understand Moisture in Wood. Forests 2021, 12, 372. [Google Scholar] [CrossRef]
- Fuchs, W. Zur Kenntnis des genuinen Lignins, I.: Die Acetylierung des Fichtenholzes. Ber. Dtsch. Chem. Ges. A B Ser. 1928, 61, 948–951. [Google Scholar] [CrossRef]
- Stamm, A.J.; Tarkow, H. Dimensional stabilization of wood. J. Phys. Chem. 1947, 51, 493–505. [Google Scholar] [CrossRef]
- Stamm, A.J.; Tarkow, H. Acetylation of Wood and Boards. U.S. Patent 2,417,995, 25 March 1947. [Google Scholar]
- Tarkow, H. Decay Resistance of Acetylated Balsa; USDA Forest Service, Forest Products Laboratory: Madison, WI, USA, 1945.
- Rowell, R.M. Chemical Modification of Wood. For. Prod. Abstr. 1983, 6, 363–382. [Google Scholar]
- Ibach, R.E.; Rowell, R.M. USDA Forest Service Forest Products Laboratory: Acetylation of Wood 1945–1966. Forests 2021, 12, 260. [Google Scholar] [CrossRef]
- Thybring, E.E. The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int. Biodeterior. Biodegrad. 2013, 82, 87–95. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Hill, C. The sorption of water vapour by anhydride modified softwood. Wood Sci. Technol. 2003, 37, 221–231. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Hill, C.; Gkaraveli, A. Analysis of the swelling behaviour of chemically modified softwood: A novel approach. Holz Roh Werkst. 2004, 62, 107–112. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Curling, S.; Ormondroyd, G.; Xie, Y. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content. J. Mater. Sci. 2013, 49, 2362–2371. [Google Scholar] [CrossRef]
- Thybring, E.E.; Piqueras, S.; Tarmian, A.; Burgert, I. Water accessibility to hydroxyls confined in solid wood cell walls. Cellulose 2020, 27, 5617–5627. [Google Scholar] [CrossRef]
- Beck, G.; Strohbusch, S.; Larnøy, E.; Militz, H.; Hill, C. Accessibility of hydroxyl groups in anhydride modified wood as measured by deuterium exchange and saponification. Holzforschung 2018, 72, 17–23. [Google Scholar] [CrossRef]
- Thygesen, L.; Elder, T. Moisture in Untreated, Acetylated, and Furfurylated Norway Spruce Studied During Drying Using Time Domain NMR. Wood Fiber Sci. 2008, 40, 309–320. [Google Scholar]
- Beck, G.; Thybring, E.E.; Thygesen, L.G.; Hill, C. Characterization of moisture in acetylated and propionylated radiata pine using low-field nuclear magnetic resonance (LFNMR) relaxometry. Holzforschung 2018, 72, 225–233. [Google Scholar] [CrossRef]
- Emmerich, L.; Brischke, C.; Sievert, M.; Schulz, M.S.; Jaeger, A.-C.; Beulshausen, A.; Humar, M. Predicting the Outdoor Moisture Performance of Wood Based on Laboratory Indicators. Forests 2020, 11, 1001. [Google Scholar] [CrossRef]
- Chang, H.-T.; Chang, S.-T. Moisture excluding efficiency and dimensional stability of wood improved by acylation. Bioresour. Technol. 2002, 85, 201–204. [Google Scholar] [CrossRef]
- Obataya, E.; Minato, K. Potassium acetate-catalyzed acetylation of wood: Extraordinarily rapid acetylation at 120 °C. Wood Sci. Technol. 2008, 42, 567. [Google Scholar] [CrossRef]
- Obataya, E. Reversible volumetric changes of acetylated wood with after-treatments. Wood Sci. Technol. 2005, 39, 472. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Brischke, C.; Richter, K. Mode of action of brown rot decay resistance in modified wood: A review. Holzforschung 2014, 68, 239–246. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Ringman, R.; Pilgård, A.; Thybring, E.E.; Jakes, J.E.; Richter, K. The role of chemical transport in the brown-rot decay resistance of modified wood. Int. Wood Prod. J. 2016, 7, 66–70. [Google Scholar] [CrossRef]
- Ringman, R.; Beck, G.; Pilgård, A. The Importance of Moisture for Brown Rot Degradation of Modified Wood: A Critical Discussion. Forests 2019, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.M. Chapter 14. Chemical Modification of Wood. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 381–420. [Google Scholar]
- Rowell, R.M.; Ibach, R.E.; McSweeny, J.; Nilsson, T. Understanding decay resistance, dimensional stability and strength changes in heat-treated and acetylated wood. Wood Mater. Sci. Eng. 2009, 4, 14–22. [Google Scholar] [CrossRef]
- Hill, C.A.; Forster, S.; Farahani, M.; Hale, M.; Ormondroyd, G.; Williams, G. An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. Int. Biodeterior. Biodegrad. 2005, 55, 69–76. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Brischke, C.; Windeisen, E.; Richter, K. Incipient brown rot decay in modified wood: Patterns of mass loss, structural integrity, moisture and acetyl content in high resolution. Int. Wood Prod. J. 2017, 8, 172–182. [Google Scholar] [CrossRef]
- Alfredsen, G.; Pilgård, A.; Fossdal, C.G. Characterisation of Postia placenta colonisation during 36 weeks in acetylated southern yellow pine sapwood at three acetylation levels including genomic DNA and gene expression quantification of the fungus. Holzforschung 2016, 70, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Beck, G.; Hegnar, O.A.; Fossdal, C.G.; Alfredsen, G. Acetylation of Pinus radiata delays hydrolytic depolymerisation by the brown-rot fungus Rhondonia placenta. Int. Biodeterior. Biodegrad. 2018, 135, 39–52. [Google Scholar] [CrossRef]
- Beck, G.; Thybring, E.E.; Thygesen, L.G. Brown-rot fungal degradation and de-acetylation of acetylated wood. Int. Biodeterior. Biodegrad. 2018, 135, 62–70. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Richter, K. Effect of wood modification on gene expression during incipient Postia placenta decay. Int. Biodeterior. Biodegrad. 2014, 86, 86–91. [Google Scholar] [CrossRef]
- Hill, C.A. Why does acetylation protect wood from microbiological attack? Wood Mater. Sci. Eng. 2009, 4, 37–45. [Google Scholar] [CrossRef]
- Ohkoshi, M.; Kato, A.; Suzuki, K.; Hayashi, N.; Ishihara, M. Characterization of acetylated wood decayed by brown-rot and white-rot fungi. J. Wood Sci. 1999, 45, 69–75. [Google Scholar] [CrossRef]
- Peterson, M.; Thomas, R. Protection of wood from decay fungi by acetylation—An ultrastructural and chemical study. Wood Fiber Sci. 1978, 10, 149–163. [Google Scholar]
- Ibach, R.E.; Rowell, R.M. Improvements in decay resistance based on moisture exclusion. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2000, 353, 23–33. [Google Scholar] [CrossRef]
- Brelid, P.L.; Simonson, R.; Bergman, Ö.; Nilsson, T. Resistance of acetylated wood to biological degradation. Holz Roh Werkst. 2000, 58, 331–337. [Google Scholar] [CrossRef]
- Brelid, P.L.; Westin, M. Acetylated wood—Results from long-term field tests. In Proceedings of the European Conference on Wood Modification, Cardiff, UK, 15–16 October 2007; pp. 71–78. [Google Scholar]
- Takahashi, M.; Imamura, Y.; Tanahashi, M. Effect of Acetylation on Decay Resistance of Wood against Brown Rot, White Rot and Soft Rot Fungi; International Research Group on Wood Preservation: Stockholm, Sweden, 1989. [Google Scholar]
- Beckers, E.; Militz, H.; Stevens, M. Resistance of Acetylated Wood to Basidiomycetes, Soft Rot and Blue Stain; International Research Group on Wood Preservation: Stockholm, Sweden, 1994. [Google Scholar]
- Papadopoulos, A.N.; Hill, C. The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana. Holz Roh Werkst. 2002, 60, 329–332. [Google Scholar] [CrossRef]
- Jakes, J.E.; Hunt, C.G.; Zelinka, S.L.; Ciesielski, P.N.; Plaza, N.Z. Effects of Moisture on Diffusion in Unmodified Wood Cell Walls: A Phenomenological Polymer Science Approach. Forests 2019, 10, 1084. [Google Scholar] [CrossRef] [Green Version]
- Zelinka, S.L.; Glass, S.; Stone, D. A Percolation Model for Electrical Conduction in Wood with Implications for Wood–Water Relations. Wood Fiber Sci. 2008, 40, 544–552. [Google Scholar]
- Jakes, J.E.; Plaza, N.; Stone, D.S.; Hunt, C.G.; Glass, S.V.; Zelinka, S.L. Mechanism of transport through wood cell wall polymers. J. For. Prod. Ind. 2013, 2, 10–13. [Google Scholar]
- Zelinka, S.L.; Gleber, S.-C.; Vogt, S.; Rodríguez López Gabriela, M.; Jakes Joseph, E. Threshold for ion movements in wood cell walls below fiber saturation observed by X-ray fluorescence microscopy (XFM). Holzforschung 2015, 69, 441–448. [Google Scholar] [CrossRef]
- Hunt, C.G.; Zelinka, S.L.; Frihart, C.R.; Lorenz, L.; Yelle, D.; Gleber, S.-C.; Vogt, S.; Jakes, J.E. Acetylation increases relative humidity threshold for ion transport in wood cell walls—A means to understanding decay resistance. Int. Biodeterior. Biodegrad. 2018, 133, 230–237. [Google Scholar] [CrossRef]
- Jakes, J.E.; Zelinka, S.L.; Hunt, C.G.; Ciesielski, P.; Frihart, C.R.; Yelle, D.; Passarini, L.; Gleber, S.-C.; Vine, D.; Vogt, S. Measurement of moisture-dependent ion diffusion constants in wood cell wall layers using time-lapse micro X-ray fluorescence microscopy. Nat. Sci. Rep. 2020, 10, 9919. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Houtman, C.J.; Hirth, K.; Lacher, S.; Lorenz, L.; Engelund Thybring, E.; Hunt, C.G. The effect of acetylation on iron uptake and diffusion in water saturated wood cell walls and implications for decay. Forests 2020, 11, 1121. [Google Scholar] [CrossRef]
- Jakes, J.E. Mechanism for Diffusion through Secondary Cell Walls in Lignocellulosic Biomass. J. Phys. Chem. B 2019, 123, 4333–4339. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Kirker, G.T.; Bishell, A.B.; Glass, S.V. Effects of Wood Moisture Content and the Level of Acetylation on Brown Rot Decay. Forests 2020, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Thybring, E.E. Water relations in untreated and modified wood under brown-rot and white-rot decay. Int. Biodeterior. Biodegrad. 2017, 118, 134–142. [Google Scholar] [CrossRef]
- Peterson, C.A.; Cowling, E.B. Influence of Various Initial Moisture Contents on Decay of Sitka Spruce and and Sweetgum Sapwood by Polyporus versicolor in the Soil-Block Test. Phytopathology 1973, 63, 235–237. [Google Scholar] [CrossRef]
- Digaitis, R.; Thybring, E.E.; Thygesen, L.G.; Fredriksson, M. Targeted acetylation of wood: A tool for tuning wood-water interactions. Cellulose 2021, 28, 8009–8025. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Raspolli Galletti, A.M. New Intensification Strategies for the Direct Conversion of Real Biomass into Platform and Fine Chemicals: What Are the Main Improvable Key Aspects? Catalysts 2020, 10, 961. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Mechanisms of Chromophore Formation and Cross-Linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Chemorheological analysis and model-free kinetics of acid catalysed furfuryl alcohol polymerization. Phys. Chem. Chem. Phys. 2007, 9, 5359–5366. [Google Scholar] [CrossRef]
- Falco, G.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. FA Polymerization Disruption by Protic Polar Solvents. Polymers 2018, 10, 529. [Google Scholar] [CrossRef] [Green Version]
- Lande, S.; Westin, M.; Schneider, M. Properties of furfurylated wood. Scand. J. For. Res. 2004, 19, 22–30. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Barsberg, S.; Venås, T.M. The fluorescence characteristics of furfurylated wood studied by fluorescence spectroscopy and confocal laser scanning microscopy. Wood Sci. Technol. 2010, 44, 51–65. [Google Scholar] [CrossRef]
- Goldstein, I.S. The impregnation of wood to impart resistance to alkali and acid. For. Prod. J. 1955, 5, 265–267. [Google Scholar]
- Schneider, M.H. New cell wall and cell lumen wood polymer composites. Wood Sci. Technol. 1995, 29, 121–127. [Google Scholar] [CrossRef]
- Westin, M.; Ohlsson, B.; Simonson, R.; Nilsson, T. New chemicals for wood preservation and new ways of chemical modification. In Proceedings of Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1996; p. 6-CELL. [Google Scholar]
- Li, W.; Ren, D.; Zhang, X.; Wang, H.; Yu, Y. The furfurylation of wood: A nanomechanical study of modified wood cells. BioResources 2016, 11, 3614–3625. [Google Scholar] [CrossRef]
- Venås, T.M.; Rinnan, Å. Determination of weight percent gain in solid wood modified with in situ cured furfuryl alcohol by near-infrared reflectance spectroscopy. Chemom. Intell. Lab. Syst. 2008, 92, 125–130. [Google Scholar] [CrossRef]
- Herold, N.; Dietrich, T.; Grigsby, W.J.; Franich, R.A.; Winkler, A.; Buchelt, B.; Pfriem, A. Effect of maleic anhydride content and ethanol dilution on the polymerization of furfuryl alcohol in wood veneer studied by differential scanning calorimetry. BioResources 2013, 8, 1064–1075. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Ehmcke, G.; Barsberg, S.; Pilgård, A. Furfurylation result of Radiata pine depends on the solvent. Wood Sci. Technol. 2020, 54, 929–942. [Google Scholar] [CrossRef]
- Barsberg, S.T.; Thygesen, L.G. A Combined Theoretical and FT-IR Spectroscopy Study of a Hybrid Poly (furfuryl alcohol)–Lignin Material: Basic Chemistry of a Sustainable Wood Protection Method. ChemistrySelect 2017, 2, 10818–10827. [Google Scholar] [CrossRef]
- Nordstierna, L.; Lande, S.; Westin, M.; Karlsson, O.; Furo, I. Towards novel wood-based materials: Chemical bonds between lignin-like model molecules and poly (furfuryl alcohol) studied by NMR. Holzforschung 2008, 62, 709–713. [Google Scholar] [CrossRef]
- Shen, X.; Guo, D.; Jiang, P.; Li, G.; Yang, S.; Chu, F. Reaction mechanisms of furfuryl alcohol polymer with wood cell wall components. Holzforschung 2021, 75, 1150–1158. [Google Scholar] [CrossRef]
- Epmeier, H.; Kliger, R. Experimental study of material properties of modified Scots pine. Holz Roh Werkst. 2005, 63, 430–436. [Google Scholar] [CrossRef]
- Epmeier, H.; Johansson, M.; Kliger, R.; Westin, M. Material properties and their interrelation in chemically modified clear wood of Scots pine. Holzforschung 2007, 61, 34–42. [Google Scholar] [CrossRef]
- Epmeier, H.; Johansson, M.; Kliger, R.; Westin, M. Bending creep performance of modified timber. Holz Roh Werkst. 2007, 65, 343–351. [Google Scholar] [CrossRef]
- Herold, N.; Pfriem, A. Shape retention of furfurylated and moulded wood veneer. BioResources 2014, 9, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Stamm, A.J. Wood and Cellulose Science; The Ronald Press Co.: New York, NY, USA, 1964. [Google Scholar]
- Westin, M. Furan Polymer Impregnated Wood. Acetylation of Wood and Boards. AU Patent 2,003,247,294 B2, 12 July 2004. [Google Scholar]
- Esteves, B.; Nunes, L.; Pereira, H. Properties of furfurylated wood (Pinus pinaster). Eur. J. Wood Wood Prod. 2011, 69, 521–525. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Tang Engelund, E.; Hoffmeyer, P. Water sorption in wood and modified wood at high values of relative humidity. Part I: Results for untreated, acetylated, and furfurylated Norway spruce. Holzforschung 2010, 64, 315–323. [Google Scholar] [CrossRef]
- Yang, T.; Cao, J.; Ma, E. How does delignification influence the furfurylation of wood? Ind. Crops Prod. 2019, 135, 91–98. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Wålinder, M.E.P.; Claesson, P.M.; Swerin, A. Wettability and swelling of acetylated and furfurylated wood analyzed by multicycle Wilhelmy plate method. Holzforschung 2016, 70, 69–77. [Google Scholar] [CrossRef]
- Skrede, I.; Solbakken, M.H.; Hess, J.; Fossdal, C.G.; Hegnar, O.; Alfredsen, G.; Master, E.R. Wood Modification by Furfuryl Alcohol Resulted in a Delayed Decomposition Response in Rhodonia (Postia) placenta. Appl. Environ. Microbiol. 2019, 85, e00338-19. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, L.G.; Beck, G.; Nagy, N.E.; Alfredsen, G. Cell wall changes during brown rot degradation of furfurylated and acetylated wood. Int. Biodeterior. Biodegrad. 2021, 162, 105257. [Google Scholar] [CrossRef]
- Hadi, Y.S.; Herliyana, E.N.; Mulyosari, D.; Abdillah, I.B.; Pari, R.; Hiziroglu, S. Termite Resistance of Furfuryl Alcohol and Imidacloprid Treated Fast-Growing Tropical Wood Species as Function of Field Test. Appl. Sci. 2020, 10, 6101. [Google Scholar] [CrossRef]
- Hadi, Y.S.; Mulyosari, D.; Herliyana, E.N.; Pari, G.; Arsyad, W.O.M.; Abdillah, I.B.; Gérardin, P. Furfurylation of wood from fast-growing tropical species to enhance their resistance to subterranean termite. Eur. J. Wood Wood Prod. 2021, 79, 1007–1015. [Google Scholar] [CrossRef]
- Hadi, Y.S.; Nawawi, D.S.; Abdillah, I.B.; Pari, G.; Pari, R. Evaluation of Discoloration and Subterranean Termite Resistance of Four Furfurylated Tropical Wood Species after One-Year Outdoor Exposure. Forests 2021, 12, 900. [Google Scholar] [CrossRef]
- Hadi, Y.S.; Westin, M.; Rasyid, E. Resistance of furfurylated wood to termite attack. For. Prod. J. 2005, 55, 85–88. [Google Scholar]
- Gascón-Garrido, P.; Oliver-Villanueva, J.V.; Ibiza-Palacios, M.S.; Militz, H.; Mai, C.; Adamopoulos, S. Resistance of wood modified with different technologies against Mediterranean termites (Reticulitermes spp.). Int. Biodeterior. Biodegrad. 2013, 82, 13–16. [Google Scholar] [CrossRef]
- Pilgård, A.; Treu, A.; van Zeeland, A.N.T.; Gosselink, R.J.A.; Westin, M. Toxic hazard and chemical analysis of leachates from furfurylated wood. Environ. Toxicol. Chem. 2010, 29, 1918–1924. [Google Scholar] [CrossRef]
- Pilgård, A.; De Vetter, L.; Van Acker, J.; Westin, M. Toxic hazard of leachates from furfurylated wood: Comparison between two different aquatic organisms. Environ. Toxicol. Chem. 2010, 29, 1067–1071. [Google Scholar] [CrossRef]
- Martin, L.S.; Jelavić, S.; Cragg, S.M.; Thygesen, L.G. Furfurylation protects timber from degradation by marine wood boring crustaceans. Green Chem. 2021, 23, 8003–8015. [Google Scholar] [CrossRef]
- Slevin, C.W.; Lande, S.; Cragg, S. Laboratory and Marine Trials of Resistance of Furfurylated Wood to Marine Borers. In Proceedings of the 8th European Conference on Wood Modification, Helsinki, Finland, 26 October 2015; pp. 464–471. [Google Scholar]
- Westin, M.; Larson Brelid, P.; Nilsson, T.; Rapp, A.O.; Dickerson, J.P.; Lande, S.; Cragg, S. Marine Borer Resistance of Acetylated and Furfurylated EWood-Results from up to 16 years of Field Exposure. In Proceedings of the 47th IRG Annual Meeting, Lisbon, Portgual, 15–19 May 2016. [Google Scholar]
- Bosq, N.; Guigo, N.; Falco, G.; Persello, J.; Sbirrazzuoli, N. Impact of Silica Nanoclusters on Furfuryl Alcohol Polymerization and Molecular Mobility. J. Phys. Chem. C 2017, 121, 7485–7494. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New insights on the thermal degradation pathways of neat poly(furfuryl alcohol) and poly(furfuryl alcohol)/SiO2 hybrid materials. Polym. Degrad. Stab. 2009, 94, 908–913. [Google Scholar] [CrossRef]
- Falco, G.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. Opening Furan for Tailoring Properties of Bio-based Poly (Furfuryl Alcohol) Thermoset. ChemSusChem 2018, 11, 1805–1812. [Google Scholar] [CrossRef]
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal Degradation of Wood: Emerging Data, New Insights and Changing Perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Arantes, V.; Goodell, B. Current understanding of brown-rot fungal biodegradation mechanisms: A review. In Deterioration and Protection of Sustainable Biomaterials; Schultz, T.P., Goodell, B., Nicholas, D., Eds.; American Chemical Society Symposium Series; American Chemical Society: Washington, DC, USA, 2014; pp. 1–21. [Google Scholar]
- Cragg, S.M.; Danjon, C.; Mansfield-Williams, H. Contribution of hardness to the natural resistance of a range of wood species to attack by the marine borer Limnoria. Holzforshung 2007, 61, 201–206. [Google Scholar] [CrossRef]
- Zimmer, K.P.; Høibø, O.A.; Vestøl, G.I.; Larnøy, E. Variation in treatability of Scots pine sapwood: A survey of 25 different northern European locations. Wood Sci. Technol. 2014, 48, 1049–1068. [Google Scholar] [CrossRef]
- Siau, J.F. Transport Processes in Wood; Springer: Berlin, Germany; New York, NY, USA, 1984. [Google Scholar]
- Sangregorio, A.; Muralidhara, A.; Guigo, N.; Thygesen, L.G.; Marlair, G.; Angelici, C.; de Jong, E.; Sbirrazzuoli, N. Humin based resin for wood modification and property improvement. Green Chem. 2020, 22, 2786–2798. [Google Scholar] [CrossRef] [Green Version]
- Sangregorio, A.; Guigo, N.; van der Waal, J.C.; Sbirrazzuoli, N. Humins from Biorefineries as Thermoreactive Macromolecular Systems. ChemSusChem 2018, 11, 4246–4255. [Google Scholar] [CrossRef]
- Liu, M.; Guo, F.; Wang, H.; Ren, W.; Cao, M.; Yu, Y. Highly Stable Wood Material with Low Resin Consumption via Vapor Phase Furfurylation in Cell Walls. ACS Sustain. Chem. Eng. 2020, 8, 13924–13933. [Google Scholar] [CrossRef]
- Liu, M.; Lyu, S.; Peng, L.; Cai, L.; Huang, Z.; Lyu, J. Improvement of Toughness and Mechanical Properties of Furfurylated Wood by Biosourced Epoxidized Soybean Oil. ACS Sustain. Chem. Eng. 2021, 9, 8142–8155. [Google Scholar] [CrossRef]
- Tiemann, H.D. Effect of different methods of drying upon the strength and the hygroscopicity of wood. In The Kiln-Drying of Lumber—A Practical and Theoretical Treatise; J.B. Lippincott Company: Philadelphia, PA, USA; London, UK, 1917; pp. 256–264. [Google Scholar]
- Stamm, A.J.; Burr, H.K.; Kline, A.A. Staybwood—Heat-stabilized wood. Ind. Eng. Chem. 1946, 38, 630–634. [Google Scholar] [CrossRef]
- Burmester, A. Einfluß einer Wärme-Druck-Behandlung halbtrockenen Holzes auf seine Formbeständigkeit. Holz Roh Werkst. 1973, 31, 237–243. [Google Scholar] [CrossRef]
- Giebeler, E. Dimensional stabilization of wood by moisture-heat-pressure-treatment. Holz Roh Werkst. 1983, 41, 87–94. [Google Scholar] [CrossRef]
- Scheiding, W. Thermoholz-20 Thermojahre. Holzkurier. 2021. Available online: https://www.holzkurier.com/holzprodukte/2021/02/20_thermojahre.html (accessed on 7 June 2022).
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Militz, H.; Altgen, M. Processes and properties of thermally modified wood manufactured in Europe. In Deterioration and Protection of Sustainable Biomaterials; ACS Symposium Series; Schultz, T.P., Goodell, B., Nicholas, D., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 269–285. [Google Scholar]
- Sandberg, D.; Kutnar, A. Thermally modified timber: Recent developments in Europe and North America. Wood Fiber Sci. 2016, 48, 28–39. [Google Scholar]
- DeGroot, W.F.; Pan, W.-P.; Rahman, M.D.; Richards, G.N. First chemical events in pyrolysis of wood. J. Anal. Appl. Pyrolysis 1988, 13, 221–231. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J. Study on the deacetylation of hemicelluloses during the hydrothermal processing of Eucalyptus wood. Holz Roh Werkst. 2001, 59, 53–59. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J. Hydrothermal processing of lignocellulosic materials. Holz Roh Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Nuopponen, M.; Vuorinen, T.; Jämsä, S.; Viitaniemi, P. Thermal modifications in softwood studied by FT-IR and UV resonance Raman spectroscopies. J. Wood Chem. Technol. 2005, 24, 13–26. [Google Scholar] [CrossRef]
- Tjeerdsma, B.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh Werkst. 1998, 56, 149. [Google Scholar] [CrossRef]
- Kotilainen, R. Chemical Changes in Wood during Heating at 150–260 °C. Ph.D. Thesis, University of Jyväskylä, Jyväskylä, Finland, 2000. [Google Scholar]
- Zaman, A.; Alén, R.; Kotilainen, R. Thermal Behavior of Scots Pine (Pinus Sylvestris) and Silver Birch (Betula Pendula) at 200–230 °C. Wood Fiber Sci. 2000, 32, 138–143. [Google Scholar]
- Winandy, J.E. Relating wood chemistry and strength: Part II. Fundamental relationships between changes in wood chemistry and strength of wood. Wood Fiber Sci. 2017, 49, 2–11. [Google Scholar]
- Sivonen, H.; Maunu, S.L.; Sundholm, F.; Jämsä, S.; Viitaniemi, P. Magnetic Resonance Studies of Thermally Modified Wood; Walter de Gruyter: Berlin, Germany, 2002. [Google Scholar]
- Kim, D.-Y.; Nishiyama, Y.; Wada, M.; Kuga, S.; Okano, T. Thermal Decomposition of Cellulose Crystallites in Wood; Walter de Gruyter: Berlin, Germany, 2001. [Google Scholar]
- Brosse, N.; El Hage, R.; Chaouch, M.; Pétrissans, M.; Dumarçay, S.; Gérardin, P. Investigation of the chemical modifications of beech wood lignin during heat treatment. Polym. Degrad. Stab. 2010, 95, 1721–1726. [Google Scholar] [CrossRef]
- Altgen, M.; Uimonen, T.; Rautkari, L. The effect of de-and re-polymerization during heat-treatment on the mechanical behavior of Scots pine sapwood under quasi-static load. Polym. Degrad. Stab. 2018, 147, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Inari, G.N.; Mounguengui, S.; Dumarçay, S.; Pétrissans, M.; Gérardin, P. Evidence of char formation during wood heat treatment by mild pyrolysis. Polym. Degrad. Stab. 2007, 92, 997–1002. [Google Scholar] [CrossRef]
- Esteves, B.; Marques, A.V.; Domingos, I.; Pereira, H. Influence of steam heating on the properties of pine (Pinus pinaster) and eucalypt (Eucalyptus globulus) wood. Wood Sci. Technol. 2007, 41, 193–207. [Google Scholar] [CrossRef]
- Viitaniemi, P.; Jamsa, S.; Ek, P.; Viitanen, H. Method for Improving Biodegradation Resistance and Dimensional Stability of Cellulosic Products. U.S. Patent 5,678,324, 21 October 1997. [Google Scholar]
- Wentzel, M.; Altgen, M.; Militz, H. Analyzing reversible changes in hygroscopicity of thermally modified eucalypt wood from open and closed reactor systems. Wood Sci. Technol. 2018, 52, 889–907. [Google Scholar] [CrossRef]
- Altgen, M.; Militz, H. Influence of process conditions on hygroscopicity and mechanical properties of European beech thermally modified in a high-pressure reactor system. Holzforschung 2016, 70, 971–979. [Google Scholar] [CrossRef]
- Endo, K.; Obataya, E.; Zeniya, N.; Matsuo, M. Effects of heating humidity on the physical properties of hydrothermally treated spruce wood. Wood Sci. Technol. 2016, 50, 1161–1179. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.A.S.; Ramsay, J.; Laine, K.; Rautkari, L.; Hughes, M. Water vapour sorption behaviour of thermally modified wood. Int. Wood Prod. J. 2013, 4, 191–196. [Google Scholar] [CrossRef]
- Obataya, E.; Tomita, B. Hygroscopicity of heat-treated wood, 2: Reversible and irreversible reductions in the hygroscopicity of wood due to heating. Mokuzai Gakkaishi 2002, 48, 288–295. [Google Scholar]
- Altgen, M.; Militz, H. Thermally modified Scots pine and Norway spruce wood as substrate for coating systems. J. Coat. Technol. Res. 2017, 14, 531–541. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; El Bakali, I.; Gerardin, P.; Zoulalian, A. Wettability changes and mass loss during heat treatment of wood. Holzforschung 2005, 59, 35–37. [Google Scholar] [CrossRef]
- Pétrissans, M.; Gérardin, P.; Bakali, I.E.; Serraj, M. Wettability of heat-treated wood. Holzforschung 2003, 57, 301–307. [Google Scholar] [CrossRef]
- Pfriem, A. Alteration of water absorption coefficient of spruce (Picea abies (L.) Karst.) due to thermal modification. Drv. Ind. Znan. Čas. Pitanja Drv. Tehnol. 2011, 62, 311–313. [Google Scholar] [CrossRef]
- Scheiding, W.; Direske, M.; Zauer, M. Water absorption of untreated and thermally modified sapwood and heartwood of Pinus sylvestris L. Eur. J. Wood Wood Prod. 2016, 74, 585–589. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S.; Antikainen, T.; Viitaniemi, P. The water absorption of sapwood and heartwood of Scots pine and Norway spruce heat-treated at 170 °C, 190 °C, 210 °C and 230 °C. Holz Roh Werkst. 2006, 64, 192–197. [Google Scholar] [CrossRef]
- Sehlstedt-Persson, M.; Johansson, D.; Morén, T. Effect of heat treatment on the microstructure of pine, spruce and birch and the influence on capillary absorption. In Proceedings of the 5th IUFRO Symposium, Conference of Wood Structure and Properties ‘06, Sliač-Sielnica, Slovakia, 3–6 September 2006; pp. 373–379. [Google Scholar]
- Čermák, P.; Rautkari, L.; Horáček, P.; Saake, B.; Rademacher, P.; Sablík, P. Analysis of dimensional stability of thermally modified wood affected by re-wetting cycles. BioResources 2015, 10, 3242–3253. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, S. Effects of heat treatment on water repellence and anti-swelling efficiency of beech wood. Paper IRG/WP 02-40223. In Proceedings of the 33rd Annual Meeting International Research Group on Wood Preservation, Cardiff, Wales, 12–17 May 2002. [Google Scholar]
- Altgen, M.; Willems, W.; Hosseinpourpia, R.; Rautkari, L. Hydroxyl accessibility and dimensional changes of Scots pine sapwood affected by alterations in the cell wall ultrastructure during heat-treatment. Polym. Degrad. Stab. 2018, 152, 244–252. [Google Scholar] [CrossRef]
- Burmester, A. Zur Dimensionsstabilisierung von Holz. Holz Roh Werkst. 1975, 33, 333–335. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Curling, S.F.; Hale, M.D. On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym. Degrad. Stab. 2009, 94, 2184–2193. [Google Scholar] [CrossRef]
- Biziks, V.; Andersons, B.; Sansonetti, E.; Andersone, I.; Militz, H.; Grinins, J. One-stage thermo-hydro treatment (THT) of hardwoods: An analysis of form stability after five soaking-drying cycles. Holzforschung 2015, 69, 563–571. [Google Scholar] [CrossRef]
- Boonstra, M.; Van Acker, J.; Kegel, E.; Stevens, M. Optimisation of a two-stage heat treatment process: Durability aspects. Wood Sci. Technol. 2007, 41, 31–57. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Gérardin, P.; Zoulalian, A. Investigations of the reasons for fungal durability of heat-treated beech wood. Polym. Degrad. Stab. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S.; Viitanen, H. Decay resistance of sapwood and heartwood of untreated and thermally modified Scots pine and Norway spruce compared with some other wood species. Wood Mater. Sci. Eng. 2009, 4, 105–114. [Google Scholar] [CrossRef]
- Welzbacher, C.R.; Rapp, A.O. Durability of thermally modified timber from industrial-scale processes in different use classes: Results from laboratory and field tests. Wood Mater. Sci. Eng. 2007, 2, 4–14. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Kölle, M.; Brischke, C.; Richter, K. Effects of thermal modification on Postia placenta wood degradation dynamics: Measurements of mass loss, structural integrity and gene expression. Wood Sci. Technol. 2016, 50, 385–397. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Fan, H.; Wang, J. Wood carbonization as a protective treatment on resistance to wood destroying fungi. Int. Biodeterior. Biodegrad. 2018, 129, 42–49. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S.; Paajanen, L.; Viitanen, H. Durability of thermally modified Norway spruce and Scots pine in above-ground conditions. Wood Mater. Sci. Eng. 2011, 6, 163–169. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S.; Viitanen, H. Durability of thermally modified sapwood and heartwood of Scots pine and Norway spruce in the modified double layer test. Wood Mater. Sci. Eng. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Kamdem, D.; Pizzi, A.; Jermannaud, A. Durability of heat-treated wood. Holz Roh Werkst. 2002, 60, 1–6. [Google Scholar] [CrossRef]
- Boonstra, M.J.; Van Acker, J.; Tjeerdsma, B.F.; Kegel, E.V. Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann. For. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Altgen, M.; Awais, M.; Altgen, D.; Kyyrö, S.; Seppäläinen, H.; Rautkari, L. Micro-tensile behavior of Scots pine sapwood after heat treatments in superheated steam or pressurized hot water. J. Mater. Sci. 2020, 55, 12621–12635. [Google Scholar] [CrossRef]
- Yildiz, S.; Gezer, E.D.; Yildiz, U.C. Mechanical and chemical behavior of spruce wood modified by heat. Build. Environ. 2006, 41, 1762–1766. [Google Scholar] [CrossRef]
- Arnold, M. Effect of moisture on the bending properties of thermally modified beech and spruce. J. Mater. Sci. 2010, 45, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Kubojima, Y.; Okano, T.; Ohta, M. Bending strength and toughness of heat-treated wood. J. Wood Sci. 2000, 46, 8–15. [Google Scholar] [CrossRef]
- van Blokland, J.; Olsson, A.; Oscarsson, J.; Daniel, G.; Adamopoulos, S. Crack formation, strain distribution and fracture surfaces around knots in thermally modified timber loaded in static bending. Wood Sci. Technol. 2020, 54, 1001–1028. [Google Scholar] [CrossRef]
- Hughes, M.; Hill, C.; Pfriem, A. The toughness of hygrothermally modified wood. Holzforschung 2015, 69, 851–862. [Google Scholar] [CrossRef]
- Phuong, L.X.; Takayama, M.; Shida, S.; Matsumoto, Y.; Aoyagi, T. Determination of the accessible hydroxyl groups in heat-treated Styrax tonkinensis (Pierre) Craib ex Hartwich wood by hydrogen-deuterium exchange and 2H NMR spectroscopy. Holzforschung 2007, 61, 488–491. [Google Scholar] [CrossRef]
- Welzbacher, C.R.; Brischke, C.; Rapp, A.O. Influence of treatment temperature and duration on selected biological, mechanical, physical and optical properties of thermally modified timber. Wood Mater. Sci. Eng. 2007, 2, 66–76. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Zoulalian, A.; Gérardin, P. Investigation of wood wettability changes during heat treatment on the basis of chemical analysis. Polym. Degrad. Stab. 2005, 89, 70–84. [Google Scholar] [CrossRef]
- Repellin, V.; Guyonnet, R. Evaluation of heat-treated wood swelling by differential scanning calorimetry in relation to chemical composition. Holzforschung 2005, 59, 28–34. [Google Scholar] [CrossRef]
- Rautkari, L.; Hill, C.A. Effect of initial moisture content on the anti-swelling efficiency of thermally modified Scots pine sapwood treated in a high-pressure reactor under saturated steam. Holzforschung 2014, 68, 323–326. [Google Scholar] [CrossRef]
- Obataya, E.; Higashihara, T. Reversible and irreversible dimensional changes of heat-treated wood during alternate wetting and drying. Wood Sci. Technol. 2017, 51, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Altgen, M.; Hofmann, T.; Militz, H. Wood moisture content during the thermal modification process affects the improvement in hygroscopicity of Scots pine sapwood. Wood Sci. Technol. 2016, 50, 1181–1195. [Google Scholar] [CrossRef]
- Willems, W.; Altgen, M.; Rautkari, L. A molecular model for reversible and irreversible hygroscopicity changes by thermal wood modification. Holzforschung 2020, 74, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Wang, L. Thermal treatment of poplar hemicelluloses at 180 to 220 °C under nitrogen atmosphere. BioResources 2017, 12, 1128–1135. [Google Scholar]
- Chen, Y.; Tshabalala, M.A.; Gao, J.; Stark, N.M.; Fan, Y.; Ibach, R.E. Thermal behavior of extracted and delignified pine wood flour. Thermochim. Acta 2014, 591, 40–44. [Google Scholar] [CrossRef]
- Candelier, K.; Dumarçay, S.; Pétrissans, A.; Desharnais, L.; Gérardin, P.; Pétrissans, M. Comparison of chemical composition and decay durability of heat treated wood cured under different inert atmospheres: Nitrogen or vacuum. Polym. Degrad. Stab. 2013, 98, 677–681. [Google Scholar] [CrossRef]
- Borrega, M.; Kärenlampi, P.P. Effect of relative humidity on thermal degradation of Norway spruce (Picea abies) wood. J. Wood Sci. 2008, 54, 323–328. [Google Scholar] [CrossRef]
- Willems, W.; Lykidis, C.; Altgen, M.; Clauder, L. Quality control methods for thermally modified wood: COST action FP0904 2010–2014: Thermo-hydro-mechanical wood behaviour and processing. Holzforschung 2015, 69, 875–884. [Google Scholar] [CrossRef]
- Candelier, K.; Dibdiakova, J. A review on life cycle assessments of thermally modified wood. Holzforschung 2021, 75, 199–224. [Google Scholar] [CrossRef]
- Graham, R.D. Service Life of Treated and Untreated Fence Posts: 1952 Progress Report on the TJ Starker Post Farm; Oregon State University: Corvallis, OR, USA, 1952. [Google Scholar]
- Miller, H. Japanese Wood Craftsmanship. Winston Churchill Memorial Fellowship Final Report; Hugh Miller Furniture Company: Liverpool, UK, 2016; Available online: https://www.hughmillerfurniture.co.uk/blog/japanese-wood-craftsmanship (accessed on 22 May 2022).
- Okamura, K.; Yasui, N.; Kaku, C.; Koshihara, M.; Imamoto, K.; Oshima, K. A research on Yakisugi-performance evaluation and feasibility study for dissemination. J. Hous. Res. Found. Jusoken 2018, 44, 13–23. [Google Scholar] [CrossRef]
- Ebner, D.H.; Barbu, M.-C.; Klaushofer, J.; Čermák, P. Surface Modification of Spruce and Fir Sawn-Timber by Charring in the Traditional Japanese Method—Yakisugi. Polymers 2021, 13, 1662. [Google Scholar] [CrossRef] [PubMed]
- Kymäläinen, M.; Hautamäki, S.; Lillqvist, K.; Segerholm, K.; Rautkari, L. Surface modification of solid wood by charring. J. Mater. Sci. 2017, 52, 6111–6119. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Turunen, H.; Čermák, P.; Hautamäki, S.; Rautkari, L. Sorption-related characteristics of surface charred spruce wood. Materials 2018, 11, 2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čermák, P.; Dejmal, A.; Paschová, Z.; Kymäläinen, M.; Dömény, J.; Brabec, M.; Hess, D.; Rautkari, L. One-sided surface charring of beech wood. J. Mater. Sci. 2019, 54, 9497–9506. [Google Scholar] [CrossRef]
- Machová, D.; Oberle, A.; Zárybnická, L.; Dohnal, J.; Šeda, V.; Dömény, J.; Vacenovská, V.; Kloiber, M.; Pěnčík, J.; Tippner, J. Surface Characteristics of One-Sided Charred Beech Wood. Polymers 2021, 13, 1551. [Google Scholar] [CrossRef] [PubMed]
- Zicherman, J.B.; Williamson, R.B. Microstructure of wood char. Wood Sci. Technol. 1981, 15, 237–249. [Google Scholar] [CrossRef]
- Gray, M.R.; Corcoran, W.H.; Gavalas, G.R. Pyrolysis of a wood-derived material. Effects of moisture and ash content. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 646–651. [Google Scholar] [CrossRef]
- Atreya, A. Pyrolysis, Ignition and Fire Spread on Horizontal Surfaces of Wood. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1983. [Google Scholar]
- Anonymous. Eurocode 5: Design of Timber Structures–Part 1-2: General–Structural Fire Design; EN 1995-1-2:2004; European Committee for Standardization (CEN): Brussels, Belgium, 2004. [Google Scholar]
- Cachim, P.B.; Franssen, J.-M. Assessment of Eurocode 5 Charring Rate Calculation Methods. Fire Technol. 2009, 46, 169. [Google Scholar] [CrossRef]
- Lingens, A.; Windeisen, E.; Wegener, G. Investigating the combustion behaviour of various wood species via their fire gases. Wood Sci. Technol. 2005, 39, 49–60. [Google Scholar] [CrossRef]
- Hietaniemi, J. A Probabilistic Approach to Wood Charring Rate; VTT Technical Research Centre: Kivimiehentie, Finland, 2005. [Google Scholar]
- Frangi, A.; Fontana, M. Charring rates and temperature profiles of wood sections. Fire Mater. 2003, 27, 91–102. [Google Scholar] [CrossRef]
- Browne, F.L. Theories of the Combustion of Wood and Its Control: A Survey of the Literature; Report 2136; Forest Products Laboratory, Forest Service, U.S. Department of Agriculture: Madison, WI, USA, 1958.
- Kurosaki, F.; Ishimaru, K.; Hata, T.; Bronsveld, P.; Kobayashi, E.; Imamura, Y. Microstructure of wood charcoal prepared by flash heating. Carbon 2003, 41, 3057–3062. [Google Scholar] [CrossRef]
- Kymäläinen. Temp Weathering Manuscript. TBP. 2022; ahead of print.
- Li, K.; Mousavi, M.; Hostikka, S. Char cracking of medium density fibreboard due to thermal shock effect induced pyrolysis shrinkage. Fire Saf. J. 2017, 91, 165–173. [Google Scholar] [CrossRef]
- Kampe, A.; Pfriem, A. A note on artificial weathering of spruce (Picea abies) with a carbonised layer. Int. Wood Prod. J. 2018, 9, 86–89. [Google Scholar] [CrossRef]
- Rabe, S.; Klack, P.; Bahr, H.; Schartel, B. Assessing the fire behavior of woods modified by N-methylol crosslinking, thermal treatment, and acetylation. Fire Mater. 2020, 44, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Kymäläinen, M.; Rautkari, L.; Hill, C.A.S. Sorption behaviour of torrefied wood and charcoal determined by dynamic vapour sorption. J. Mater. Sci. 2015, 50, 7673–7680. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Dömény, J. Temporary Sorption Paper. TBP. 2022; ahead of print. [Google Scholar]
- Kymäläinen, M.; Havimo, M.; Keriö, S.; Kemell, M.; Solio, J. Biological degradation of torrefied wood and charcoal. Biomass Bioenergy 2014, 71, 170–177. [Google Scholar] [CrossRef]
- Hasburgh, L.E.; Zelinka, S.L.; Bishell, A.B.; Kirker, G.T. Durability and Fire Performance of Charred Wood Siding (Shou Sugi Ban). Forests 2021, 12, 1262. [Google Scholar] [CrossRef]
- Fakoussa, R.M.; Hofrichter, M. Biotechnology and microbiology of coal degradation. Appl. Microbiol. Biotechnol. 1999, 52, 25–40. [Google Scholar] [CrossRef]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood—The state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Evans, P.D. Review of the weathering and photostability of modified wood. Wood Mater. Sci. Eng. 2009, 4, 2–13. [Google Scholar] [CrossRef]
- Feist, W.C.; Hon, D.N.S. Chemistry of Weathering and Protection. In The Chemistry of Solid Wood; American Chemical Society: Washington, DC, USA, 1984; Volume 207, pp. 401–451. [Google Scholar]
- Windeisen, E.; Wegener, G. Behaviour of lignin during thermal treatments of wood. Ind. Crops Prod. 2008, 27, 157–162. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Turunen, H.; Rautkari, L. Effect of Weathering on Surface Functional Groups of Charred Norway Spruce Cladding Panels. Forests 2020, 11, 1373. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Lourencon, T.V.; Lillqvist, K. Natural weathering of soft- and hardwoods modified by contact charring and flame. Wood Sci. Technol. 2022, in press. [Google Scholar]
- Akizuki, M.; Hasemi, Y.; Yasui, N.; Kinoshita, K.; Yammamoto, K.; Yoshida, M.; Tamura, Y.; Takeda, M. Fire Safety Studies in the Restoration of a Historic Wooden Townhouse in Kyoto-Fire Safety Experiments on Japanese Traditional Wood-Based Constructions. In Proceedings of the Fire Safety Science Proceedings, 5th AOSFST, Newcastle, Australia, 3–7 March 1997; pp. 329–340. [Google Scholar]
- Tran, H.C.; White, R.H. Burning rate of solid wood measured in a heat release rate calorimeter. Fire Mater. 1992, 16, 197–206. [Google Scholar] [CrossRef]
- Pastor-Villegas, J.; Durán-Valle, C.J.; Valenzuela-Calahorro, C.; Gómez-Serrano, V. Organic chemical structure and structural shrinkage of chars prepared from rockrose. Carbon 1998, 36, 1251–1256. [Google Scholar] [CrossRef]
- Candelier, K.; Thevenon, M.-F.; Petrissans, A.; Dumarcay, S.; Gerardin, P.; Petrissans, M. Control of wood thermal treatment and its effects on decay resistance: A review. Ann. For. Sci. 2016, 73, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Rousset, P.; Lapierre, C.; Pollet, B.; Quirino, W.; Perre, P. Effect of severe thermal treatment on spruce and beech wood lignins. Ann. For. Sci. 2009, 66, 110. [Google Scholar] [CrossRef] [Green Version]
- Toth, P.; Vikström, T.; Molinder, R.; Wiinikka, H. Structure of carbon black continuously produced from biomass pyrolysis oil. Green Chem. 2018, 20, 3981–3992. [Google Scholar] [CrossRef]
- Schaffert, S. Steuerung und Optimierung von Holzvernetzungsprozessen. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2006. [Google Scholar]
- Mahnert, K.-C. Entwicklung Eines Nichttragenden Bodenbelages für den Schiffbau auf Basis Ausgewählter Verfahren der Holzmodifizierung. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2013. [Google Scholar]
- Behr, G.; Bollmus, S.; Gellerich, A.; Militz, H. Improvement of mechanical properties of thermally modified hardwood through melamine treatment. Wood Mater. Sci. Eng. 2018, 13, 262–270. [Google Scholar] [CrossRef]
- Behr, G. The Influence of Melamine Treatment in Combination with Thermal Modification on the Properties and Performance of Native Hardwoods. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2020. [Google Scholar]
- Wepner, F. Entwicklung Eines Modifizierungsverfahrens für Buchenfurniere (Fagus sylvatica L.) auf Basis von Zyklischen N-Methylol-Verbindungen. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2006. [Google Scholar]
- Dieste, A.; Krause, A.; Bollmus, S.; Militz, H. Physical and mechanical properties of plywood produced with 1.3-dimethylol-4.5-dihydroxyethyleneurea (DMDHEU)-modifiedveneers of Betula sp. and Fagus sylvatica. Holz Roh Werkst. 2008, 66, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Fleckenstein, M.; Biziks, V.; Mai, C.; Militz, H. Modification of beech veneers with lignin phenol formaldehyde resins in the production of laminated veneer lumber (LVL). Eur. J. Wood Wood Prod. 2018, 76, 843–851. [Google Scholar] [CrossRef]
- Bicke, S. Dimensionsstabile und Pilzresistente Furnierwerkstoffe Durch Zellwand-Modifizierung mit Niedermolekularem Phenol-Formaldehyd. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2019. [Google Scholar]
- Stamm, A.J.; Seborg, R.M. Resin-Treated Plywood. Ind. Eng. Chem. 1939, 31, 897–902. [Google Scholar] [CrossRef]
- Weaver, J.; Nielson, J.; Goldstein, I. Dimensional stabilization of wood with aldehydes and related compounds. For. Prod. J. 1960, 10, 306–310. [Google Scholar]
- Stefanowski, B.; Spear, M.; Pitman, A. Review of the use of PF and related resins for modification of solid wood. In Timber 2018; Prifysgol Bangor University: London, UK, 2018; pp. 165–179. [Google Scholar]
- Imamura, Y.; Kajita, H.; Higuchi, N. Modification of wood by treatment with low molecular phenol-formaldehyde resin (1). Influence of neutral and alkaline resins. In Proceedings of the 48th Annual Meeting of Japan Wood Research society, Shizuoka, Japan, 3–5 April 1998. [Google Scholar]
- Biziks, V.; Bicke, S.; Militz, H. Penetration depth of phenol-formaldehyde (PF) resin into beech wood studied by light microscopy. Wood Sci. Technol. 2019, 53, 165–176. [Google Scholar] [CrossRef]
- Altgen, M.; Awais, M.; Altgen, D.; Klüppel, A.; Mäkelä, M.; Rautkari, L. Distribution and curing reactions of melamine formaldehyde resin in cells of impregnation-modified wood. Sci. Rep. 2020, 10, 3366. [Google Scholar] [CrossRef] [Green Version]
- Krause, A. Holzmodifizierung mit N-Methylolvernetzern. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2006. [Google Scholar]
- Lukowsky, D. Holzschutz mit Melaminharzen. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1999. [Google Scholar]
- Behr, G.; Gellerich, A.; Bollmus, S.; Brinker, S.; Militz, H. The influence of curing conditions on properties of melamine modified wood. Eur. J. Wood Wood Prod. 2018, 76, 1263–1272. [Google Scholar] [CrossRef]
- He, G.; Riedl, B. Curing kinetics of phenol formaldehyde resin and wood-resin interactions in the presence of wood substrates. Wood Sci. Technol. 2004, 38, 69–81. [Google Scholar] [CrossRef]
- Krause, A.; Jones, D.; Van der Zee, M.; Militz, H. Interlace treatment—Wood modification with N-methylol compounds. In Proceedings of the First European Conference on Wood Modification, Ghent, Belgium, 3–4 April 2003; pp. 317–327. [Google Scholar]
- Klüppel, A.; Mai, C. The influence of curing conditions on the chemical distribution in wood modified with thermosetting resins. Wood Sci. Technol. 2013, 47, 643–658. [Google Scholar] [CrossRef] [Green Version]
- Bollmus, S. Biologische und Technologische Eigenschaften von Buchenholz Nach Einer Modifizierung mit 1,3-Dimethylol-4,5-Dihydroxyethyleneurea (DMDHEU). Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2011. [Google Scholar]
- ChemVerbots, V. Verordnung über Verbote und Beschränkungen des Inverkehrbringens und über die Abgabe Bestimmter Stoffe, Gemische und Erzeugnisse Nach dem Chemikaliengesetz [Chemicals Prohibition Order]; BMJV (Federal Ministry of Justice and Consumer Protection): Berlin, Germany, 2017.
- Derham, B.; Singh, T.; Militz, H. Commercialisation of DMDHEU Modified Wood in Australasia. In Proceedings of the International Research Group on Wood Preservation, IRG/WP/17-40772; International Research Group on Wood Preservation: Ghent, Belgium, 2017. [Google Scholar]
- Emmerich, L.; Militz, H. Added value and utilization of untreated and heat-treated poplar (Populus spp. L.) with and without treatment with N-methylol compounds. In Proceedings of the 8th Hardwood Conference, Sopron, Hungary, 25–26 October 2018. [Google Scholar]
- Emmerich, L.; Militz, H. Study on the impregnation quality of rubberwood (Hevea brasiliensis Müll. Arg.) and English oak (Quercus robur L.) sawn veneers after treatment with 1,3-dimethylol-4,5- dihydroxyethyleneurea (DMDHEU). Holzforschung 2020, 74, 362–371. [Google Scholar] [CrossRef]
- Gindl, W.; Gupta, H.S. Cell-wall hardness and Young’s modulus of melamine-modified spruce wood by nano-indentation. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1141–1145. [Google Scholar] [CrossRef]
- Gindl, W.; Zargar-Yaghubi, F.; Wimmer, R. Impregnation of softwood cell walls with melamine-formaldehyde resin. Bioresour. Technol. 2003, 87, 325–330. [Google Scholar] [CrossRef]
- Rapp, A.O.; Bestgen, H.; Adam, W.; Peek, R.-D. Electron Energy Loss Spectroscopy (EELS) for Quantification of Cell-Wall Penetration of a Melamine Resin. Holzforschung 1999, 53, 111–117. [Google Scholar] [CrossRef]
- Mahnert, K.-C.; Adamopoulos, S.; Koch, G.; Militz, H. Topochemistry of heat-treated and N-methylol melamine-modified wood of koto (Pterygota macrocarpa K. Schum.) and limba (Terminalia superba Engl. et. Diels). Holzforschung 2013, 67, 137–146. [Google Scholar] [CrossRef]
- Kielmann, B.C.; Adamopoulos, S.; Militz, H.; Mai, C. Decay resistance of ash, beech and maple wood modified with N-methylol melamine and a metal complex dye. Int. Biodeterior. Biodegrad. 2014, 89, 110–114. [Google Scholar] [CrossRef]
- Devallencourt, C.; Saiter, J.M.; Capitaine, D. Reactions between melamine formaldehyde resin and cellulose: Influence of pH. J. Appl. Polym. Sci. 2000, 78, 1884–1896. [Google Scholar] [CrossRef]
- Schindler, W.D.; Hauser, P.J. Chemical Finishing of Textiles; Woodhead Publishing Ltd.: Cambridge, UK, 2004. [Google Scholar] [CrossRef]
- Emmerich, L.; Altgen, M.; Rautkari, L.; Militz, H. Sorption behavior and hydroxyl accessibility of wood treated with different cyclic N-methylol compounds. J. Mater. Sci. 2020, 55, 16561–16575. [Google Scholar] [CrossRef]
- Pizzi, A. Melamine-Formaldehyde Adhesives. In Handbook of Adhesive Technology; Pizzi, A., Mittal, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- West, H.; Watt, W. Processes of Preparing Water-Soluble Melamine-Formaldehyde Resins and Products Thereof. U.S. Patent 2529856A, 4 November 1950. [Google Scholar]
- Okano, M.; Ogata, Y. Kinetics of the condensation of melamine with formaldehyde. J. Am. Chem. Soc. 1952, 74, 5728–5731. [Google Scholar] [CrossRef]
- Nastke, R.; Dietrich, K.; Reinisch, G.; Rafler, G.; Gajewski, H. The initial stage of the reaction of melamine with formaldehyde. J. Macromol. Sci.—Chem. 1986, 23, 579–596. [Google Scholar] [CrossRef]
- Merline, D.J.; Vukusic, S.; Abdala, A.A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar] [CrossRef]
- Emmerich, L.; Brischke, C.; Bollmus, S.; Militz, H. Dynamic strength properties and structural integrity of wood modified with cyclic N-methylol and N-methyl compounds. Holzforschung 2021, 75, 932–944. [Google Scholar] [CrossRef]
- Emmerich, L.; Ehrmann, A.; Brischke, C.; Militz, H. Comparative studies on the durability and moisture performance of wood modified with cyclic N-methylol and N-methyl compounds. Wood Sci. Technol. 2021, 55, 1531–1554. [Google Scholar] [CrossRef]
- Andrews, B.; Trask-Morrell, B. Long Term Formaldehyde Emissions from DMDHEU-Finished Cotton Fabrics. Text. Chem. Colorist 1997, 29, 16–19. [Google Scholar]
- Inoue, M.; Ogata, S.; Nishikawa, M.; Otsuka, Y.; Kawai, S.; Norimoto, M. Dimensional stability, mechanical properties, and color changes of a low molecular weight melamine-formaldehyde resin impregnated wood. Mokuzai Gakkaishi 1993, 39, 181–189. [Google Scholar]
- Pittman, C.U.; Kim, M.G.; Nicholas, D.D.; Wang, L.; Kabir, F.R.A.; Schultz, T.P.; Ingram, L.L. Wood Enhancement Treatments I. Impregnation of Southern Yellow Pine with Melamine-Formaldehyde and Melamine-Ammeline-Formaldehyde Resins. J. Wood Chem. Technol. 1994, 14, 577–603. [Google Scholar] [CrossRef]
- Rapp, A.O.; Peek, R.D. Melaminharzimprägniertes sowie mit Wetterschutzlasur oberflächenbehandeltes und unbehandeltes Vollholz während zweijähriger Freilandbewitterung. Holz Roh Werkst. 1999, 57, 331–339. [Google Scholar] [CrossRef]
- Kielmann, B.C.; Adamopoulos, S.; Militz, H.; Koch, G.; Mai, C. Modification of three hardwoods with an N-methylol melamine compound and a metal-complex dye. Wood Sci. Technol. 2014, 48, 123–136. [Google Scholar] [CrossRef]
- Kielmann, B.C.; Militz, H.; Adamopoulos, S. Combined N-methylol melamine-colouring agent modification of hardwoods to improve their performance under use class 3. In Proceedings of the Sixth European Conference on Wood Modification, Ljubljana, Slovenia, 17–18 September 2012; pp. 437–446. [Google Scholar]
- Sint, K.M.; Adamopoulos, S.; Koch, G.; Hapla, F.; Militz, H. Impregnation of Bombax ceiba and Bombax insigne wood with a N-methylol melamine compound. Wood Sci. Technol. 2013, 47, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Wang, X.; Liu, J. Changes in dimensional stability and mechanical properties of Eucalyptus pellita by melamine–urea–formaldehyde resin impregnation and heat treatment. Eur. J. Wood Wood Prod. 2013, 71, 557–562. [Google Scholar] [CrossRef]
- Verma, P.; Junga, U.; Militz, H.; Mai, C. Protection mechanisms of DMDHEU treated wood against white and brown rot fungi. Holzforschung 2009, 63, 371–378. [Google Scholar] [CrossRef]
- Emmerich, L.; Militz, H.; Brischke, C. Long-term performance of DMDHEU-treated wood installed in different test set-ups in ground, above ground and in the marine environment. Int. Wood Prod. J. 2020, 11, 27–37. [Google Scholar] [CrossRef]
- Meyer, L.; Brischke, C.; Melcher, E.; Brandt, K.; Lenz, M.T.; Soetbeer, A. Durability of English oak (Quercus robur L.)–Comparison of decay progress and resistance under various laboratory and field conditions. Int. Biodeterior. Biodegrad. 2014, 86, 79–85. [Google Scholar] [CrossRef]
- Gellerich, A.; Brischke, C.; Militz, H.; Klüppel, A. Resistance of modified wood against marine borers. Holztechnologie 2018, 59, 5–11. [Google Scholar]
- Emmerich, L.; Brischke, C.; Bicke, S.; Militz, H. Performance of resin-treated solid wood and laminated veneer lumber (LVL) under marine conditions. IRG/WP 21-10973. In Proceedings of the 52nd Annual Meeting of the International Research Group on Wood Protection, Online Meeting, Heidelberg, Germany, 23–26 June 2021. [Google Scholar]
- Emmerich, L.; Gascón-Garrido, P.; Militz, H. Resistance of modified wood to termite attack assessed in laboratory and field testing: A review of internal research. IRG/WP 18-40824. In Proceedings of the 49th Annual Meeting of the International Research Group on Wood Protection, Johannesburg, South Africa, 29 April–3 May 2018. [Google Scholar]
- Xie, Y.; Krause, A.; Militz, H.; Mai, C. Weathering of uncoated and coated wood treated with methylated 1,3-dimethylol-4,5-dihydroxyethyleneurea(mDMDHEU). Holz Roh Werkst. 2008, 66, 455–464. [Google Scholar] [CrossRef]
- Pfeffer, A.; Dieste, A.; Mai, C.; Militz, H. Effects of water glass and DMDHEU treatment on the colonisation of wood by Aureobasidium pullulans. Eur. J. Wood Wood Prod. 2011, 69, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, D.; Williams, A.D. Dimensional stabilization of wood with dimethylol compounds. IRG-WP 87-3412. In Proceedings of the 18th Annual Meeting of the International Research Group on Wood Protection, Honey Harbour, ON, Canada, 17–22 May 1987. [Google Scholar]
- Militz, H. Treatment of timber with water soluble dimethylol resins to improve their dimensional stability and durability. Wood Sci. Technol. 1993, 27, 347–355. [Google Scholar] [CrossRef]
- Biziks, V.; Bicke, S.; Koch, G.; Militz, H. Effect of phenol-formaldehyde (PF) resin oligomer size on the decay resistance of beech wood. Holzforschung 2021, 75, 574–583. [Google Scholar] [CrossRef]
- Emmerich, L.; Bleckmann, M.; Strohbusch, S.; Brischke, C.; Bollmus, S.; Militz, H. Growth behavior of wood-destroying fungi in chemically modified wood: Wood degradation and translocation of nitrogen compounds. Holzforschung 2021, 75, 786–797. [Google Scholar] [CrossRef]
- Altgen, M.; Altgen, D.; Klüppel, A.; Rautkari, L. Effect of curing conditions on the water vapor sorption behavior of melamine formaldehyde resin and resin-modified wood. J. Mater. Sci. 2020, 55, 11253–11266. [Google Scholar] [CrossRef]
- Spear, M.J.; Curling, S.F.; Dimitriou, A.; Ormondroyd, G.A. Review of Functional Treatments for Modified Wood. Coatings 2021, 11, 327. [Google Scholar] [CrossRef]
- Berglund, L.A.; Burgert, I. Bioinspired Wood Nanotechnology for Functional Materials. Adv. Mater. 2018, 30, 1704285. [Google Scholar] [CrossRef]
- Chen, C.J.; Kuang, Y.D.; Zhu, S.Z.; Burgert, I.; Keplinger, T.; Gong, A.; Li, T.; Berglund, L.; Eichhorn, S.J.; Hu, L.B. Structure-property-function relationships of natural and engineered wood. Nat. Rev. Mater. 2020, 5, 642–666. [Google Scholar] [CrossRef]
- Panzarasa, G.; Burgert, I. Designing functional wood materials for novel engineering applications. Holzforschung 2022, 76, 211–222. [Google Scholar] [CrossRef]
- Keplinger, T.; Wang, X.Q.; Burgert, I. Nanofibrillated cellulose composites and wood derived scaffolds for functional materials. J. Mater. Chem. A 2019, 7, 2981–2992. [Google Scholar] [CrossRef] [Green Version]
- Burgert, I.; Cabane, E.; Zollfrank, C.; Berglund, L. Bio-inspired functional wood-based materials-hybrids and replicates. Int. Mater. Rev. 2015, 60, 431–450. [Google Scholar] [CrossRef]
- Zhu, M.W.; Song, J.W.; Li, T.; Gong, A.; Wang, Y.B.; Dai, J.Q.; Yao, Y.G.; Luo, W.; Henderson, D.; Hu, L.B. Highly Anisotropic, Highly Transparent Wood Composites. Adv. Mater. 2016, 28, 5181. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.L.; Tu, K.K.; Goldhahn, C.; Keplinger, T.; Adobes-Vidal, M.; Sorieul, M.; Burgert, I. Luminescent and Hydrophobic Wood Films as Optical Lighting Materials. ACS Nano 2020, 14, 13775–13783. [Google Scholar] [CrossRef] [PubMed]
- Fink, S. Transparent wood—A new approach in the functional-study of wood structure. Holzforschung 1992, 46, 403–408. [Google Scholar] [CrossRef]
- Li, Y.Y.; Fu, Q.L.; Yu, S.; Yan, M.; Berglund, L. Optically Transparent Wood from a Nanoporous Cellulosic Template: Combining Functional and Structural Performance. Biomacromolecules 2016, 17, 1358–1364. [Google Scholar] [CrossRef]
- Li, Y.Y.; Fu, Q.L.; Rojas, R.; Yan, M.; Lawoko, M.; Berglund, L. Lignin-Retaining Transparent Wood. Chemsuschem 2017, 10, 3445–3451. [Google Scholar] [CrossRef] [Green Version]
- Montanari, C.; Olsen, P.; Berglund, L.A. Interface tailoring by a versatile functionalization platform for nanostructured wood biocomposites. Green Chem. 2020, 22, 8012–8023. [Google Scholar] [CrossRef]
- Montanari, C.; Ogawa, Y.; Olsen, P.; Berglund, L.A. High Performance, Fully Bio-Based, and Optically Transparent Wood Biocomposites. Adv. Sci. 2021, 8, 2100559. [Google Scholar] [CrossRef]
- Lang, A.W.; Li, Y.Y.; De Keersmaecker, M.; Shen, D.E.; Osterholm, A.M.; Berglund, L.; Reynolds, J.R. Transparent Wood Smart Windows: Polymer Electrochromic Devices Based on Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) Electrodes. ChemSusChem 2018, 11, 854–863. [Google Scholar] [CrossRef]
- Montanari, C.; Li, Y.Y.; Chen, H.; Yan, M.; Berglund, L.A. Transparent Wood for Thermal Energy Storage and Reversible Optical Transmittance. ACS Appl. Mater. Interfaces 2019, 11, 20465–20472. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity as a fundamental property of wood. Wood Sci. Technol. 1968, 2, 299–307. [Google Scholar] [CrossRef]
- Sun, J.G.; Guo, H.Y.; Ribera, J.; Wu, C.S.; Tu, K.K.; Binelli, M.; Panzarasa, G.; Schwarze, F.; Wang, Z.L.; Burgert, I. Sustainable and Biodegradable Wood Sponge Piezoelectric Nanogenerator for Sensing and Energy Harvesting Applications. ACS Nano 2020, 14, 14665–14674. [Google Scholar] [CrossRef]
- Sun, J.G.; Guo, H.Z.; Schadli, G.N.; Tu, K.K.; Schar, S.; Schwarze, F.; Panzarasa, G.; Ribera, J.; Burgert, I. Enhanced mechanical energy conversion with selectively decayed wood. Sci. Adv. 2021, 7, eabd9138. [Google Scholar] [CrossRef]
- Sun, J.G.; Tu, K.K.; Buchele, S.; Koch, S.M.; Ding, Y.; Ramakrishna, S.N.; Stucki, S.; Guo, H.Y.; Wu, C.S.; Keplinger, T.; et al. Functionalized wood with tunable tribopolarity for efficient triboelectric nanogenerators. Matter 2021, 4, 3049–3066. [Google Scholar] [CrossRef]
- Boutilier, M.S.H.; Lee, J.H.; Chambers, V.; Venkatesh, V.; Karnik, R. Water Filtration Using Plant Xylem. PLoS ONE 2014, 9, e89934. [Google Scholar] [CrossRef] [Green Version]
- Vitas, S.; Beckmann, P.; Skibinski, B.; Goldhahn, C.; Muff, L.F.; Cabane, E. Rejection of micron-sized particles using beech wood xylem. Environ. Sci.-Water Res. Technol. 2019, 5, 944–955. [Google Scholar] [CrossRef] [Green Version]
- Ramchander, K.; Hegde, M.; Antony, A.P.; Wang, L.D.; Leith, K.; Smith, A.; Karnik, R. Engineering and characterization of gymnosperm sapwood toward enabling the design of water filtration devices. Nat. Commun. 2021, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Büchele, S.; Mitchell, S.; Stricker, L.; Liu, C.; Goldhahn, C.; Allaz, J.; Ding, Y.; Günther, R.; Zhang, Z. Natural Wood-Based Catalytic Membrane Microreactors for Continuous Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 8417–8426. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Jiang, J.; Li, Y.; Gan, Z.; Su, S.; Ding, S.; Li, Z.; Hou, L. Distribution and leaching behavior of organophosphorus and brominated flame retardants in soil in Chengdu. Environ. Sci. Processes Impacts 2020, 22, 1295–1305. [Google Scholar] [CrossRef]
- Merk, V.; Chanana, M.; Gaan, S.; Burgert, I. Mineralization of wood by calcium carbonate insertion for improved flame retardancy. Holzforschung 2016, 70, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Merk, V.; Chanana, M.; Keplinger, T.; Gaan, S.; Burgert, I. Hybrid wood materials with improved fire retardance by bio-inspired mineralisation on the nano-and submicron level. Green Chem. 2015, 17, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Luković, M.; Mendoza, M.; Schlepütz, C.M.; Griffa, M.; Xu, B.; Gaan, S.; Herrmann, H.; Burgert, I. Bioinspired struvite mineralization for fire-resistant wood. ACS Appl. Mater. Interfaces 2019, 11, 5427–5434. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In Situ Polymerization of Furfuryl Alcohol with Ammonium Dihydrogen Phosphate in Poplar Wood for Improved Dimensional Stability and Flame Retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Kong, L.; Tu, K.; Guan, H.; Wang, X. Growth of high-density ZnO nanorods on wood with enhanced photostability, flame retardancy and water repellency. Appl. Surf. Sci. 2017, 407, 479–484. [Google Scholar] [CrossRef]
- Fu, Q.; Medina, L.; Li, Y.; Carosio, F.; Hajian, A.; Berglund, L.A. Nanostructured Wood Hybrids for Fire-Retardancy Prepared by Clay Impregnation into the Cell Wall. ACS Appl. Mater. Interfaces 2017, 9, 36154–36163. [Google Scholar] [CrossRef]






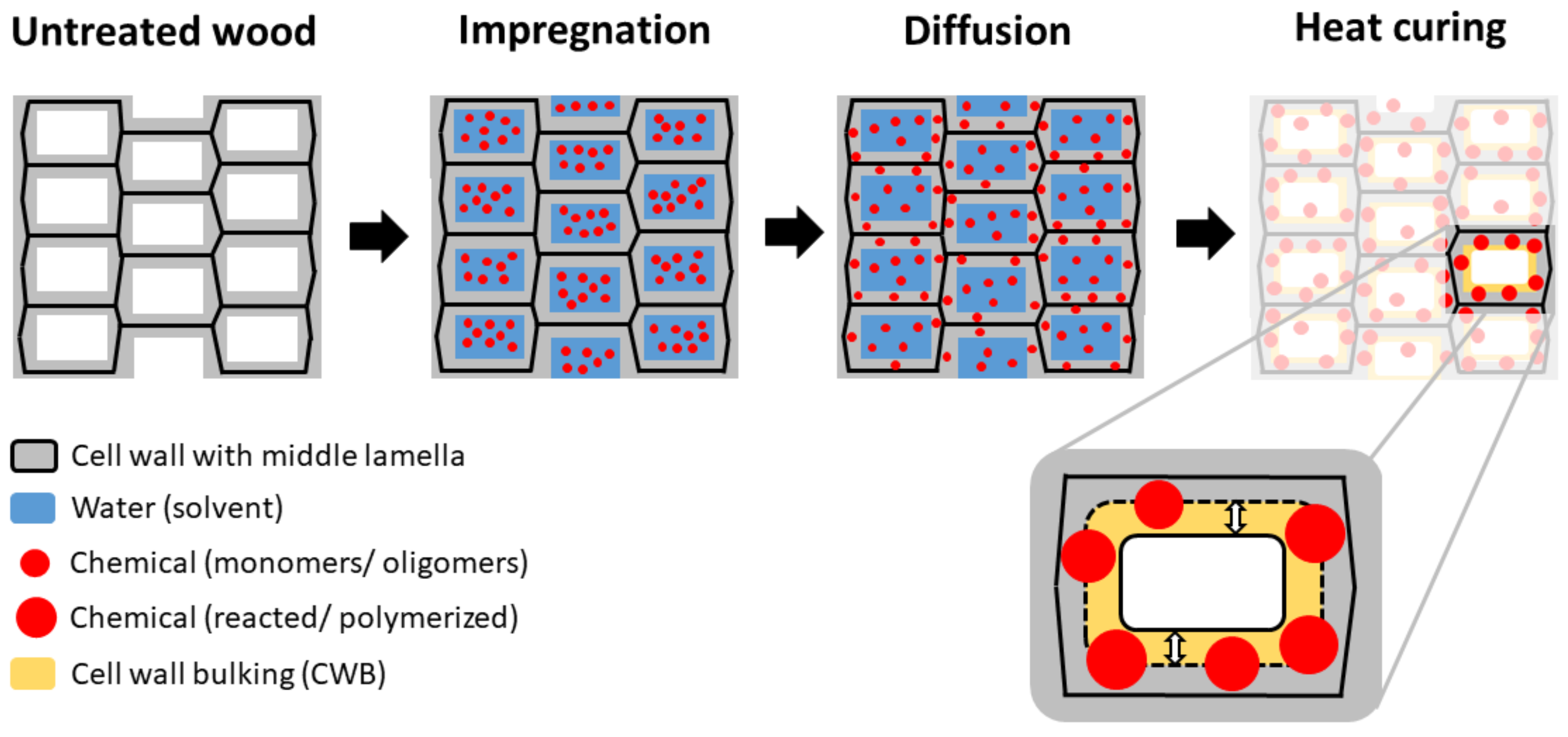
| Parameter | Original | Corrected |
|---|---|---|
| Moisture content, u (g g−1) | * | |
| Moisture exclusion efficiency, ηu (-) | * | |
| Swelling, Ssw (m3 m−3) | ||
| Anti-swelling efficiency, ηsw (-) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelinka, S.L.; Altgen, M.; Emmerich, L.; Guigo, N.; Keplinger, T.; Kymäläinen, M.; Thybring, E.E.; Thygesen, L.G. Review of Wood Modification and Wood Functionalization Technologies. Forests 2022, 13, 1004. https://doi.org/10.3390/f13071004
Zelinka SL, Altgen M, Emmerich L, Guigo N, Keplinger T, Kymäläinen M, Thybring EE, Thygesen LG. Review of Wood Modification and Wood Functionalization Technologies. Forests. 2022; 13(7):1004. https://doi.org/10.3390/f13071004
Chicago/Turabian StyleZelinka, Samuel L., Michael Altgen, Lukas Emmerich, Nathanael Guigo, Tobias Keplinger, Maija Kymäläinen, Emil E. Thybring, and Lisbeth G. Thygesen. 2022. "Review of Wood Modification and Wood Functionalization Technologies" Forests 13, no. 7: 1004. https://doi.org/10.3390/f13071004
APA StyleZelinka, S. L., Altgen, M., Emmerich, L., Guigo, N., Keplinger, T., Kymäläinen, M., Thybring, E. E., & Thygesen, L. G. (2022). Review of Wood Modification and Wood Functionalization Technologies. Forests, 13(7), 1004. https://doi.org/10.3390/f13071004









