Nursery Roosts Used by Barbastelle Bats, Barbastella barbastellus (Schreber, 1774) (Chiroptera: Vespertilionidae) in European Lowland Mixed Forest Transformed by Spruce Bark Beetle, Ips typographus (Linnaeus, 1758) (Coleoptera: Curculionidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
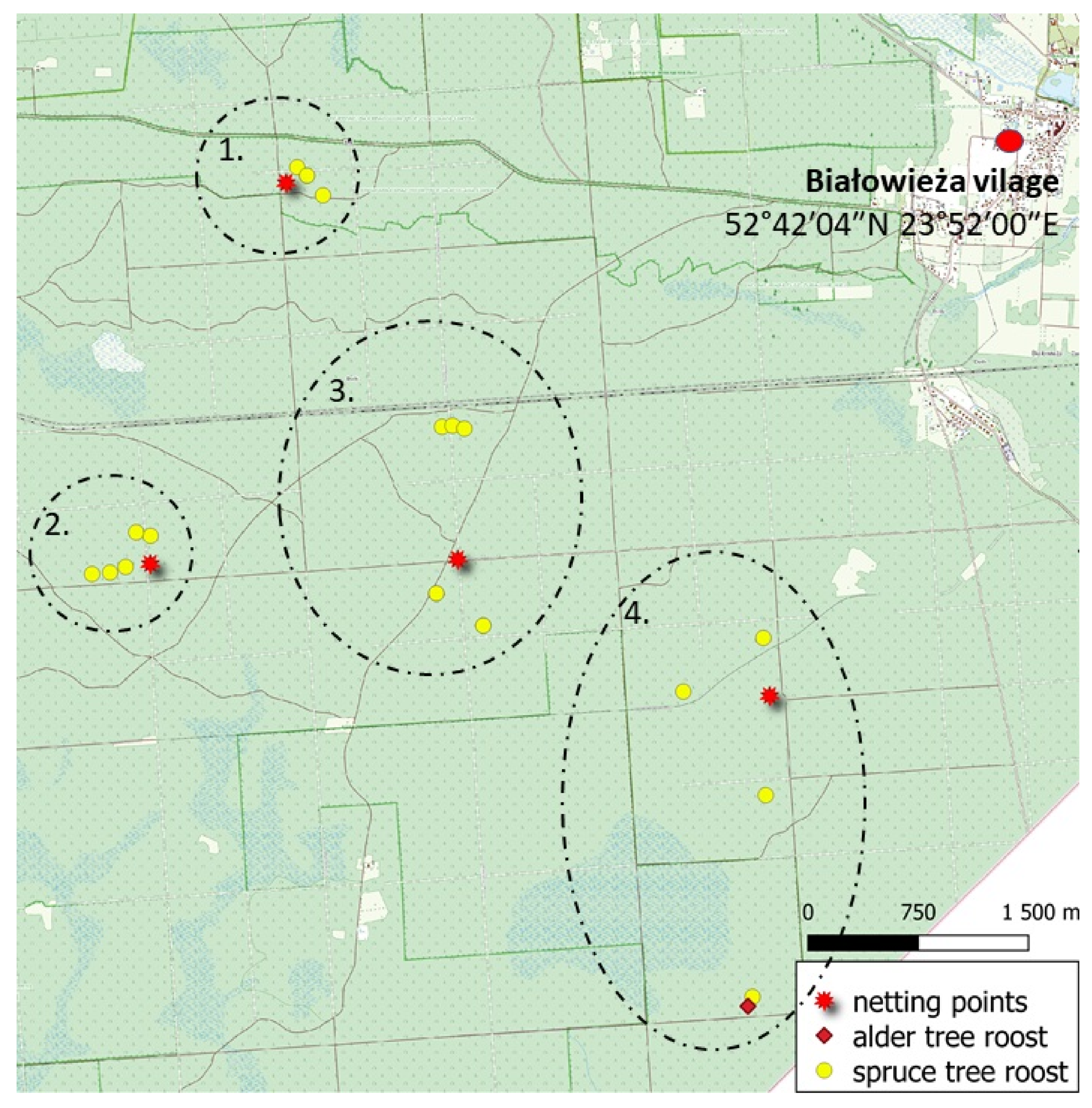
2.2. Study Design and Collecting Data
2.3. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czeszczewik, D.; Walankiewicz, W. Logging affects the white-backed wood-pecker Dendrocopos leucotos distribution in Białowieża Forest. Ann. Zool. Fenn. 2006, 43, 221–227. [Google Scholar]
- Ruczyński, I.; Nicholls, B.; MacLeod, C.D.; Racey, P.A. Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Białowieża Forest—Adaptive response to forest management? Forest Ecol. Manag. 2010, 259, 1633–1641. [Google Scholar] [CrossRef]
- Kahl, T.; Bauhus, J. An index of forest management intensity based on assessment of harvested tree volume, tree species composition and dead wood origin. Nat. Conserv. 2014, 7, 15–27. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Paillet, Y.; Archaux, F.; du Puy, S.; Bouget, C.; Boulanger, V.; Debaive, N.; Guilbert, E. The indicator side of tree microhabitats: A multi-taxon approach based on bats, birds and saproxylic beetles. J. Appl. Ecol. 2018, 55, 2147–2159. [Google Scholar] [CrossRef]
- Courbaud, B.; Larrieu, L.; Kozak, D.; Kraus, D.; Lachat, T.; Ladet, S.; Müller, J.; Paillet, Y.; Sagheb-Talebi, K.; Schuck, A.; et al. Factors influencing the rate of formation of tree-related microhabitats and implications for biodiversity conservation and forest management. J. Appl. Ecol. 2021, 59, 492–503. [Google Scholar] [CrossRef]
- Kunz, T.H. Roosting ecology of bats. In Ecology of Bats, 1st ed.; Kunz, T.H., Ed.; Plenum Press: New York, NY, USA, 1982; pp. 1–55. [Google Scholar]
- Rydell, J.; Entwistle, A.; Racey, P.A. Timing of foraging flights in three species of bats in relation to insect activity and predation risk. Oikos 1996, 76, 243–252. [Google Scholar] [CrossRef]
- Vonhof, M.J.; Barclay, R.M.R. Roost-site selection and roosting ecology of forest-dwelling bats in southern British Columbia. Can. J. Zool. 1996, 74, 1797–1805. [Google Scholar] [CrossRef]
- Willis, C.K.R.; Brigham, R.M. Roost switching, roost sharing and social cohesion: Forest-dwelling big brown bats, Eptesicus fuscus, conform to the fission-fusion model. Anim. Behav. 2004, 68, 495–505. [Google Scholar] [CrossRef]
- Barclay, R.M.R.; Kurta, A. Ecology and Behaviour of Bats Roosting in Tree Cavities and under Bark. In Bats in Forests; Lacki, M.J., Hayes, J.P., Kurta, A., Eds.; Johns Hopkins University Press: Baltimore, MA, USA, 2007; pp. 17–59. [Google Scholar]
- Krzanowski, A. Wyniki rozwieszania skrzynek dla nietoperzy w Białowieskim Parku Narodowym. Chrońmy Przyr. Ojczystą 1961, 17, 29–32. (In Polish) [Google Scholar]
- Crampton, L.H.; Barclay, R.M.R. Selection of roosting and foraging habitat by bats in different-aged aspen mixedwood stands. Conserv. Biol. 1998, 12, 1347–1358. [Google Scholar] [CrossRef]
- Ruczyński, I.; Bogdanowicz, W. Roost cavity selection by Nyctalus noctula and N. leisleri (Vespertilionidae, Chiroptera) in Białowieża Primeval Forest, Eastern Poland. J. Mammal. 2005, 86, 921–930. [Google Scholar] [CrossRef]
- Sierro, A.; Arlettaz, R. Barbastelle bats (Barbastella spp.) specialize in the predation of moths: Implicationsfor foraging tactics and conservation. Acta Oecol. 1997, 18, 91–106. [Google Scholar] [CrossRef]
- Carr, A.; Weatherall, A.; Fialas, P.; Zeale, M.; Clare, E.; Jones, G. Moths Consumed by the Barbastelle Barbastella barbastellus Require Larval Host Plants that Occur within the Bat's Foraging Habitats. Acta Chiropterol. 2021, 22, 257–269. [Google Scholar] [CrossRef]
- Sierro, A. Habitat selection by barbastelle bats (Barbastella barbastellus) in the Swiss Alps (Valais). J. Zool. 2006, 248, 429–432. [Google Scholar] [CrossRef]
- Gottfried, I.; Gottfried, T.; Fuszara, E.; Fuszara, M.; Ignaczak, M.; Jaros, R.; Piskorski, M. Breeding sites of the barbastelle Barbastella barbastellus (Schreber, 1774) in Poland. N. W. J. Zool. 2015, 11, 194–203. [Google Scholar]
- Kühnert, E.; Schönbächler, C.; Arlettaz, R.; Christe, P. Roost selection and switching in two forest-dwelling bats: Implications for forest management. Eur. J. Wildlife Res. 2016, 62, 497–500. [Google Scholar] [CrossRef]
- Apoznański, G.; Kokurewicz, T.; Petterson, S.; Sánchez-Navarro, S.; Górska, M.; Rydell, J. Barbastelles in a Production Landscape: Where Do They Roost? Acta Chiropterol. 2021, 23, 225–232. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Jones, G.; Mazzoleni, S. Roost selection by barbastelle bats (Barbastella barbastellus, Chiroptera: Vespertilionidae) in beech woodlands of central Italy: Consequences for conservation. Biol. Conserv. 2004, 117, 73–81. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Jones, G. Spatial and temporal patterns of roost use by tree-dwelling barbastelle bats Barbastella barbastellus. Ecography 2005, 28, 769–776. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Garonna, A.P.; Jones, G. Reconsidering the importance of harvested forests for the conservation of tree-dwelling bats. Biodivers. Conserv. 2010, 19, 2501–2515. [Google Scholar] [CrossRef]
- Görföl, T.; Hága, K.; Dombi, I. Roost selection of barbastelle bats (Barbastella barbastellus) in an intensively managed floodplain forest: Implications for conservation. North-West. J. Zool. 2019, 15, 184–186. [Google Scholar]
- Wesołowski, T. “Lifespan” of woodpecker-made holes in a primeval temperate forest: A thirty year study. Forest Ecol. Manag. 2011, 262, 1846–1852. [Google Scholar] [CrossRef]
- Wesołowski, T. “Lifespan” of non-excavated holes in a primeval temperate forest: A 30 year study. Biol. Conserv. 2012, 153, 118–126. [Google Scholar]
- Walankiewicz, W.; Czeszczewik, D.; Stański, T.; Sahel, M.; Ruczyński, I. Tree Cavity Resources in Spruce-Pine Managed and Protected Stands of the Białowieża Forest, Poland. Nat. Area. J. 2014, 34, 423–428. [Google Scholar] [CrossRef]
- Ruprecht, A.L. Nowe obserwacje nad nietoperzami (Chiroptera) Białowieży. Przegląd Zool. 1976, 20, 115–123. (In Polish) [Google Scholar]
- Kurskov, A.N. Research on bats in Belovezhskaia pushcha. Zapovedniki Belorussii. Issledovanija 1981, 5, 87–93. (In Russian) [Google Scholar]
- Rachwald, A.; Boratyński, P.; Nowakowski, W. Species composition and activity of bats flying over rivers in the Białowieża Primeval Forest. Acta Theriol. 2001, 46, 235–242. [Google Scholar] [CrossRef]
- Dietz. M.; Brombacher, M.; Erasmy, M.; Fenchuk, V.; Simon, O. Bat community and roost site selection of tree-dwelling bats in a well-preserved European lowland forest. Acta Chiropterol. 2018, 20, 117–127. [Google Scholar] [CrossRef]
- Rachwald. A.; Boratyński, J.S.; Krawczyk, J.; Szurlej, M.; Nowakowski, W.K. Natural and Anthropogenic Factors Influencing the Bat Community in Commercial Tree Stands in a Temperate Lowland Forest of Natural Origin (Białowieża Forest). Forest Ecol. Manag. 2021, 479, 118544. [Google Scholar] [CrossRef]
- Faliński, J.B. Vegetation Dynamics in Temperate Lowland Primeval Forest Ecological Studies in Białowieża Forest, 1st ed.; Junk, W., Ed.; Springer: Dordrecht, The Netherlands, 1986; pp. 1–537. [Google Scholar]
- Latałowa, M.; Zimny, M.; Jędrzejewska, B.; Samojlik, T. Białowieża Primeval Forest: A 2000-year interplay of environmental and cultural forces in Europe’s best preserved temperate woodland. In Europe’s Changing Woods and Forests: From Wildwood to Managed Landscapes; Kirby, K.J., Watkins, C., Eds.; CABI: Wallingford, UK, 2015; pp. 243–264. [Google Scholar]
- Müller, J.; Bußler, H.; Goßner, M.; Rettelbach, T.; Duelli, P. The European spruce bark beetle Ips typographus in a national park: From pest to keystone species. Biodivers. Conserv. 2008, 17, 2979–3001. [Google Scholar] [CrossRef]
- Martin, K.; Norris, A.R.; Drever, M.C. Effects of bark beetle outbreaks on avian biodiversity in the British Columbia interior: Implications for critical habitat management. J. Ecosyst. Manag. 2006, 7, 10–24. [Google Scholar]
- Matsuoka, S.M.; Handel, C.M. Nesting ecology of boreal forest birds following a massive outbreak of spruce beetles. J. Wildlife Manage. 2007, 71, 51–63. [Google Scholar] [CrossRef]
- Vaillancourt, M.-A.; Drapeau, P.; Gauthier, S.; Robert, M. Availability of standing trees for large cavity-nesting birds in the eastern boreal forest of Quebec, Canada. Forest Ecol. Manag. 2008, 255, 2272–2285. [Google Scholar] [CrossRef]
- Norris, A.R.; Drever, M.C.; Martini, K. Insect outbreaks increase populations and facilitate reproduction in a cavity-dependent songbird, the Mountain Chickadee Poecile gambeli. Ibis 2013, 155, 165–176. [Google Scholar] [CrossRef]
- Janousek, W.M.; Hicke, J.A.; Meddens, A.J.H.; Dreitz, V.J. The effects of mountain pine beetle outbreaks on avian communities in lodgepole pine forests across the greater Rocky Mountain region. Forest Ecol. Manag. 2019, 444, 374–381. [Google Scholar] [CrossRef]
- Latif, Q.S.; Ivan, J.S.; Seglund, A.E.; Pavlacky, D.L.; Truex, R.L. Avian relationships with bark beetle outbreaks and underlying mechanisms in lodgepole pine and spruce-fir forests of Colorado. Forest Ecol. Manag. 2020, 464, 118043. [Google Scholar] [CrossRef]
- Przepióra, F.; Loch, J.; Ciach, M. Bark beetle infestation spots as biodiversity hotspots: Canopy gaps resulting from insect outbreaks enhance the species richness, diversity and abundance of birds breeding in coniferous forests. Forest Ecol. Manag. 2020, 473, 118280. [Google Scholar] [CrossRef]
- Saab, V.A.; Latif, Q.S.; Rowland, M.M.; Johnson, T.N.; Chalfoun, A.D.; Buskirk, S.W.; Heyward, J.E.; Dresser, M.A. Ecological consequences of mountain pine beetle outbreaks for wildlife in western North American forests. Forest Sci. 2014, 60, 539–559. [Google Scholar] [CrossRef]
- Ivan, J.S.; Seglund, A.E.; Truex, R.L.; Newkirk, E.S. Mammalian responses to changed forest conditions resulting from bark beetle outbreaks in the southern Rocky Mountains. Ecosphere 2018, 9, e02369. [Google Scholar] [CrossRef]
- Randall, L.A.; Barclay, R.M.R.; Reid, M.L.; Jung, T.S. Recent infestation of forest stands by spruce beetles does not predict habitat use by little brown bats (Myotis lucifugus) in southwestern Yukon, Canada. Forest Ecol. Manag. 2011, 261, 1950–1956. [Google Scholar] [CrossRef]
- Lawson, K.J.; Lausen, C.L.; Mancuso, K.A.; Volkmann, L.A.; Gooliaff, T.J.; Hutchen, J.; Teichman, K.J.; Kelly, A.J.; Hodges, K.E. Bat activity and richness in beetle-killed forests in southern British Columbia. J. Mammal. 2019, 100, 510–517. [Google Scholar] [CrossRef]
- Thomas, J.P.; Reid, M.L.; Jung, T.S.; Barclay, R.M.R. Site occupancy of little brown bats (Myotis lucifugus) in response to salvage logging in the boreal forest. Forest Ecol. Manag. 2019, 451, 117501. [Google Scholar] [CrossRef]
- Mehr, M.; Brandl, R.; Kneib, T.; Muller, J. The effect of bark beetle infestation and salvage logging on bat activity in a national park. Biodivers. Conserv. 2012, 21, 2775–2786. [Google Scholar] [CrossRef]
- Kortmann, M.; Hurst, J.; Brinkmann, R.; Heurich, M.; Silveyra González, R.; Müller, J.; Thorn, S. Beauty and the beast: How a bat utilizes forests shaped by outbreaks of an insect pest. Anim. Conserv. 2018, 21, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Rachwald, A.; Ciesielski, M.; Szurlej, M.; Żmihorski, M. Following the damage: Increasing western barbastelle bat activity in bark beetle infested stands in Białowieża Primeval Forest. Forest Ecol. Manag. 2022, 503, 119803. [Google Scholar] [CrossRef]
- Grodzki, W. Mass outbreaks of the spruce bark beetle Ips typographus in the context of the controversies around the Białowieża Primeval Forest. For. Res. Pap. 2016, 77, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Berg, E.E.; Henry, J.D.; Fastie, C.L.; de Volder, A.D.; Matsuoka, S.M. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: Relationship to summer temperatures and regional differences in disturbance regimes. Forest Ecol. Manag. 2006, 227, 219–232. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Change Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Kolb, T.E. Responses of tree-killing bark beetles to a changing climate. In Climate Change and Insect Pests; Bjorkman, C., Niemela, P., Eds.; Centre for Agriculture and Biosciences International (CABI): Wallingford, UK, 2015; pp. 173–201. [Google Scholar]
- Boczoń, A.; Kowalska, A.; Ksepko, M.; Sokołowski, K. Climate warming and drought in the Bialowieza Forest from 1950–2015 and their impact on the dieback of Norway spruce stands. Water 2018, 10, 1502. [Google Scholar] [CrossRef] [Green Version]
- Gross, M. Europe’s last wilderness threatened. Curr. Biol. 2016, 26, R641–R666. [Google Scholar] [CrossRef]
- Stokstad, E. Last stands. Foresters and ecologists face over the future of Europe’s oldest forest. Science 2017, 358, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Blicharska, M.; Smithers, R.J. Białowieża Forest: Political stands. Science 2018, 359, 646. [Google Scholar] [CrossRef] [PubMed]
- Mikusiński, G.; Bubnicki, J.W.; Churski, M.; Czeszczewik, D.; Walankiewicz, W.; Kuijper, D.P.J. Is the impact of loggings in the last primeval lowland forest in Europe underestimated? The conservation issues of Białowieża Forest. Biol. Conserv. 2018, 227, 266–274. [Google Scholar] [CrossRef]
- Schiermeier, Q. EU’s top court says logging in Poland’s ancient forest was illegal. Nature 2018. [Google Scholar] [CrossRef]
- European Court of Justice Judgment of the Court (Grand Chamber) of 17 April 2018. European Commission vs. Republic of Poland. Case C-441/17. Available online: https://eur-lex.europa.eu/legalcontent/EN/ALL/?uri=CELEX:62017CJ0441 (accessed on 17 April 2019).
- Regnery, B.; Couvet, D.; Kubarek, L.; Julien, J.-F.; Kerbiriou, C. Tree microhabitats as indicators of bird and bat communities in Mediterranean forests. Ecol. Indic. 2013, 34, 221–230. [Google Scholar] [CrossRef]
- Samojlik, T.; Fedotova, A.; Daszkiewicz, P.; Rotherham, I.D. Białowieża Primeval Forest: Nature and Culture in the Nineteenth Century; Springer International Publishing: New York, NY, USA, 2020; pp. 1–223. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Zajączkowski, J.; Drozdowski, S.; Gawron, L.; Bielak, K.; Szeligowski, H.; Dzwonkowski, M.; Ostrowski, J.; Widawska, Z.; Keczyński, A. Operat dynamiki ekosystemów leśnych Białowieskiego Parku Narodowego. Raport; Białowieża National Park: Białowieża, Poland, 2010. (In Polish) [Google Scholar]
- Gottfried, I. No. 1308: Mopek Barbastella barbastellus, SCHREBER (1774). Monitoring Gatunków Zwierząt; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2012; Volume 3, pp. 604–633. (In Polish) [Google Scholar]
- Rolfes, J.W.; Encarnacao, J.A.; Becker, N.I. Going bald—The hairy affair of timing in telemetry studies: Moulting activity in European bat species. Acta Chiropterol. 2022, 23, 513–523. [Google Scholar] [CrossRef]
- Aldridge, H.D.J.N.; Brigham, R.M. Load carrying and maneuverability in an insectivorous bat: A test of the 5% “rule” of radio-telemetry. J. Mammal. 1988, 69, 379–382. [Google Scholar] [CrossRef]
- Finch, D.M. Habitat use and habitat overlap of riparian birds in three elevational zones. Ecology 1989, 70, 866–880. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Richter, T.; Jestädt, K.; Leitl, R.; Linner, J.; Müller, J.; Hagge, J. Quartiernutzung der Mopsfledermaus (Barbastella barbastellus) im Nationalpark Bayerischer Wald und eine Evaluation von Erfassungsmethoden. Nyctalus (NF) 2019, 19, 270–284. [Google Scholar]
- Heurich, M.; Englmaier, K.H. The development of tree species composition in the Rachel–Lusen region of the Bavarian Forest National Park. Silva Gabreta 2010, 16, 165–186. [Google Scholar]
- Cieśliński, S.; Czyżewska, K.; Faliński, J.B.; Klama, H.; Mułenko, W.; Żarnowiec, J. Relicts of the Primeval (Virgin) Forest. Relict Phenomena. In Cryptogamous Plants in the Forest Communities of Białowieża National Park (Project Crypto 3); Phytocoenosis 8 (N.S.) Archiwum Geobotanicum; Faliński, J.B., Mułenko, W., Eds.; Polish Botanical Society: Kraków, Poland, 1996; Volume 6, pp. 197–216. [Google Scholar]
- Blicharska, M.; Angelstam, P. Conservation at risk: Conflict analysis in the Białowieża Forest, an European biodiversity hotspot. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2010, 6, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Hillen, J.; Kiefer, A.; Veith, M. Interannual fidelity to roosting habitat and flight paths by female western barbastelle bats. Acta Chiropterol. 2010, 12, 187–195. [Google Scholar] [CrossRef]
- Carr, A.; Zeale, M.R.K.; Weatherall, A.; Froidevaux, J.S.P.; Jones, G. Ground-based and LiDAR-derived measurements reveal scale-dependent selection of roost characteristics by the rare tree-dwelling bat Barbastella barbastellus. Forest Ecol. Manag. 2018, 417, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Kerth, G.; Koenig, B. Fission, fusion and non-random associations in female Bechstein’s bats (Myotis bechsteinii). Behaviour 1999, 136, 1187–1202. [Google Scholar] [CrossRef]
- Rydell, J.; Bogdanowicz, W. Barbastella barbastellus. Mamm. Species 1997, 557, 1. [Google Scholar] [CrossRef]
- Hillen, J.; Kiefer, A.; Veith, M. Foraging site fidelity shapes the spatial organisation of a population of female western barbastelle bats. Biol. Conserv. 2009, 142, 817–823. [Google Scholar] [CrossRef]
- Denzinger, A.; Schnitzler, H.U. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 2013, 4, 164. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, L.; Bailey, S.; Park, K. Negative impacts of felling in exotic spruce plantations on moth diversity mitigated by remnants of deciduous tree cover. Forest Ecol. Manag. 2017, 404, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Watts, K.; Quine, C.P.; Aycott, A.E.; Moseley, D.; Humphrey, J.W.; Ray, D. Conserving Forest Biodiversity: Recent Approaches in UK Forest Planning and Management. In Patterns and Processes in Forest Landscapes; Lafortezza, R., Sanesi, G., Chen, J., Crow, T.R., Eds.; Springer Nature: Berlin, Germany, 2008; pp. 373–398. [Google Scholar]
- Controversial Aspects of Nature Conservation Management in Šumava National Park: Submission from Hnutí DUHA/Friends of the Earth Czech Republic to the IUCN Mission to the National Park, September 2002. Report. Sponsored by DUHA. Available online: http://sumava.tadytoje.cz/info/studieadokumenty/studieainfo/podklad_pro_iucn_en.pdf (accessed on 13 April 2022).
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.; Cahall, R.; Campell, J.; Castro, J.; Choi, C.-Y.; Cobb, T.; Donato, D.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Svoboda, M.; Müller, J. Effects of natural disturbances and salvage logging on biodiversity—Lessons from the Bohemian Forest. Forest Ecol. Manag. 2017, 388, 113–119. [Google Scholar] [CrossRef]
- Busse, A.; Cizek, L.; Čížková, P.; Drag, L.; Dvorak, V.; Foit, J.; Heurich, M.; Hubený, P.; Kašák, J.; Kittler, F.; et al. Forest dieback in a protected area triggers the return of the primeval forest specialist Peltis grossa (Coleoptera, Trogossitidae). Conserv. Sci. Pract. 2022, 4, e612. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.M.; Samojlik, T.; Zimny, M.; Latałowa, M. Białowieża Forest—A Relic of the High Naturalness of European Forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]


| Day in Roost | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bat | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 1 | ||||||||||||||||
| 2 |  | |||||||||||||||
| 3 |  | |||||||||||||||
| 4 | ||||||||||||||||
| 5 |  | |||||||||||||||
| 6 | ||||||||||||||||
| 7 | ||||||||||||||||
| 8 | ||||||||||||||||
| 9 |  | |||||||||||||||
| Tree No. | Species | DBH (cm) | Height (m) | DistDecid (m) | Dom./Age (yrs) | Four Trees | Status |
|---|---|---|---|---|---|---|---|
| 1 | P. abies | 42 | 22 | 963 | Psyl/101 | Pabi/Pabi/Pabi/Pabi | D/D/D/D |
| 2 | P. abies | 60 | 28 | 915 | Psyl/101 | Psyl/Pabi/Pabi/Pabi | L/D/D/D |
| 3 | P. abies | 50 | 25 | 903 | Psyl/40 | Bver/Pabi/Pabi/Pabi | L/D/D/D |
| 4 | P. abies | 53 | 29 | 1088 | Pabi/120 | Pabi/Pabi/Pabi/Pabi | D/D/D/D |
| 5 | P. abies | 51 | 17 | 1126 | Psyl/90 | Pabi/Pabi/Pabi/Pabi | D/D/D/D |
| 6 | P. abies | 65 | 22 | 373 | Pabi/155 | Pabi/Pabi/Pabi/Qrob | D/D/D/L |
| 7 | P. abies | 53 | 22 | 208 | Pabi/150 | Pabi/Pabi/Pabi/Qrob | D/D/D/L |
| 8 | P. abies | 55 | 25 | 89 | Psyl/105 | Pabi/Pabi/Psyl/Psyl | D/D/L/L |
| 9 | P. abies | 62 | 30 | 85 | Pabi/160 | Pabi/Pabi/Psyl/Pabi | D/D/L/D |
| 10 | P. abies | 47 | 23 | 145 | Pabi/160 | Pabi/Pabi/Qrob/Pabi | L/D/L/D |
| 11 | P. abies | 55 | 27 | 240 | Pabi/160 | Pabi/Pabi/Pabi/Pabi | D/D/D/D |
| 12 | P. abies | 62 | 29 | 250 | Pabi/180 | Pabi/Pabi/Bver/Pabi | D/D/L/D |
| 13 | A. glutinosa | 24 | 15 | 0 | Aglu/70 | Aglu/Aglu/Aglu/Aglu | L/D/L/L |
| 14 | P. abies | 62 | 30 | 37 | Pabi/150 | Ptre/Pabi/Cbet/Ptre | L/D/L/L |
| 15 | P. abies | 58 | 28 | 105 | Pabi/90 | Psyl/Pabi/Pabi/Pabi | L/D/D/D |
| 15 | P. abies | 46 | 25 | 15 | Pabi/105 | Psyl/Pabi/Pabi/Pabi | L/D/D/D |
| 17 | P. abies | 52 | 27 | 248 | Pabi/105 | Bver/Pabi/Pabi/Bver | L/D/D/L |
| 18 | P. abies | 56 | 25 | 148 | Pabi/160 | Psyl/Pabi/Pabi/Pabi | L/D/D/D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachwald, A.; Apoznański, G.; Thor, K.; Więcek, M.; Zapart, A. Nursery Roosts Used by Barbastelle Bats, Barbastella barbastellus (Schreber, 1774) (Chiroptera: Vespertilionidae) in European Lowland Mixed Forest Transformed by Spruce Bark Beetle, Ips typographus (Linnaeus, 1758) (Coleoptera: Curculionidae). Forests 2022, 13, 1073. https://doi.org/10.3390/f13071073
Rachwald A, Apoznański G, Thor K, Więcek M, Zapart A. Nursery Roosts Used by Barbastelle Bats, Barbastella barbastellus (Schreber, 1774) (Chiroptera: Vespertilionidae) in European Lowland Mixed Forest Transformed by Spruce Bark Beetle, Ips typographus (Linnaeus, 1758) (Coleoptera: Curculionidae). Forests. 2022; 13(7):1073. https://doi.org/10.3390/f13071073
Chicago/Turabian StyleRachwald, Alek, Grzegorz Apoznański, Katarzyna Thor, Mirosław Więcek, and Aneta Zapart. 2022. "Nursery Roosts Used by Barbastelle Bats, Barbastella barbastellus (Schreber, 1774) (Chiroptera: Vespertilionidae) in European Lowland Mixed Forest Transformed by Spruce Bark Beetle, Ips typographus (Linnaeus, 1758) (Coleoptera: Curculionidae)" Forests 13, no. 7: 1073. https://doi.org/10.3390/f13071073
APA StyleRachwald, A., Apoznański, G., Thor, K., Więcek, M., & Zapart, A. (2022). Nursery Roosts Used by Barbastelle Bats, Barbastella barbastellus (Schreber, 1774) (Chiroptera: Vespertilionidae) in European Lowland Mixed Forest Transformed by Spruce Bark Beetle, Ips typographus (Linnaeus, 1758) (Coleoptera: Curculionidae). Forests, 13(7), 1073. https://doi.org/10.3390/f13071073






