Multiple-Temporal Scale Variations in Nighttime Sap Flow Response to Environmental Factors in Ficus concinna over a Subtropical Megacity, Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Descriptions and Tree Species
2.2. Sap Flow and Transpiration Measurements
2.3. Hydrometeorological Parameters
2.4. Date Treatment and Gap-Filling
2.5. Statistical Analysis
3. Results
3.1. Variations in Hydrometeorological Parameters
3.2. Nocturnal Variation in Sap Flow Rates and Its Regulating Parameters
3.3. Seasonal Variation in Nighttime Sap Flow Rates and Its Regulating Parameters
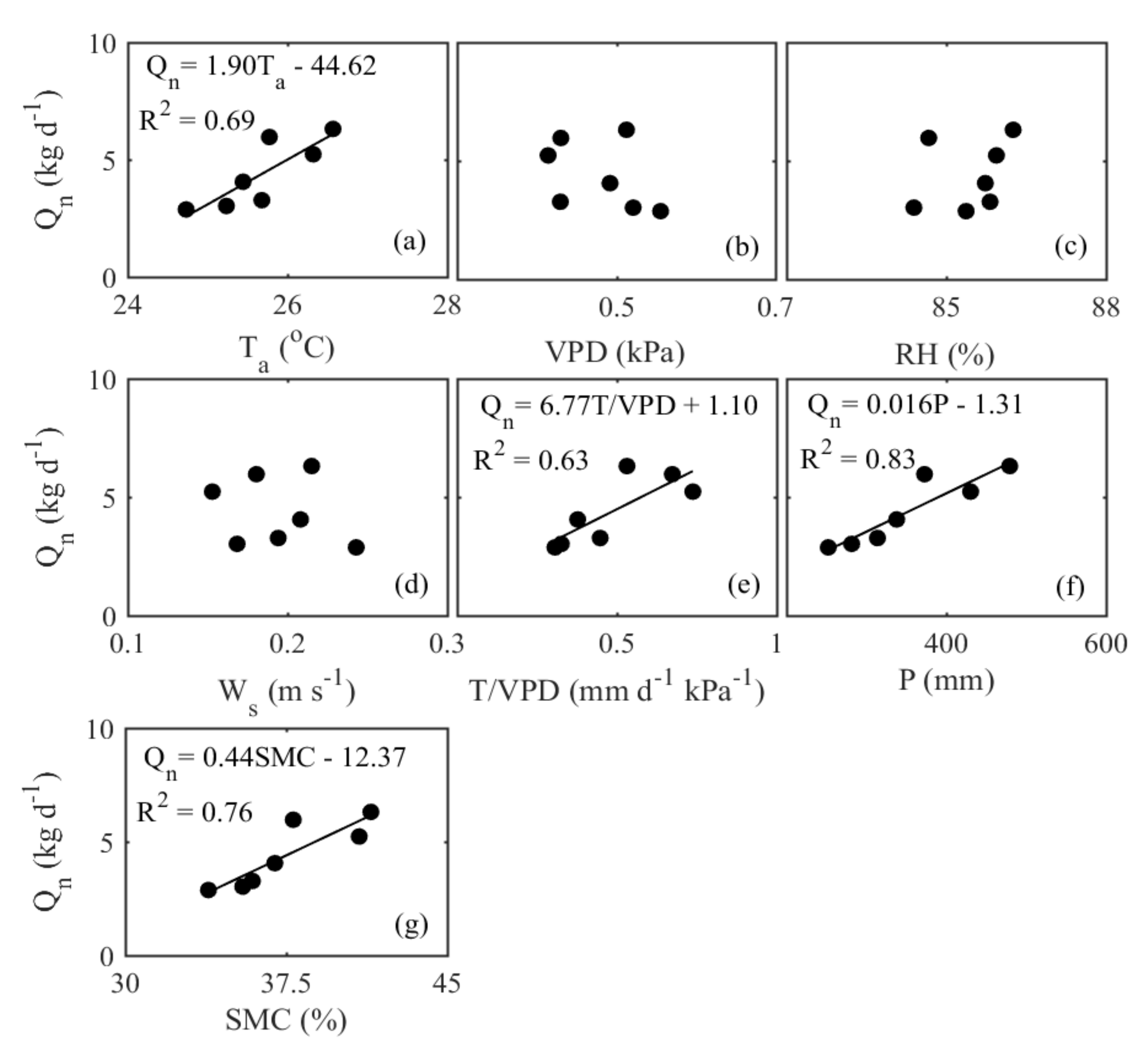
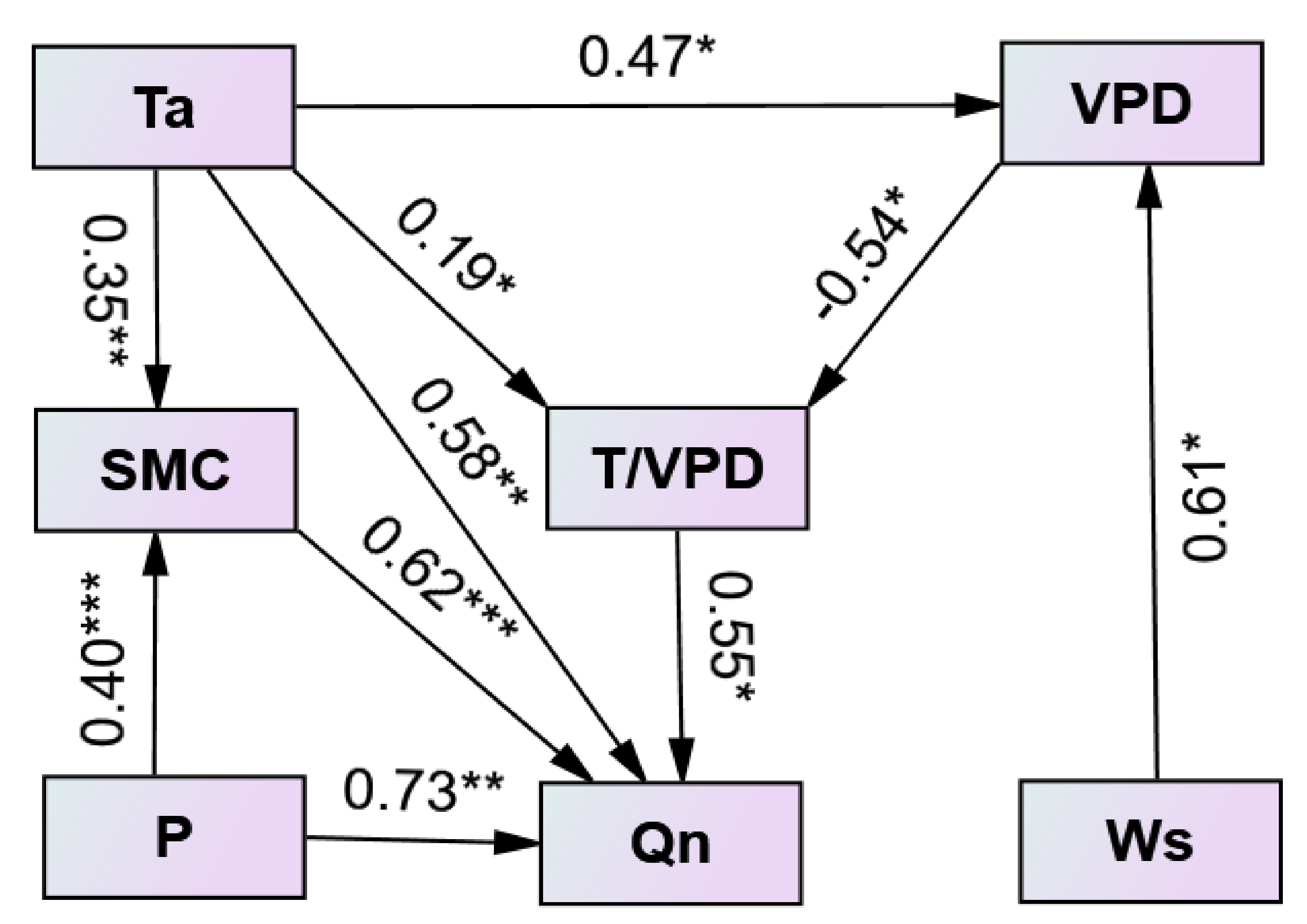
3.4. Interannual Variation in Nighttime Sap Flow Rates and Its Regulating Parameters
4. Discussion
4.1. Environmental Control on Sap Flow Rates at the Hourly/Nocturnal Scale
4.2. Environmental Control on Nighttime Sap Flow Rates at the Seasonal Scale
4.3. Environmental Control on Nighttime Sap Flow Rates at the Interannual Scale
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahn, M.; Reichstein, M.; Guan, K.; Moreno, J.M.; Williams, C. Preface: Climate extremes and biogeochemical cycles in the terrestrial biosphere: Impacts and feedbacks across scales. Biogeosciences 2015, 12, 4827–4830. [Google Scholar] [CrossRef]
- Domec, J.C.; Ogée, J.; Noormets, A.; Jouangy, J.; Gavazzi, M.; Treasure, E.; King, J.S. Interactive effects of nocturnal transpiration and climate change on the root hydraulic redistribution and carbon and water budgets of southern United States pine plantations. Tree Physiol. 2012, 32, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davy, R.; Esau, I.; Chernokulsky, A.; Outten, S.; Zilitinkevich, S. Diurnal asymmetry to the observed global warming. Int. J. Climatol. 2017, 37, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Siddiq, Z.; Cao, K.F. Nocturnal transpiration in 18 broadleaf timber species under a tropical seasonal climate. For. Ecol. Manag. 2018, 418, 47–54. [Google Scholar] [CrossRef]
- Hayat, M.; Iqbal, S.; Zha, T.; Jia, X.; Qian, D.; Bourque, C.P.A.; Yang, R. Biophysical control on nighttime sap flow in Salix psammophila in a semiarid shrubland ecosystem. Agric. For. Meteorol. 2021, 300, 108329. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Lewis, J.D.; Phillips, N.G.; Tissue, D.T. Consequences of nocturnal water loss: A synthesis of regulating factors and implications for capacitance, embolism and use in models. Tree Physiol. 2014, 34, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.H.; Liu, Y.; De Boeck, H.J.; Menzel, A.; Nijs, I.; Peaucelle, M.; Janssens, I.A. Three times greater weight of daytime than of night-time temperature on leaf unfolding phenology in temperate trees. New Phytol. 2016, 212, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Daley, M.J.; Phillips, N.G. Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol. 2006, 26, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, Z.; Sun, G.; Chen, L.; Xu, H.; Chen, S. Biophysical controls on nocturnal sap flow in plantation forests in a semi-arid region of northern China. Agric. For. Meteorol. 2020, 284, 107904. [Google Scholar] [CrossRef]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, G.; Bucci, S.J.; Scholz, F.G. Why do trees adjust water relations and hydraulic architecture in response to nutrient availability? Tree Physiol. 2013, 33, 238–240. [Google Scholar] [CrossRef] [Green Version]
- Gansert, D. Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Ehr.). Plant Cell Environ. 2003, 26, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Resco de Dios, V.; Loik, M.E.; Smith, R.; Aspinwall, M.J.; Tissue, D.T. Genetic variation in circadian regulation of nocturnal stomatal conductance enhances carbon assimilation and growth. Plant Cell Environ. 2016, 39, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Benyon, R.G. Nighttime water use in an irrigated Eucalyptus grandis plantation. Tree Physiol. 1999, 19, 853–859. [Google Scholar] [CrossRef]
- Fisher, J.B.; Baldocchi, D.D.; Misson, L.; Dawson, T.E.; Goldstein, A.H. What the towers don’t see at night: Nocturnal sap flow in trees and shrubs at two AmeriFlux sites in California. Tree Physiol. 2007, 27, 597–610. [Google Scholar] [CrossRef] [Green Version]
- Marks, C.O.; Lechowicz, M.J. The ecological and functional correlates of nocturnal transpiration. Tree Physiol. 2007, 27, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Si, J.; Feng, Q.; Yu, T.; Li, P.; Forster, M.A. Nighttime transpiration of Populus euphratica during different phenophases. J. For. Res. 2019, 30, 435–444. [Google Scholar] [CrossRef]
- Snyder, K.A.; Richards, J.H.; Donovan, L.A. Night-time conductance in C3 and C4 species: Do plants lose water at night? J. Exp. Bot. 2003, 54, 861–865. [Google Scholar] [CrossRef]
- Lombardozzi, D.L.; Zeppel, M.J.; Fisher, R.A.; Tawfik, A. Representing nighttime and minimum conductance in CLM4. 5: Global hydrology and carbon sensitivity analysis using observational constraints. Geosci. Model Dev. 2017, 10, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T.E.; Burgess, S.S.; Tu, K.P.; Oliveira, R.S.; Santiago, L.S.; Fisher, J.B.; Ambrose, A.R. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol. 2007, 27, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Forster, M.A. How significant is nocturnal sap flow? Tree Physiol. 2014, 34, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.; McJannet, D. Processes controlling transpiration in the rainforests of north Queensland, Australia. J. Hydrol. 2010, 384, 107–117. [Google Scholar] [CrossRef]
- Rosado, B.H.; Oliveira, R.S.; Joly, C.A.; Aidar, M.P.; Burgess, S.S. Diversity in nighttime transpiration behavior of woody species of the Atlantic Rain Forest, Brazil. Agric. For. Meteorol. 2012, 158, 13–20. [Google Scholar] [CrossRef]
- Hayat, M.; Xiang, J.; Yan, C.; Xiong, B.; Wang, B.; Qin, L.; Qiu, G.Y. Environmental control on transpiration and its cooling effect of Ficus concinna in a subtropical city Shenzhen, southern China. Agric. For. Meteorol. 2022, 312, 108715. [Google Scholar] [CrossRef]
- Bosch, D.D.; Marshall, L.K.; Teskey, R. Forest transpiration from sap flux density measurements in a Southeastern Coastal Plain riparian buffer system. Agric. For. Meteorol. 2014, 187, 72–82. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Roy, J.; Ferrio, J.P.; Alday, J.G.; Landais, D.; Milcu, A.; Gessler, A. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Sci. Rep. 2015, 5, 10975. [Google Scholar] [CrossRef]
- Rahman, M.A.; Moser, A.; Rötzer, T.; Pauleit, S. Microclimatic differences and their influence on transpirational cooling of Tilia cordata in two contrasting street canyons in Munich, Germany. Agric. For. Meteorol. 2017, 232, 443–456. [Google Scholar] [CrossRef]
- Hayat, M.; Zha, T.; Jia, X.; Iqbal, S.; Qian, D.; Bourque, C.P.A.; Yang, R. A multiple-temporal scale analysis of biophysical control of sap flow in Salix psammophila growing in a semiarid shrubland ecosystem of northwest China. Agric. For. Meteorol. 2020, 288, 107985. [Google Scholar] [CrossRef]
- Iqbal, S.; Zha, T.; Jia, X.; Hayat, M.; Qian, D.; Bourque, C.P.A.; Khan, A. Interannual variation in sap flow response in three xeric shrub species to periodic drought. Agric. For. Meteorol. 2021, 297, 108276. [Google Scholar] [CrossRef]
- Phillips, N.G.; Lewis, J.D.; Logan, B.A.; Tissue, D.T. Inter-and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol. 2010, 30, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Feng, Q.; Si, J.; Zhang, X.; Alec, D.; Zhao, C. Evidences and magnitude of nighttime transpiration derived from Populus euphratica in the extreme arid region of China. J. Plant Biol. 2016, 59, 648–657. [Google Scholar] [CrossRef]
- Ma, F.; Kang, S.; Li, F.; Zhang, J.; Du, T.; Hu, X.; Wang, M. Effect of water deficit in different growth stages on stem sap flux of greenhouse grown pear-jujube tree. Agric. Water Manag. 2007, 90, 190–196. [Google Scholar] [CrossRef]
- Zha, T.; Qian, D.; Jia, X.; Bai, Y.; Tian, Y.; Bourque, C.P.A.; Peltola, H. Soil moisture control of sap-flow response to biophysical factors in a desert-shrub species, Artemisia ordosica. Biogeosciences 2017, 14, 4533–4544. [Google Scholar] [CrossRef] [Green Version]
- Roth, M. Review of urban climate research in (sub) tropical regions. Int. J. Climatol. J. R. Meteorol. Soc. 2007, 27, 1859–1873. [Google Scholar] [CrossRef]
- Fang, W.; Lu, N.; Zhang, Y.; Jiao, L.; Fu, B. Responses of nighttime sap flow to atmospheric and soil dryness and its potential roles for shrubs on the Loess Plateau of China. J. Plant Ecol. 2018, 11, 717–729. [Google Scholar] [CrossRef]
- Rahman, M.A.; Stratopoulos, L.M.; Moser-Reischl, A.; Zölch, T.; Häberle, K.H.; Rötzer, T.; Pauleit, S. Traits of trees for cooling urban heat islands: A meta-analysis. Build. Environ. 2020, 170, 106606. [Google Scholar] [CrossRef]
- Rahman, M.A.; Moser, A.; Gold, A.; Rötzer, T.; Pauleit, S. Vertical air temperature gradients under the shade of two contrasting urban tree species during different types of summer days. Sci. Total Environ. 2018, 633, 100–111. [Google Scholar] [CrossRef]
- Young, M.H.; Wierenga, P.J.; Mancino, C.F. Monitoring near-surface soil water storage in turfgrass using time domain reflectometry and weighing lysimetry. Soil Sci. Soc. Am. J. 1997, 61, 1138–1146. [Google Scholar] [CrossRef]
- Gill, S.E.; Rahman, M.A.; Handley, J.F.; Ennos, A.R. Modelling water stress to urban amenity grass in Manchester UK under climate change and its potential impacts in reducing urban cooling. Urban For. Urban Green. 2013, 12, 350–358. [Google Scholar] [CrossRef]
- Konarska, J.; Uddling, J.; Holmer, B.; Lutz, M.; Lindberg, F.; Pleijel, H.; Thorsson, S. Transpiration of urban trees and its cooling effect in a high latitude city. Int. J. Biometeorol. 2016, 60, 159–172. [Google Scholar] [CrossRef]
- Qiu, G.Y.; Yu, X.; Wen, H.; Yan, C. An advanced approach for measuring the transpiration rate of individual urban trees by the 3D three-temperature model and thermal infrared remote sensing. J. Hydrol. 2020, 587, 125034. [Google Scholar] [CrossRef]
- Goulden, M.L.; Field, C.B. Three methods for monitoring the gas exchange of individual tree canopies: Ventilated-chamber, sap-flow and Penman-Monteith measurements on evergreen oaks. Funct. Ecol. 1994, 8, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Granier, A.; Biron, P.; Breda, N.; Pontailler, J.Y.; Saugier, B. Transpiration of trees and forest stands: Short and long-term monitoring using sapflow methods. Glob. Change Biol. 1996, 2, 265–274. [Google Scholar] [CrossRef]
- Litvak, E.; McCarthy, H.R.; Pataki, D.E. A method for estimating transpiration of irrigated urban trees in California. Landsc. Urban Plan. 2017, 158, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhao, P.; Hu, Y.; Ouyang, L.; Zhu, L.; Ni, G. Canopy transpiration and its cooling effect of three urban tree species in a subtropical city-Guangzhou, China. Urban For. Urban Green. 2019, 43, 126368. [Google Scholar] [CrossRef]
- Zou, Z.; Yan, C.; Yu, L.; Jiang, X.; Ding, J.; Qin, L.; Qiu, G. Impacts of land use/land cover types on interactions between urban heat island effects and heat waves. Build. Environ. 2021, 204, 108138. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, D.; Xu, Y.; Xiao, J.; Wang, J.; He, F.; Pan, H. Arrangement of landscape plants in parks of Shenzhen. J. Northeast. For. Univ. 2011, 39, 102–105. [Google Scholar]
- Qiu, G.; Tan, S.; Wang, Y.; Yu, X.; Yan, C. Characteristics of evapotranspiration of urban lawns in a sub-tropical megacity and its measurement by the ‘Three Temperature Model+ Infrared Remote Sensing’method. Remote Sens. 2017, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.Y.; Sack, L.; Wang, A.Y.; Cao, K.F.; Goldstein, G. Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Funct. Ecol. 2010, 24, 731–740. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Lundblad, M.; Lagergren, F.; Lindroth, A. Evaluation of heat balance and heat dissipation methods for sapflow measurements in pine and spruce. Ann. For. Sci. 2001, 58, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Delzon, S.; Sartore, M.; Granier, A.; Loustau, D. Radial profiles of sap flow with increasing tree size in maritime pine. Tree Physiol. 2004, 24, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Urban, L.; Zhao, P. Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: Theory and practice. Acta Bot. Sin. 2004, 46, 631–646. [Google Scholar]
- Oishi, A.C.; Hawthorne, D.A.; Oren, R. Baseliner: An open-source, interactive tool for processing sap flux data from thermal dissipation probes. SoftwareX 2016, 5, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Whitehead, D. Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiol. 1998, 18, 633–644. [Google Scholar] [CrossRef]
- Tetens, O. Uber einige meteorologische Begriffe. Z. Geophys. 1930, 6, 297–309. [Google Scholar]
- Wang, H.; Zhao, P.; Hölscher, D.; Wang, Q.; Lu, P.; Cai, X.A.; Zeng, X.P. Nighttime sap flow of Acacia mangium and its implications for nighttime transpiration and stem water storage. J. Plant Ecol. 2012, 5, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Chu, X.; Han, G.; Xing, Q.; Xia, J.; Sun, B.; Yu, J.; Li, D. Dual effect of precipitation redistribution on net ecosystem CO2 exchange of a coastal wetland in the Yellow River Delta. Agric. For. Meteorol. 2018, 249, 286–296. [Google Scholar] [CrossRef]
- Van Gorsel, E.; Harman, I.N.; Finnigan, J.J.; Leuning, R. Decoupling of air flow above and in plant canopies and gravity waves affect micrometeorological estimates of net scalar exchange. Agric. For. Meteorol. 2011, 151, 927–933. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H. Gap filling strategies for long term energy flux data sets. Agric. For. Meteorol. 2001, 107, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wen, X.; Sun, X.; Zhang, X.; Wang, H. The limiting effect of deep soil water on evapotranspiration of a subtropical coniferous plantation subjected to seasonal drought. Adv. Atmos. Sci. 2014, 31, 385–395. [Google Scholar] [CrossRef]
- Jeon, J. The strengths and limitations of the statistical modeling of complex social phenomenon: Focusing on SEM, path analysis, or multiple regression models. Int. J. Econ. Manag. Eng. 2015, 9, 1634–1642. [Google Scholar]
- Jia, X.; Zha, T.S.; Gong, J.N.; Wu, B.; Zhang, Y.Q.; Qin, S.G.; Peltola, H. Energy partitioning over a semi-arid shrubland in northern China. Hydrol. Processes 2016, 30, 972–985. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol. 2007, 27, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Novick, K.A.; Oren, R.; Stoy, P.C.; Siqueira, M.B.S.; Katul, G.G. Nocturnal evapotranspiration in eddy-covariance records from three co-located ecosystems in the Southeastern US: Implications for annual fluxes. Agric. For. Meteorol. 2009, 149, 1491–1504. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Shang, G.; Tian, Y.; Jia, X.; Zha, T.; Li, C.; Zhang, X. Dynamics of Nocturnal Evapotranspiration and Its Biophysical Controls over a Desert Shrubland of Northwest China. Forests 2021, 12, 1296. [Google Scholar] [CrossRef]
- Yan, C.; Guo, Q.; Li, H.; Li, L.; Qiu, G.Y. Quantifying the cooling effect of urban vegetation by mobile traverse method: A local-scale urban heat island study in a subtropical megacity. Build. Environ. 2020, 169, 106541. [Google Scholar] [CrossRef]
- Montaldo, N.; Oren, R. The way the wind blows matters to ecosystem water use efficiency. Agric. For. Meteorol. 2016, 217, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.Y.; Si, J.H.; Feng, Q.; Yu, T.F.; Du Li, P. Comparative study of daytime and nighttime sap flow of Populus euphratica. Plant Growth Regul. 2017, 82, 353. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Xu, L.; Kiang, N. How plant functional-type, weather, seasonal drought, and soil physical properties alter water and energy fluxes of an oak–grass savanna and an annual grassland. Agric. For. Meteorol. 2004, 123, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, J.; Zhao, P.; Zhu, L.; Ouyang, L.; Ni, G.; Zhao, X. Biotic-and abiotic-driven variations of the night-time sap flux of three co-occurring tree species in a low subtropical secondary broadleaf forest. AoB Plants 2018, 10, ply025. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Liu, B. The response of sap flow in shrubs to rainfall pulses in the desert region of China. Agric. For. Meteorol. 2010, 150, 1297–1306. [Google Scholar] [CrossRef]
- Zhu, L.W.; Zhao, P. Temporal variation in sap-flux-scaled transpiration and cooling effect of a subtropical Schima superba plantation in the urban area of Guangzhou. J. Integr. Agric. 2013, 12, 1350–1356. [Google Scholar] [CrossRef]
- Zha, T.; Barr, A.G.; van der Kamp, G.; Black, T.A.; McCaughey, J.H.; Flanagan, L.B. Interannual variation of evapotranspiration from forest and grassland ecosystems in western Canada in relation to drought. Agric. For. Meteorol. 2010, 150, 1476–1484. [Google Scholar] [CrossRef]
- Alvarado-Barrientos, M.S.; Asbjornsen, H.; Holwerda, F. Is nighttime transpiration enhanced after fog events? Acta. Hort. 2013, 991, 133–139. [Google Scholar] [CrossRef]
- Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Campanello, P.; Scholz, F.G. Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in Neotropical savanna trees. Trees 2005, 19, 296–304. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Sack, L.; Savage, J. Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiol. 2007, 27, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Zeppel, M.; Tissue, D.; Taylor, D.; Macinnis-Ng, C.; Eamus, D. Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiol. 2010, 30, 988–1000. [Google Scholar] [CrossRef] [Green Version]
- Barbeta, A.; Ogaya, R.; Peñuelas, J. Comparative study of diurnal and nocturnal sap flow of Quercus ilex and Phillyrea latifolia in a Mediterranean holm oak forest in Prades (Catalonia, NE Spain). Trees 2012, 26, 1651–1659. [Google Scholar] [CrossRef]
- Pfautsch, S.; Keitel, C.; Turnbull, T.L.; Braimbridge, M.J.; Wright, T.E.; Simpson, R.R.; Adams, M.A. Diurnal patterns of water use in Eucalyptus victrix indicate pronounced desiccation–rehydration cycles despite unlimited water supply. Tree Physiol. 2011, 31, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, K.L.; Pangle, R.; Schotzko, A.D. Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiol. 2007, 27, 621–629. [Google Scholar] [CrossRef]
- Yoshifuji, N.; Kumagai, T.O.; Tanaka, K.; Tanaka, N.; Komatsu, H.; Suzuki, M.; Tantasirin, C. Inter-annual variation in growing season length of a tropical seasonal forest in northern Thailand. For. Ecol. Manag. 2006, 229, 333–339. [Google Scholar] [CrossRef]
- Liu, H.; Park Williams, A.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Badmaeva, N.K. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Change Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Li, X.; Liang, E.; Beck, P.S.; Huang, Y. Seasonal divergence in the interannual responses of Northern Hemisphere vegetation activity to variations in diurnal climate. Sci. Rep. 2016, 6, 19000. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zha, T.; Jia, X.; Tian, Y.; Bourque, C.P.A.; Liu, P.; Chen, W. Energy and water vapor exchange over a young plantation in northern China. Agric. For. Meteorol. 2018, 263, 334–345. [Google Scholar] [CrossRef]
- Xie, J.; Zha, T.; Zhou, C.; Jia, X.; Yu, H.; Yang, B.; Peltola, H. Seasonal variation in ecosystem water use efficiency in an urban-forest reserve affected by periodic drought. Agric. For. Meteorol. 2016, 221, 142–151. [Google Scholar] [CrossRef]
- Wang, H.; Tetzlaff, D.; Dick, J.J.; Soulsby, C. Assessing the environmental controls on Scots pine transpiration and the implications for water partitioning in a boreal headwater catchment. Agric. For. Meteorol. 2017, 240, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Meinzer, F.C.; James, S.A.; Goldstein, G. Dynamics of transpiration, sap flow and use of stored water in tropical forest canopy trees. Tree Physiol. 2004, 24, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Verbeeck, H.; Steppe, K.; Nadezhdina, N.; de Beeck, M.O.; Deckmyn, G.; Meiresonne, L.; Janssens, I.A. Stored water use and transpiration in Scots pine: A modeling analysis with ANAFORE. Tree Physiol. 2007, 27, 1671–1685. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tetzlaff, D.; Soulsby, C. Hysteretic response of sap flow in Scots pine (Pinus sylvestris) to meteorological forcing in a humid low-energy headwater catchment. Ecohydrology 2019, 12, e2125. [Google Scholar] [CrossRef]
- Ewers, B.E.; Oren, R.; Phillips, N.; Strömgren, M.; Linder, S. Mean canopy stomatal conductance responses to water and nutrient availabilities in Picea abies and Pinus taeda. Tree Physiol. 2001, 21, 841–850. [Google Scholar] [CrossRef] [PubMed]
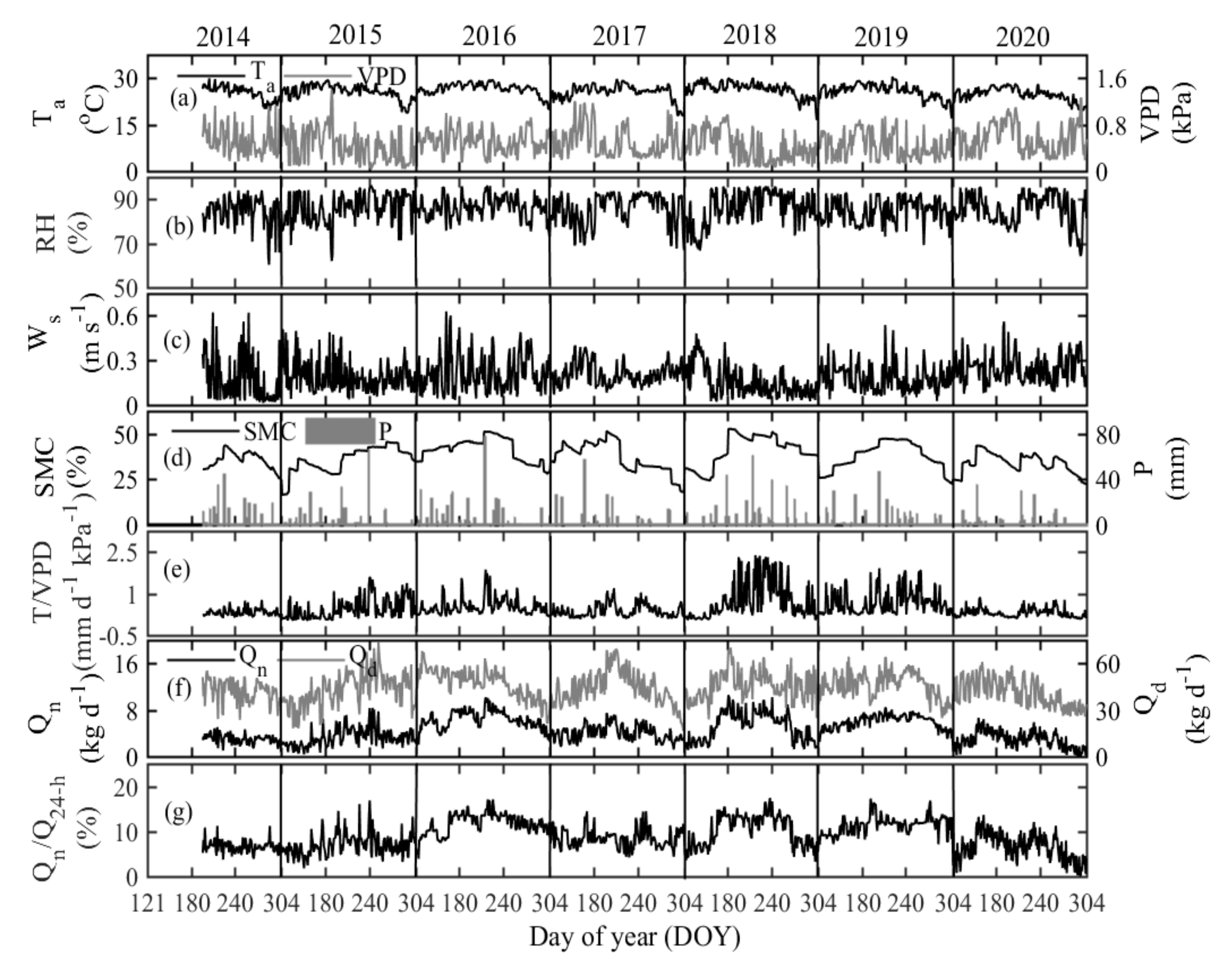

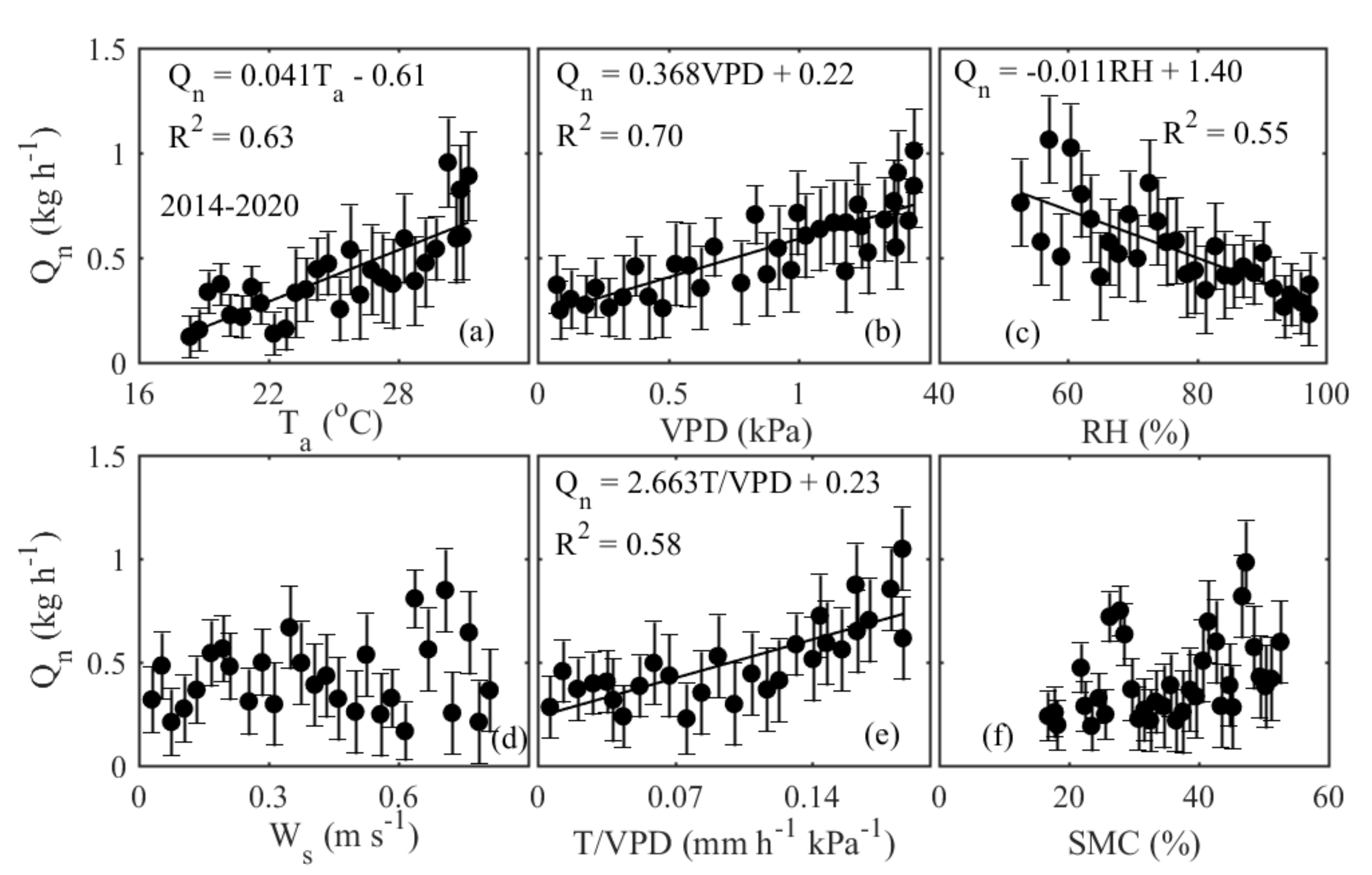
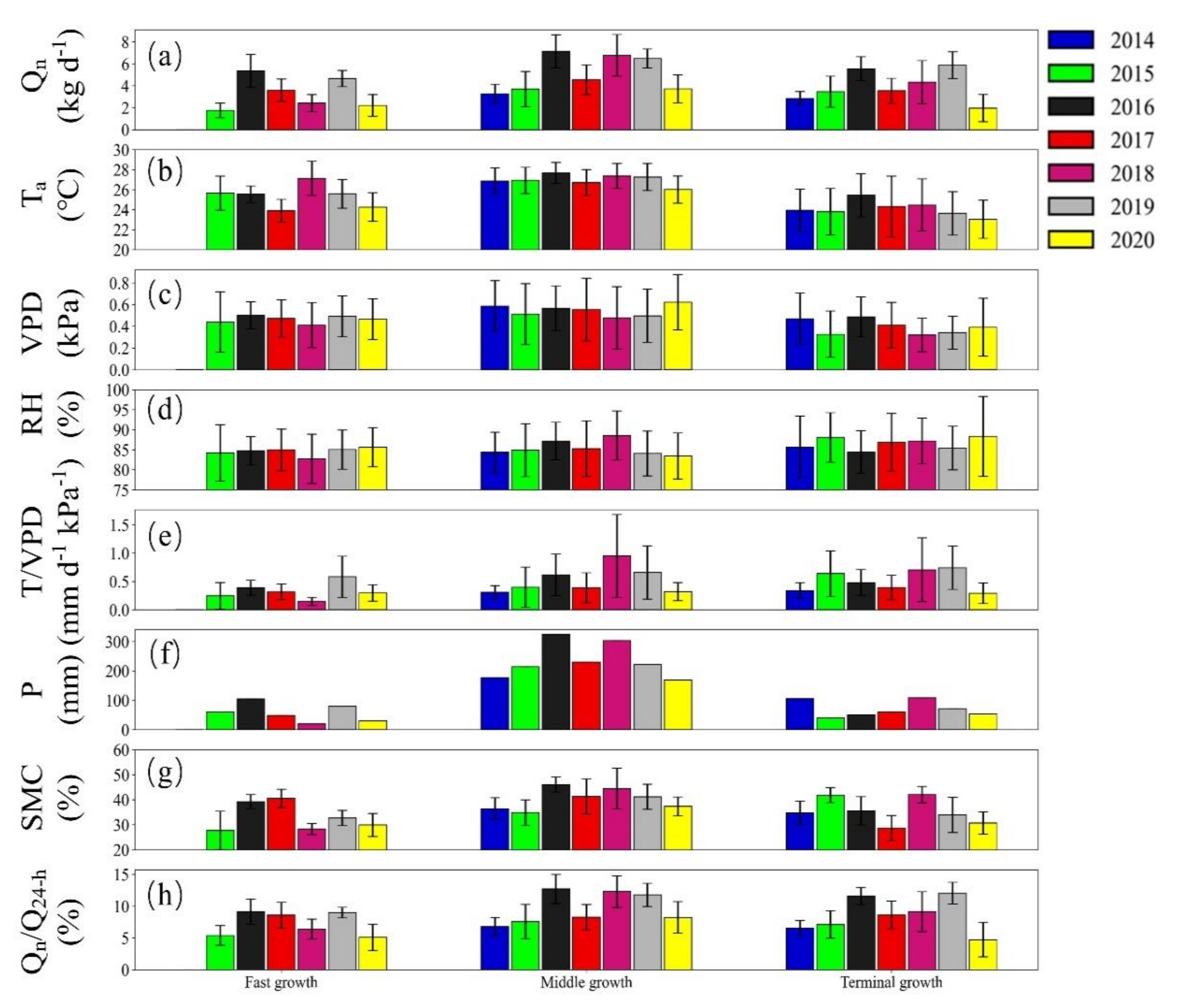
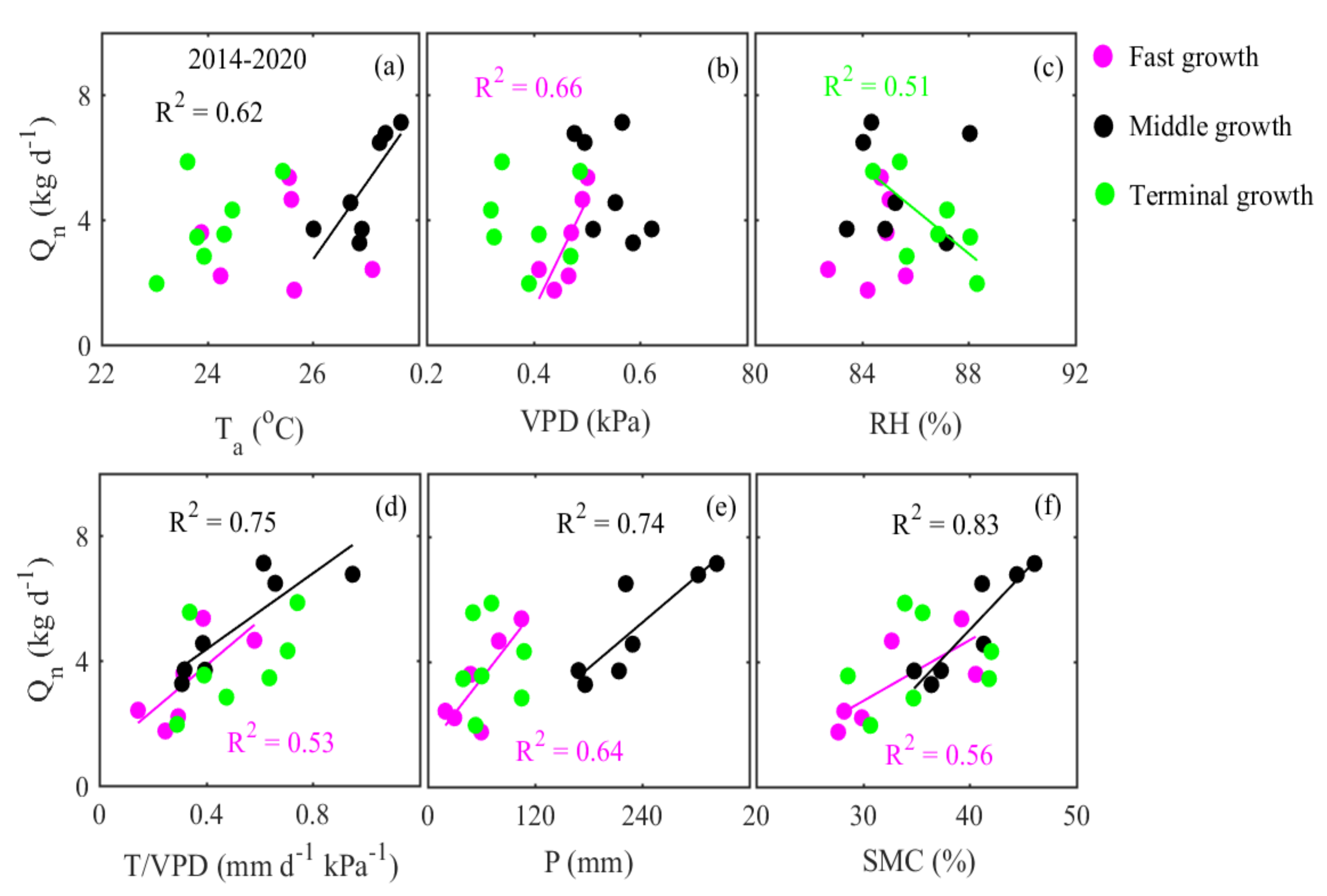

| Variables | Year | May | Jun | Jul | Aug | Sep | Oct | Average or Sum | CV |
|---|---|---|---|---|---|---|---|---|---|
| Ta (°C) | 2014 | NA | NA | 27.04 | 26.59 | 25.21 | 22.08 | 25.23 | 8.87 |
| 2015 | 25.64 | 27.63 | 26.96 | 26.20 | 25.45 | 22.21 | 25.68 | 7.34 | |
| 2016 | 25.55 | 27.57 | 27.86 | 27.55 | 26.67 | 24.22 | 26.57 | 5.37 | |
| 2017 | 23.89 | 26.84 | 26.20 | 27.08 | 25.88 | 22.80 | 25.45 | 6.75 | |
| 2018 | 27.12 | 27.22 | 27.71 | 27.18 | 26.20 | 22.51 | 26.32 | 7.33 | |
| 2019 | 25.59 | 27.47 | 27.40 | 26.91 | 24.64 | 22.64 | 25.78 | 7.35 | |
| 2020 | 24.25 | 26.18 | 27.00 | 24.85 | 24.57 | 21.56 | 24.73 | 7.57 | |
| Mean | 25.34 | 27.15 | 27.17 | 26.62 | 25.52 | 22.57 | 25.68 | ||
| VPD (kPa) | 2014 | NA | NA | 0.60 | 0.57 | 0.42 | 0.47 | 0.52 | 16.13 |
| 2015 | 0.43 | 0.62 | 0.54 | 0.37 | 0.33 | 0.26 | 0.42 | 31.11 | |
| 2016 | 0.50 | 0.58 | 0.52 | 0.44 | 0.48 | 0.53 | 0.51 | 9.57 | |
| 2017 | 0.47 | 0.80 | 0.36 | 0.49 | 0.30 | 0.50 | 0.49 | 35.36 | |
| 2018 | 0.41 | 0.73 | 0.40 | 0.32 | 0.28 | 0.32 | 0.41 | 39.14 | |
| 2019 | 0.49 | 0.58 | 0.46 | 0.42 | 0.31 | 0.35 | 0.43 | 21.84 | |
| 2020 | 0.46 | 0.63 | 0.81 | 0.41 | 0.36 | 0.62 | 0.55 | 30.53 | |
| Mean | 0.46 | 0.66 | 0.53 | 0.43 | 0.36 | 0.44 | 0.48 | ||
| RH (%) | 2014 | NA | NA | 83.83 | 84.64 | 87.22 | 81.85 | 84.39 | 2.63 |
| 2015 | 84.21 | 82.35 | 83.79 | 88.40 | 88.21 | 87.90 | 85.81 | 3.10 | |
| 2016 | 84.72 | 85.30 | 86.80 | 89.65 | 87.12 | 83.89 | 86.25 | 2.40 | |
| 2017 | 84.93 | 78.52 | 87.03 | 89.99 | 91.10 | 82.74 | 85.72 | 5.47 | |
| 2018 | 82.73 | 84.23 | 87.11 | 89.84 | 88.26 | 83.48 | 85.94 | 3.34 | |
| 2019 | 85.03 | 81.89 | 84.39 | 85.81 | 86.89 | 83.99 | 84.67 | 2.02 | |
| 2020 | 85.64 | 84.56 | 80.77 | 89.75 | 90.59 | 80.93 | 85.37 | 4.91 | |
| Mean | 84.54 | 82.81 | 85.24 | 87.88 | 88.44 | 83.58 | 85.45 | ||
| Ws (m s−1) | 2014 | NA | NA | 0.24 | 0.14 | 0.20 | 0.07 | 0.16 | 45.51 |
| 2015 | 0.25 | 0.18 | 0.22 | 0.15 | 0.16 | 0.18 | 0.19 | 19.28 | |
| 2016 | 0.17 | 0.26 | 0.20 | 0.17 | 0.24 | 0.22 | 0.21 | 17.38 | |
| 2017 | 0.17 | 0.29 | 0.16 | 0.19 | 0.17 | 0.23 | 0.20 | 23.89 | |
| 2018 | 0.29 | 0.15 | 0.13 | 0.12 | 0.11 | 0.09 | 0.15 | 48.24 | |
| 2019 | 0.21 | 0.19 | 0.15 | 0.19 | 0.15 | 0.16 | 0.18 | 12.72 | |
| 2020 | 0.24 | 0.23 | 0.27 | 0.20 | 0.22 | 0.27 | 0.24 | 11.57 | |
| Mean | 0.226 | 0.221 | 0.200 | 0.170 | 0.184 | 0.180 | 0.194 | ||
| P (mm) | 2014 | NA | NA | 50.70 | 125.60 | 72.00 | 33.30 | 281.60 | 14.22 |
| 2015 | 48.00 | 52.10 | 90.50 | 83.60 | 32.90 | 6.80 | 313.90 | 9.97 | |
| 2016 | 105.00 | 104.60 | 44.10 | 175.00 | 16.50 | 34.20 | 479.40 | 12.40 | |
| 2017 | 60.00 | 97.90 | 103.10 | 16.50 | 29.40 | 31.00 | 337.90 | 10.97 | |
| 2018 | 19.78 | 100.21 | 39.51 | 184.15 | 72.82 | 14.10 | 430.57 | 14.87 | |
| 2019 | 79.60 | 51.20 | 69.40 | 101.20 | 37.60 | 33.80 | 372.80 | 7.00 | |
| 2020 | 29.98 | 57.00 | 14.40 | 97.40 | 44.80 | 8.80 | 252.38 | 12.91 | |
| Mean | 57.06 | 77.16 | 58.81 | 111.92 | 43.71 | 23.14 | 352.65 | ||
| SMC (%) | 2014 | NA | NA | 33.29 | 39.02 | 38.33 | 31.24 | 35.47 | 10.71 |
| 2015 | 27.69 | 32.13 | 31.62 | 40.53 | 43.57 | 40.10 | 35.94 | 17.49 | |
| 2016 | 39.23 | 43.02 | 45.30 | 49.78 | 39.27 | 32.02 | 41.44 | 14.69 | |
| 2017 | 40.56 | 42.86 | 47.56 | 33.54 | 32.18 | 25.12 | 36.97 | 22.13 | |
| 2018 | 28.24 | 34.15 | 49.72 | 49.04 | 44.57 | 39.56 | 40.88 | 20.90 | |
| 2019 | 32.69 | 35.16 | 42.13 | 45.98 | 40.52 | 30.50 | 37.83 | 15.83 | |
| 2020 | 29.90 | 40.91 | 33.78 | 37.36 | 33.88 | 27.37 | 33.87 | 14.45 | |
| Mean | 33.05 | 38.04 | 40.49 | 42.18 | 38.90 | 32.27 | 37.48 | ||
| T/VPD (mm d−1 kPa−1) | 2014 | NA | NA | 0.29 | 0.31 | 0.35 | 0.32 | 0.32 | 7.03 |
| 2015 | 0.24 | 0.19 | 0.36 | 0.62 | 0.57 | 0.67 | 0.44 | 45.85 | |
| 2016 | 0.38 | 0.47 | 0.54 | 0.82 | 0.55 | 0.40 | 0.53 | 30.16 | |
| 2017 | 0.31 | 0.21 | 0.60 | 0.33 | 0.51 | 0.27 | 0.37 | 40.61 | |
| 2018 | 0.14 | 0.44 | 0.97 | 1.44 | 0.97 | 0.44 | 0.73 | 64.74 | |
| 2019 | 0.58 | 0.47 | 0.68 | 0.81 | 0.90 | 0.58 | 0.67 | 23.59 | |
| 2020 | 0.29 | 0.36 | 0.19 | 0.39 | 0.39 | 0.19 | 0.30 | 30.02 | |
| Mean | 0.32 | 0.35 | 0.52 | 0.67 | 0.61 | 0.41 | 0.48 | ||
| Qn (kg d−1) | 2014 | NA | NA | 3.47 | 3.15 | 2.78 | 2.69 | 3.02 | 11.87 |
| 2015 | 1.74 | 2.59 | 3.79 | 4.37 | 3.94 | 3.16 | 3.27 | 29.75 | |
| 2016 | 5.36 | 6.08 | 7.09 | 8.16 | 6.13 | 4.99 | 6.30 | 18.44 | |
| 2017 | 3.59 | 4.31 | 5.27 | 4.07 | 4.11 | 2.99 | 4.05 | 18.76 | |
| 2018 | 2.41 | 5.76 | 6.88 | 7.62 | 5.60 | 3.07 | 5.22 | 39.65 | |
| 2019 | 4.65 | 5.76 | 6.78 | 6.87 | 6.83 | 4.91 | 5.97 | 16.93 | |
| 2020 | 1.95 | 4.62 | 3.04 | 3.49 | 2.74 | 1.40 | 2.87 | 40.56 | |
| Mean | 3.28 | 4.85 | 5.19 | 5.39 | 4.59 | 3.32 | 4.39 | ||
| Qn/Q24-h (%) | 2014 | NA | NA | 7.07 | 6.64 | 6.42 | 6.60 | 6.68 | 4.14 |
| 2015 | 5.39 | 6.32 | 7.99 | 8.40 | 7.01 | 7.20 | 7.05 | 15.54 | |
| 2016 | 9.13 | 11.03 | 12.96 | 14.04 | 12.25 | 10.95 | 11.73 | 14.76 | |
| 2017 | 8.58 | 8.88 | 8.57 | 7.35 | 8.91 | 8.32 | 8.43 | 6.79 | |
| 2018 | 6.40 | 11.07 | 12.41 | 13.34 | 11.36 | 6.96 | 10.26 | 28.16 | |
| 2019 | 8.99 | 10.93 | 12.72 | 11.64 | 12.05 | 12.05 | 11.40 | 11.55 | |
| 2020 | 5.09 | 7.96 | 7.99 | 8.80 | 6.31 | 3.09 | 6.54 | 32.95 | |
| Mean | 7.26 | 9.36 | 9.96 | 10.03 | 9.19 | 7.88 | 8.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, M.; Yan, C.; Xiang, J.; Xiong, B.; Qin, L.; Khan, A.; Wang, B.; Khan, M.; Zou, Z.; Qiu, G. Multiple-Temporal Scale Variations in Nighttime Sap Flow Response to Environmental Factors in Ficus concinna over a Subtropical Megacity, Southern China. Forests 2022, 13, 1059. https://doi.org/10.3390/f13071059
Hayat M, Yan C, Xiang J, Xiong B, Qin L, Khan A, Wang B, Khan M, Zou Z, Qiu G. Multiple-Temporal Scale Variations in Nighttime Sap Flow Response to Environmental Factors in Ficus concinna over a Subtropical Megacity, Southern China. Forests. 2022; 13(7):1059. https://doi.org/10.3390/f13071059
Chicago/Turabian StyleHayat, Muhammad, Chunhua Yan, Jiao Xiang, Bowen Xiong, Longjun Qin, Alamgir Khan, Bei Wang, Mohsin Khan, Zhendong Zou, and Guoyu Qiu. 2022. "Multiple-Temporal Scale Variations in Nighttime Sap Flow Response to Environmental Factors in Ficus concinna over a Subtropical Megacity, Southern China" Forests 13, no. 7: 1059. https://doi.org/10.3390/f13071059







