Ecological Safety and Spatial Distribution of Mercury and Arsenic in Qinghai Spruce Ecosystems in Remote Plateau Mountains, Northwest China
Abstract
:1. Introduction
2. Materials and Methods
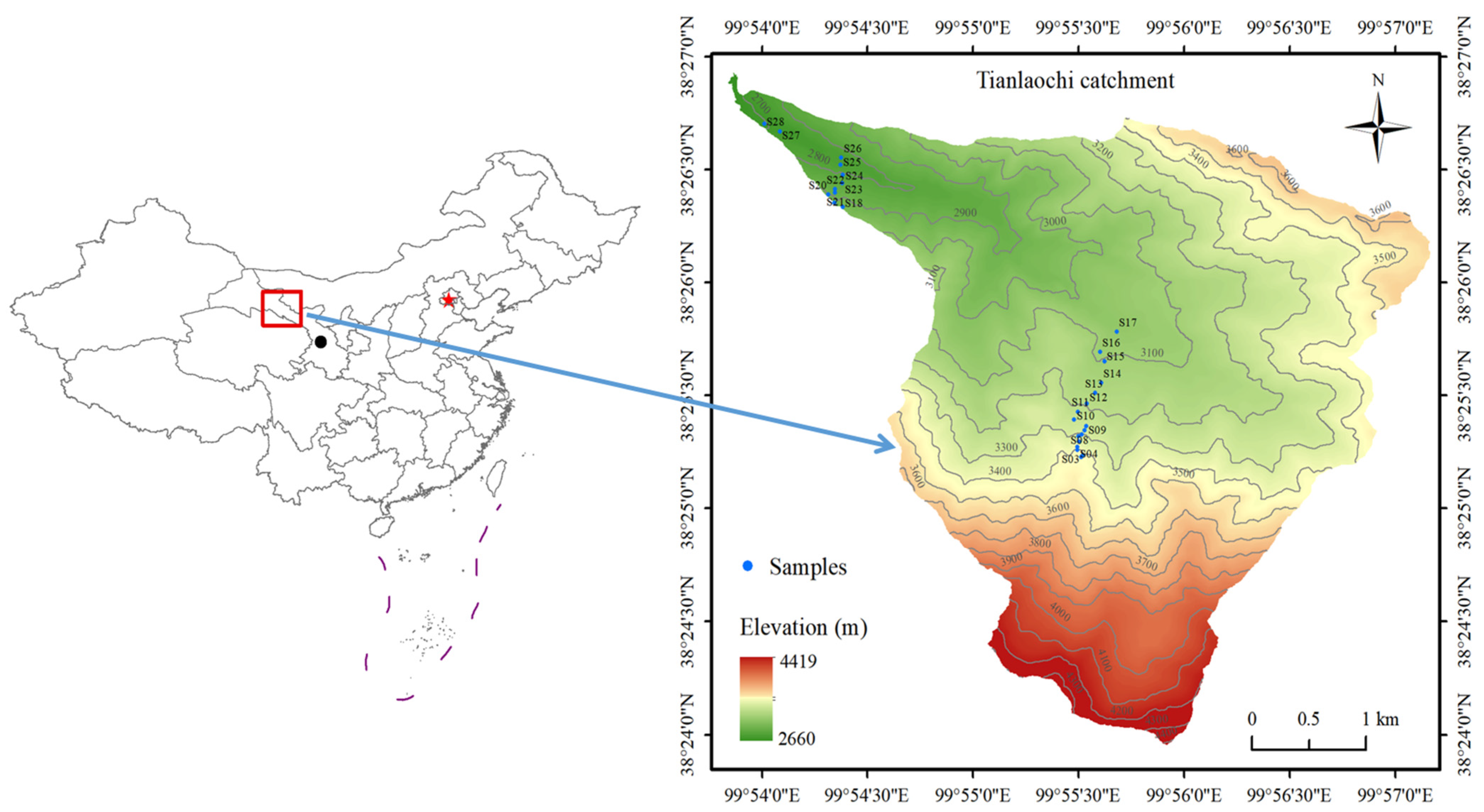
2.1. Site Description
2.2. Sample Collection
2.3. Chemical Analysis
2.4. Pollution Level Assessment
2.4.1. Bioconcentration Factor (BCF)
2.4.2. Enrichment Factor
2.4.3. Potential Ecological Risk
2.5. Statistical Analysis
3. Results and Discussion
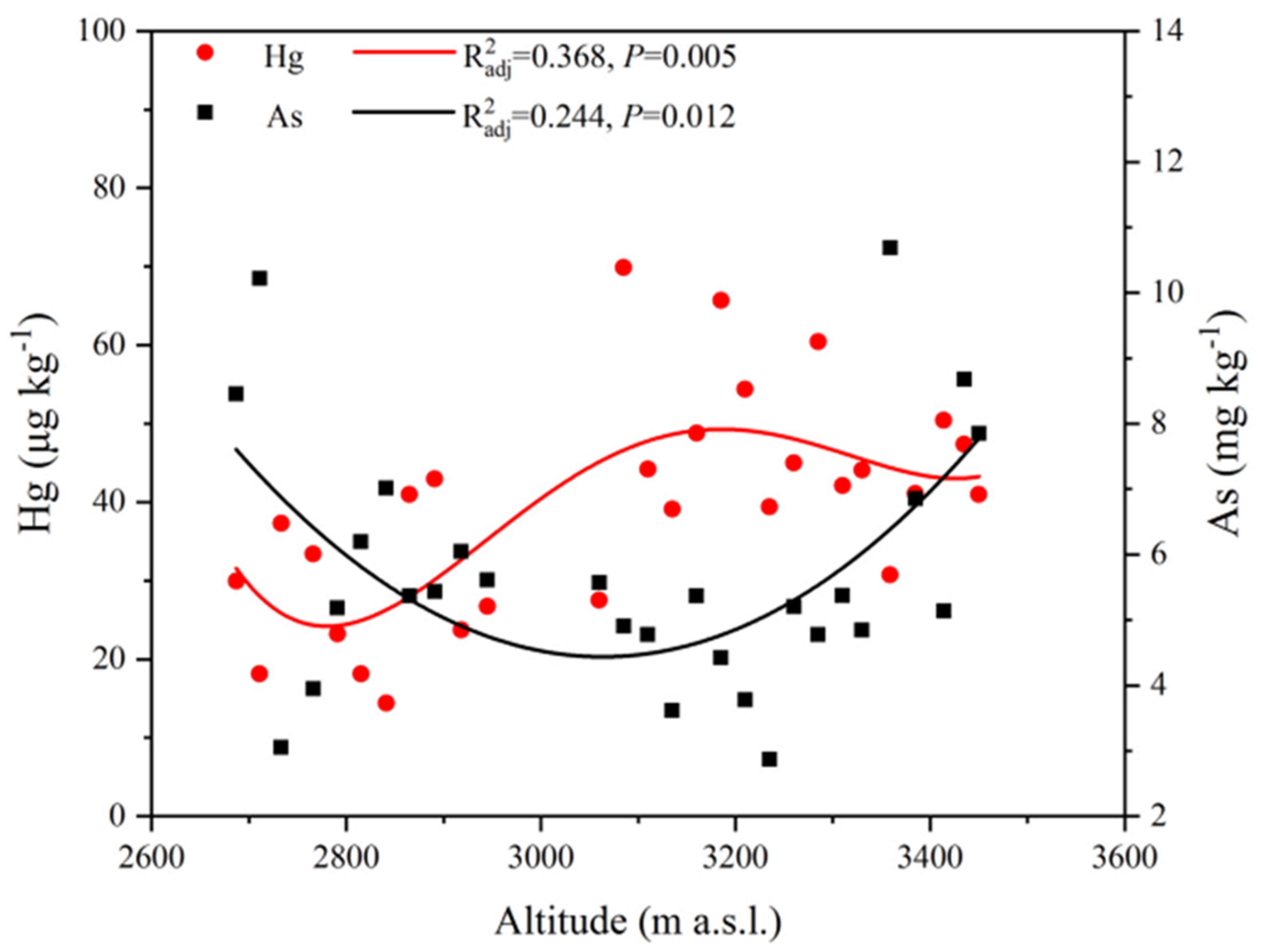
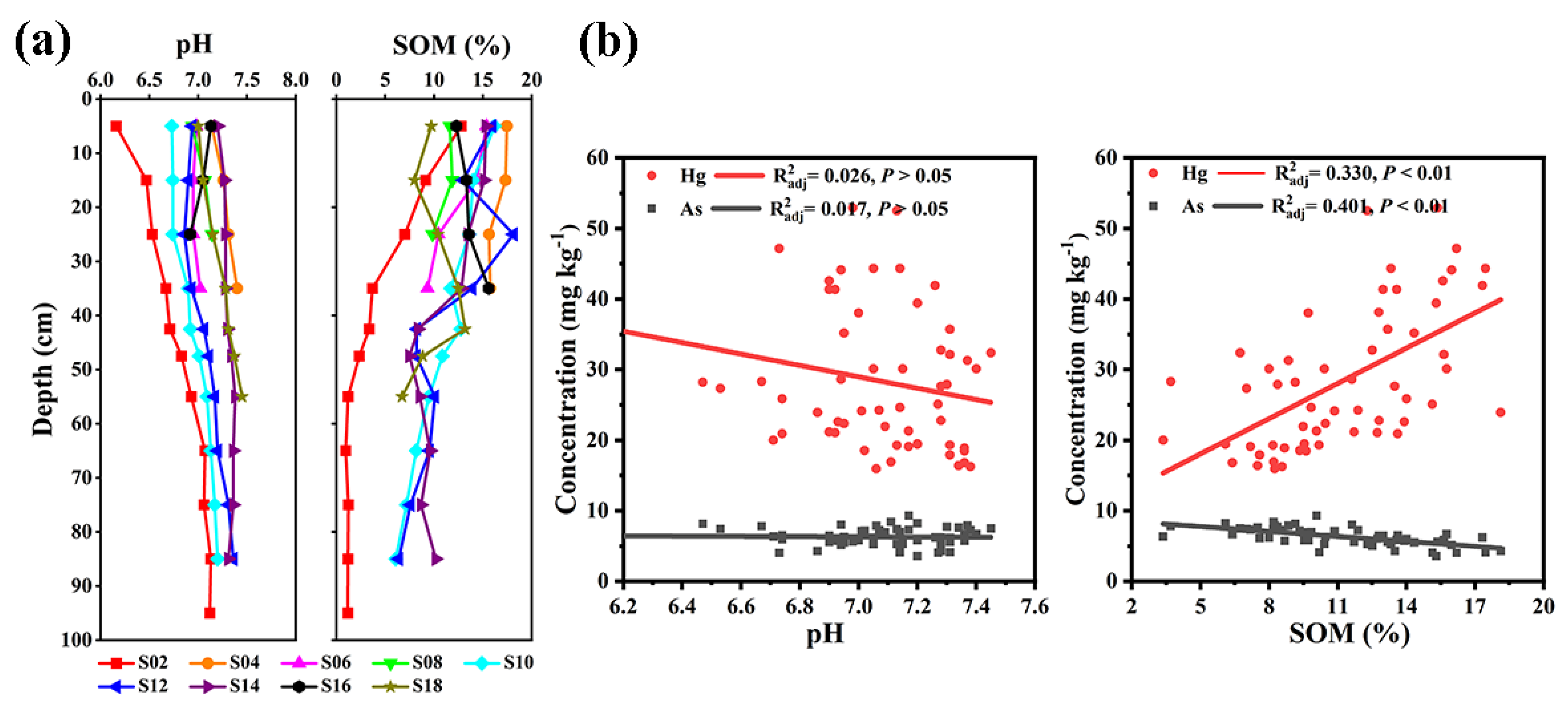
3.1. The Physicochemical Properties and Hg and As Concentrations in Topsoil
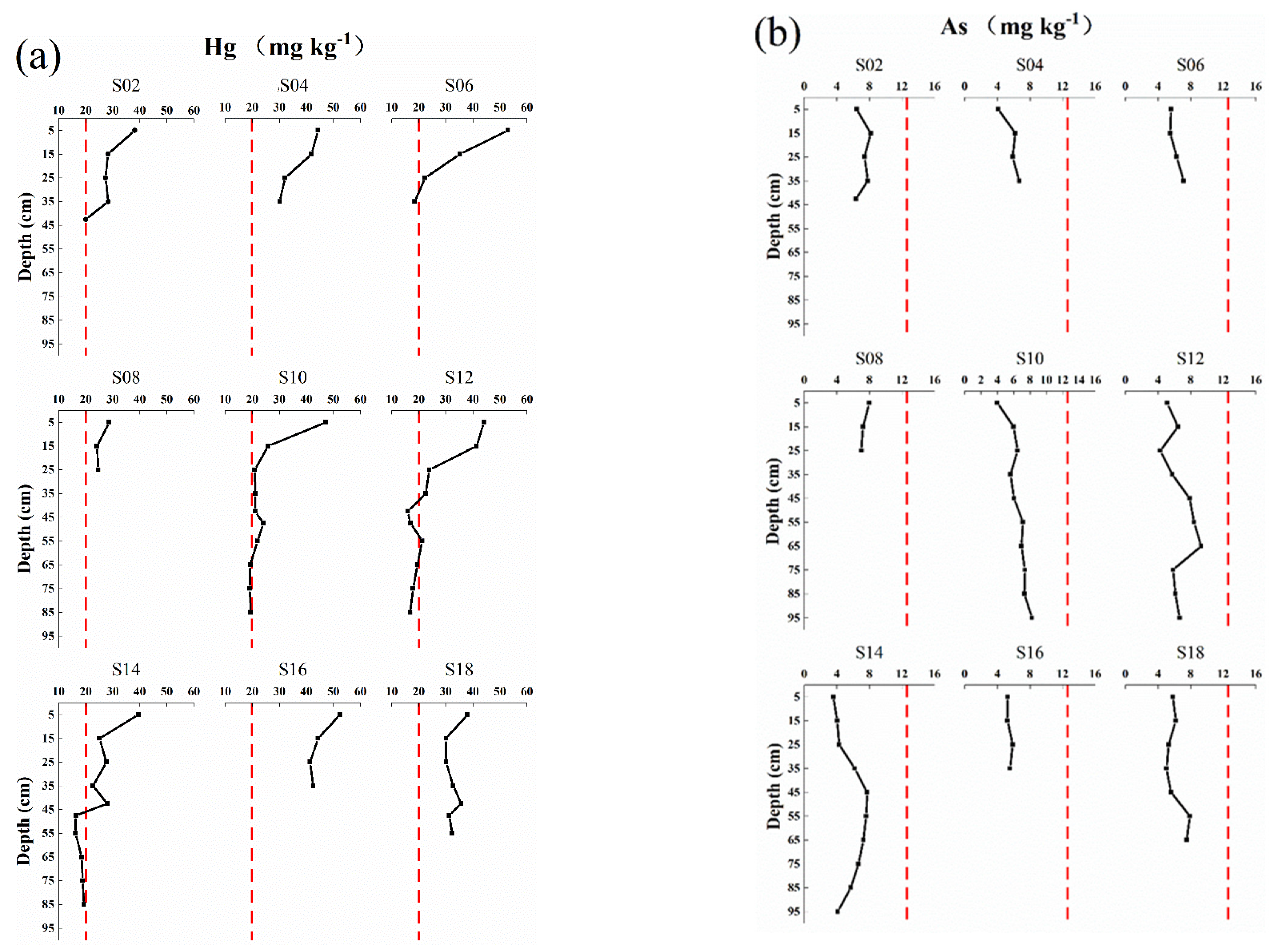
3.2. Soil Hg and As Spatial Distribution
3.3. Mercury (Hg) and As Concentrations in Plants
3.4. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, R.; Wang, H.; Luo, J.; Sun, S.; Gong, Y.; She, J.; Chen, Y.; Dandan, Y.; Zhou, J. Spatial distribution and temporal trends of mercury and arsenic in remote timberline coniferous forests, eastern of the Tibet Plateau, China. Environ. Sci. Pollut. Res. 2015, 22, 11658–11668. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Z.; Lin, C.-J.; Yuan, W.; Feng, X. Assessment of Global Mercury Deposition through Litterfall. Environ. Sci. Technol. 2016, 50, 8548–8557. [Google Scholar] [CrossRef] [PubMed]
- Bing, H.; Zhou, J.; Wu, Y.; Luo, X.-S.; Xiang, Z.; Sun, H.; Wang, J.; Zhu, H. Barrier effects of remote high mountain on atmospheric metal transport in the eastern Tibetan Plateau. Sci. Total Environ. 2018, 628, 687–696. [Google Scholar] [CrossRef]
- Li, R.; Bing, H.; Wu, Y.; Zhou, J.; Xiang, Z. Altitudinal patterns and controls of trace metal distribution in soils of a remote high mountain, Southwest China. Environ. Geochem. Health 2018, 40, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Stankwitz, C.; Kaste, J.M.; Friedland, A.J. Threshold Increases in Soil Lead and Mercury from Tropospheric Deposition Across an Elevational Gradient. Environ. Sci. Technol. 2012, 46, 8061–8068. [Google Scholar] [CrossRef]
- Gruba, P.; Socha, J.; Pietrzykowski, M.; Pasichnyk, D. Tree species affects the concentration of total mercury (Hg) in forest soils: Evidence from a forest soil inventory in Poland. Sci. Total Environ. 2019, 647, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, J.; Yin, R.; Yuan, W.; Lin, C.-J.; Sommar, J.; Feng, X.; Wang, H.; Lin, C. Using Mercury Isotopes To Understand Mercury Accumulation in the Montane Forest Floor of the Eastern Tibetan Plateau. Environ. Sci. Technol. 2017, 51, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, R.-S.; Feng, X.-B.; Sommar, J.; Anderson, C.W.N.; Sapkota, A.; Fu, X.; Larssen, T. Atmospheric mercury inputs in montane soils increase with elevation: Evidence from mercury isotope signatures. Sci. Rep. 2013, 3, 3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.-L.; Zhao, Y.-P.; Li, J.-S.; Beiyuan, J.-Z.; Tsang, D.C.; Poon, C.-S.; Chan, T.-S.; Wang, W.-X.; Li, X.-D. Speciation, mobilization, and bioaccessibility of arsenic in geogenic soil profile from Hong Kong. Environ. Pollut. 2018, 232, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lin, Z.; Wan, X.; Feng, L. Risk assessment for the mercury polluted site near a pesticide plant in Changsha, Hunan, China. Chemosphere 2017, 169, 333–341. [Google Scholar] [CrossRef]
- Bargagli, R. Moss and lichen biomonitoring of atmospheric mercury: A review. Sci. Total Environ. 2016, 572, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Zhao, H.; Peros, M.; Yang, Q.; Liu, S.; Li, H.; Wang, S.; Bu, Z. Mercury and arsenic in the surface peat soils of the Changbai Mountains, northeastern China: Distribution, environmental controls, sources, and ecological risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 34595–34609. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kang, S.; Ma, M.; Guo, J.; Cong, Z.; Dong, Z.; Yin, R.; Xu, J.; Tripathee, L.; Ram, K.; et al. Accumulation of Atmospheric Mercury in Glacier Cryoconite over Western China. Environ. Sci. Technol. 2019, 53, 6632–6639. [Google Scholar] [CrossRef]
- Liu, H.-W.; Shao, J.-J.; Yu, B.; Liang, Y.; Duo, B.; Fu, J.-J.; Yang, R.-Q.; Shi, J.-B.; Jiang, G.-B. Mercury isotopic compositions of mosses, conifer needles, and surface soils: Implications for mercury distribution and sources in Shergyla Mountain, Tibetan Plateau. Ecotoxicol. Environ. Saf. 2019, 172, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metal(loid)s. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef] [Green Version]
- Outridge, P.M.; Mason, R.P.; Wang, F.; Guerrero, S.; Heimbürger-Boavida, L.E. Updated Global and Oceanic Mercury Budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef]
- Kang, H.; Liu, X.; Guo, J.; Wang, B.; Xu, G.; Wu, G.; Kang, S.; Huang, J. Characterization of mercury concentration from soils to needle and tree rings of Schrenk spruce (Picea schrenkiana) of the middle Tianshan Mountains, northwestern China. Ecol. Indic. 2019, 104, 24–31. [Google Scholar] [CrossRef]
- Zang, F.; Wang, H.; Zhao, C.; Nan, Z.; Wang, S.; Yang, J.; Li, N. Atmospheric wet deposition of trace elements to forest ecosystem of the Qilian Mountains, northwest China. CATENA 2021, 197, 104966. [Google Scholar] [CrossRef]
- Wang, S.; Xing, D.; Wei, Z.; Jia, Y. Spatial and seasonal variations in soil and river water mercury in a boreal forest, Changbai Mountain, Northeastern China. Geoderma 2013, 206, 123–132. [Google Scholar] [CrossRef]
- Zhou, J.; Du, B.; Wang, Z.; Zhang, W.; Xu, L.; Fan, X.; Liu, X.; Zhou, J. Distributions and pools of lead (Pb) in a terrestrial forest ecosystem with highly elevated atmospheric Pb deposition and ecological risks to insects. Sci. Total Environ. 2019, 647, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Bing, H.; Wu, Y.; Li, J.; Xiang, Z.; Luo, X.; Zhou, J.; Sun, H.; Zhang, G. Biomonitoring trace element contamination impacted by atmospheric deposition in China’s remote mountains. Atmos. Res. 2019, 224, 30–41. [Google Scholar] [CrossRef]
- Bing, H.; Wu, Y.; Zhou, J.; Sun, H. Biomonitoring trace metal contamination by seven sympatric alpine species in Eastern Tibetan Plateau. Chemosphere 2016, 165, 388–398. [Google Scholar] [CrossRef]
- Dong, Z.; Kang, S.; Qin, X.; Li, X.; Qin, D.; Ren, J. New insights into trace elements deposition in the snow packs at remote alpine glaciers in the northern Tibetan Plateau, China. Sci. Total Environ. 2015, 529, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wang, F.; Xu, C.; Pan, N.; Lin, J.; Zhao, R.; Yang, Y.; Luo, H. Source apportionment of heavy metals in agricultural soil based on PMF: A case study in Hexi Corridor, northwest China. Chemosphere 2018, 193, 189–197. [Google Scholar] [CrossRef]
- Luo, J.; She, J.; Yang, P.; Sun, S.; Li, W.; Gong, Y.; Tang, R. Heavy metal concentrations in timberline trees of eastern Tibetan Plateau. Ecotoxicology 2014, 23, 1086–1098. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zhao, S.; Zhang, W. Age and Climate Sensitivity of Radial Growth of Picea crassifoliato Climate in a Transitional Climatic Zone in Northwest China. Pol. J. Ecol. 2018, 66, 114–125. [Google Scholar] [CrossRef]
- Liang, E.; Shao, X.; Eckstein, D.; Liu, X. Spatial variability of tree growth along a latitudinal transect in the Qilian Mountains, northeastern Tibetan Plateau. Can. J. For. Res. 2010, 40, 200–211. [Google Scholar] [CrossRef]
- Lu, R. Analysis Methods of Soil Agricultural Chemistry; Agricultural Science and Technology Publishing House: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Liu, B.; Ai, S.; Zhang, W.; Huang, D.; Zhang, Y. Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Sci. Total Environ. 2017, 609, 822–829. [Google Scholar] [CrossRef]
- Bargagli, R.; Brown, D.; Nelli, L. Metal biomonitoring with mosses: Procedures for correcting for soil contamination. Environ. Pollut. 1995, 89, 169–175. [Google Scholar] [CrossRef]
- Gujre, N.; Mitra, S.; Soni, A.; Agnihotri, R.; Rangan, L.; Rene, E.R.; Sharma, M.P. Speciation, contamination, ecological and human health risks assessment of heavy metals in soils dumped with municipal solid wastes. Chemosphere 2021, 262, 128013. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, H.; Wu, Y.; Zhou, J.; Ming, L.; Sun, S.; Li, X. Atmospheric deposition of lead in remote high mountain of eastern Tibetan Plateau, China. Atmos. Environ. 2014, 99, 425–435. [Google Scholar] [CrossRef]
- Chai, Y.; Guo, J.; Chai, S.; Cai, J.; Xue, L.; Zhang, Q. Source identification of eight heavy metal(loid)s in grassland soils by multivariate analysis from the Baicheng–Songyuan area, Jilin Province, Northeast China. Chemosphere 2015, 134, 67–75. [Google Scholar] [CrossRef] [PubMed]
- CNEMC (China National Environmental Monitoring Center). The Soil Background Values of China; China Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Yang, Y.; Yanai, R.D.; Driscoll, C.T.; Montesdeoca, M.; Smith, K.T. Concentrations and content of mercury in bark, wood, and leaves in hardwoods and conifers in four forested sites in the northeastern USA. PLoS ONE 2018, 13, e0196293. [Google Scholar] [CrossRef]
- Liu, Y.-R.; He, Z.-Y.; Yang, Z.-M.; Sun, G.-X.; He, J.-Z. Variability of heavy metal content in soils of typical Tibetan grasslands. RSC Adv. 2016, 6, 105398–105405. [Google Scholar] [CrossRef]
- Ericksen, J.; Gustin, M.; Schorran, D.; Johnson, D.; Lindberg, S.; Coleman, J. Accumulation of atmospheric mercury in forest foliage. Atmos. Environ. 2003, 37, 1613–1622. [Google Scholar] [CrossRef]
- Luo, T.; Pan, Y.; Ouyang, H.; Shi, P.; Luo, J.; Yu, Z.; Lu, Q. Leaf area index and net primary productivity along subtropical to alpine gradients in the Tibetan Plateau. Glob. Ecol. Biogeogr. 2004, 13, 345–358. [Google Scholar] [CrossRef]
- Evans, C.A.; Hutchinson, T.C. Mercury accumulation in transplanted moss and lichens at high elevation sites in Quebec. Water Air Soil Pollut. 1996, 90, 475–488. [Google Scholar] [CrossRef]
- Graydon, J.A.; Louis, V.L.S.; Lindberg, S.E.; Hintelmann, H.; Krabbenhoft, D.P. Investigation of Mercury Exchange between Forest Canopy Vegetation and the Atmosphere Using a New Dynamic Chamber. Environ. Sci. Technol. 2006, 40, 4680–4688. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhou, J.; Zhou, L.; Fan, X.; Zhou, J. Mercury distribution in the foliage and soil profiles of a subtropical forest: Process for mercury retention in soils. J. Geochem. Explor. 2019, 205, 106337. [Google Scholar] [CrossRef]
- Brun, C.B.; Peltola, P.; Åström, M.E.; Johansson, M.-B. Spatial distribution of major, trace and ultra trace elements in three Norway spruce (Picea abies) stands in boreal forests, Forsmark, Sweden. Geoderma 2010, 159, 252–261. [Google Scholar] [CrossRef]
- Şen, A.; Pereira, H.; Olivella, M.A.; Villaescusa, I. Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Urošević, M.A.; Vuković, G.; Vasić, P.; Jakšić, T.; Nikolić, D.; Škrivanj, S.; Popović, A. Environmental implication indices from elemental characterisations of collocated topsoil and moss samples. Ecol. Indic. 2018, 90, 529–539. [Google Scholar] [CrossRef]
- Chiarantini, L.; Rimondi, V.; Benvenuti, M.; Beutel, M.W.; Costagliola, P.; Gonnelli, C.; Lattanzi, P.; Paolieri, M. Black pine (Pinus nigra) barks as biomonitors of airborne mercury pollution. Sci. Total Environ. 2016, 569, 105–113. [Google Scholar] [CrossRef]
- Fleck, J.A.; Grigal, D.F.; Nater, E.A. Mercury Uptake by Trees: An Observational Experiment. Water Air Soil Pollut. 1999, 115, 513–523. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Feng, X.; Wang, D.; Luo, J. Moss facilitating mercury, lead and cadmium enhanced accumulation in organic soils over glacial erratic at Mt. Gongga, China. Environ. Pollut. 2019, 254, 112974. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-J.; Liu, C.-B.; Zhang, Q.-H.; Fu, J.-J.; Yang, R.-Q.; Shi, J.-B.; Cai, Y.; Jiang, G.-B. Characterization and speciation of mercury in mosses and lichens from the high-altitude Tibetan Plateau. Environ. Geochem. Health 2017, 39, 475–482. [Google Scholar] [CrossRef]


| Min | Mean | Median | Max | SD a | CV b (%) | Skewness | K–S Test | Soil Background c | |
|---|---|---|---|---|---|---|---|---|---|
| pH | 6.5 | 7.1 | 7.1 | 7.7 | 0.3 | 4.4 | 0.04 | 0.200 | 8.5 |
| EC (μS cm−1) | 80 | 208 | 187 | 587 | 119 | 57.0 | 1.33 | 0.168 | ― |
| CaCO3 (%) | 1.4 | 3.9 | 4.1 | 6.6 | 1.3 | 34.0 | 0.10 | 0.200 | 11.8 |
| OM (%) | 4.5 | 18.9 | 21.8 | 30.9 | 8.5 | 45.1 | −0.31 | 0.037 | 2.7 |
| TN (mg kg−1) | 106 | 456 | 561 | 815 | 239 | 52.4 | −0.17 | 0.002 | ― |
| TP (mg kg−1) | 83 | 344 | 330 | 648 | 131 | 38.2 | 0.30 | 0.200 | ― |
| Hg (μg kg−1) | 14.4 | 39.3 | 41.0 | 69.9 | 13.9 | 35.3 | 0.23 | 0.200 | 20 |
| As (mg kg−1) | 2.9 | 5.5 | 5.4 | 10.7 | 1.67 | 30.1 | 1.17 | 0.007 | 12.6 |
| Fe (%) | 2.65 | 3.28 | 3.21 | 4.46 | 0.32 | 9.76 | 1.76 | 0.039 | 3.09 |
| N | Min | Mean | Median | Max | Standard Deviation | |
|---|---|---|---|---|---|---|
| Hg Concentration (μg kg−1) | ||||||
| Moss | 28 | 32.4 | 58.4 | 58.7 | 75.8 | 10.7a |
| Caragana jubata | 5 | 7.52 | 13.7 | 13.9 | 18.3 | 4.04b |
| Salix gilashanica | 3 | 8.83 | 16.4 | 15.6 | 24.8 | 8.01b |
| Bark | 23 | 15.9 | 62.9 | 62.0 | 109 | 21.6b |
| Litterfall | 23 | 12.1 | 32.6 | 28.1 | 132 | 23.9b |
| Foliage | 23 | 2.64 | 20.1 | 20.7 | 42.1 | 8.13b |
| Cone | 23 | 1.17 | 11.8 | 11.8 | 22.0 | 5.78b |
| As Concentration (mg kg−1) | ||||||
| Moss | 28 | 1.42 | 2.83 | 2.57 | 8.63 | 1.37a |
| Caragana jubata | 5 | 0.05 | 0.23 | 0.20 | 0.55 | 1.93bd |
| Salix gilashanica | 3 | 0.14 | 0.19 | 0.18 | 0.25 | 0.53bd |
| Bark | 23 | 0.77 | 1.47 | 1.43 | 2.08 | 0.36c |
| Litterfall | 23 | 0.32 | 1.00 | 1.01 | 1.85 | 0.41bc |
| Foliage | 23 | 0.19 | 0.39 | 0.34 | 0.74 | 0.15d |
| Cone | 23 | 0.17 | 0.31 | 0.29 | 0.50 | 0.11d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, S.; Zhao, C.; Nan, Z.; Zhao, C. Ecological Safety and Spatial Distribution of Mercury and Arsenic in Qinghai Spruce Ecosystems in Remote Plateau Mountains, Northwest China. Forests 2022, 13, 1269. https://doi.org/10.3390/f13081269
Wu Y, Wang S, Zhao C, Nan Z, Zhao C. Ecological Safety and Spatial Distribution of Mercury and Arsenic in Qinghai Spruce Ecosystems in Remote Plateau Mountains, Northwest China. Forests. 2022; 13(8):1269. https://doi.org/10.3390/f13081269
Chicago/Turabian StyleWu, Yi, Shengli Wang, Cuicui Zhao, Zhongren Nan, and Chuanyan Zhao. 2022. "Ecological Safety and Spatial Distribution of Mercury and Arsenic in Qinghai Spruce Ecosystems in Remote Plateau Mountains, Northwest China" Forests 13, no. 8: 1269. https://doi.org/10.3390/f13081269





