Increases in the Methane Uptake of Upland Forest Soil in China Could Significantly Contribute to Climate Change Mitigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. CH4 Flux Data
2.2. Environmental Data
2.3. Statistical Analysis
3. Results
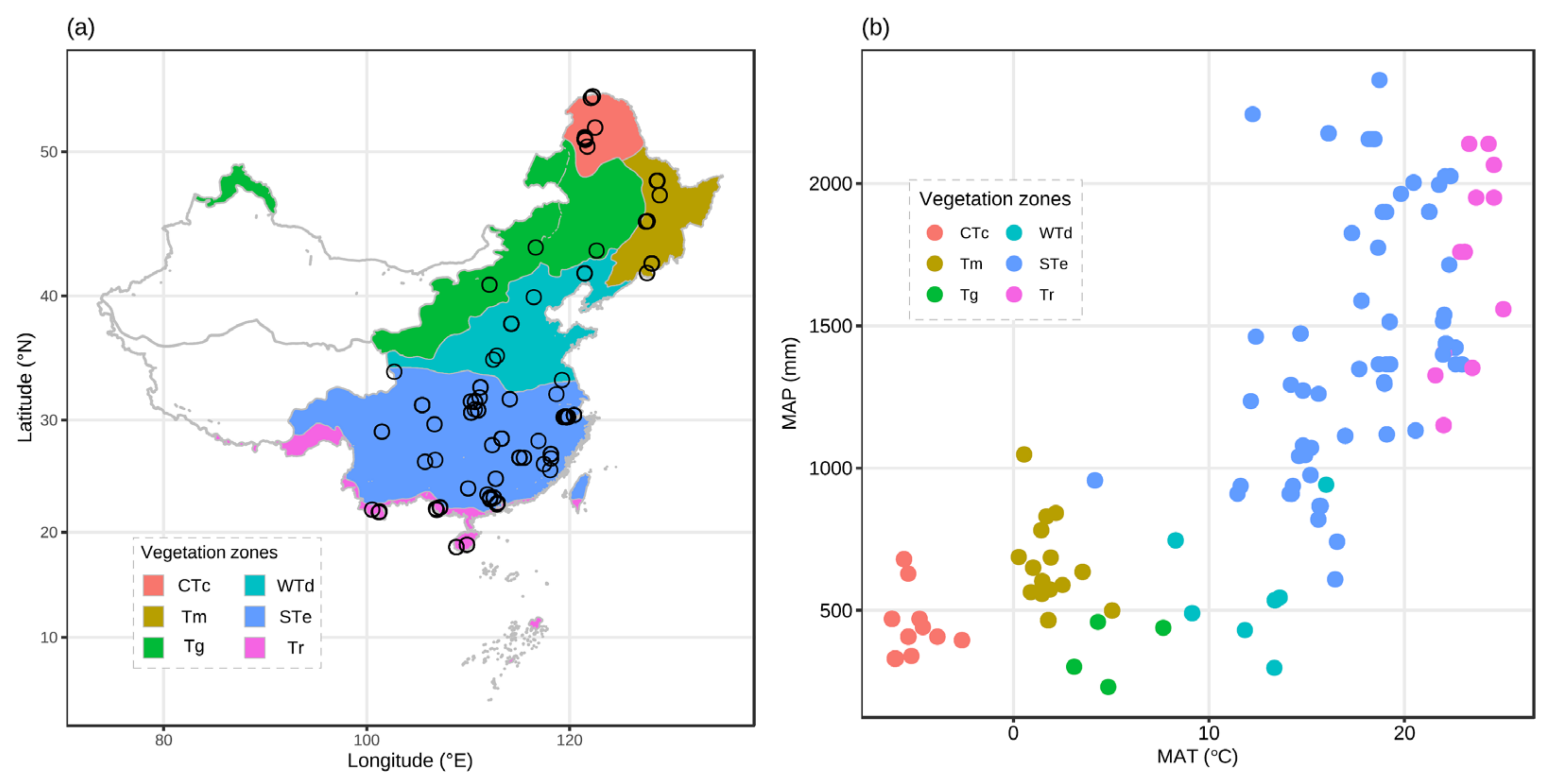
3.1. Spatial Variation in Forest Soil CH4 Flux
3.2. Long-Term Changes in Upland Forest Soil CH4 Uptake
3.3. Relationships between Environmental Factors and CH4 Flux
4. Discussion
4.1. Upland Forest Soil in China Is an Important CH4 Sink
4.2. CH4 Uptake of Upland Forest Soil Is Increasing
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Szopa, S.; Naik, V.; Adhikary, B.; Artaxo, P.; Berntsen, T.; Collins, W.D.; Fuzzi, S.; Gallardo, L.; Kiendler-Scharr, A.; Klimont, Z.; et al. Short-Lived Climate Forcers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 817–922. [Google Scholar]
- Yu, L.; Huang, Y.; Zhang, W.; Li, T.; Sun, W. Methane uptake in global forest and grassland soils from 1981 to 2010. Sci. Total Environ. 2017, 607–608, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chen, G.; Lu, C.; Xu, X.; Ren, W.; Zhang, B.; Banger, K.; Tao, B.; Pan, S.; Liu, M.; et al. Global methane and nitrous oxide emissions from terrestrial ecosystems due to multiple environmental changes. Ecosyst. Health Sustain. 2015, 1, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Qin, Z.C.; Deng, X.; Griscom, B.; Huang, Y.; Li, T.T.; Smith, P.; Yuan, W.P.; Zhang, W. Natural Climate Solutions for China: The Last Mile to Carbon Neutrality. Adv. Atmos. Sci. 2021, 38, 889–895. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Peñuelas, J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma 2019, 355, 113920. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Han, M.; Wang, W.; Peng, C.; Jin, J.; Song, X.; Yu, S. A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For. Ecol. Manag. 2020, 455, 117702. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, F.; Hanson, P.J.; Wullschleger, S.D.; Thornton, P.E.; Riley, W.J.; Song, X.; Graham, D.E.; Song, C.; Tian, H. Reviews and syntheses: Four decades of modeling methane cycling in terrestrial ecosystems. Biogeosciences 2016, 13, 3735–3755. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xie, S.; Han, A.; Rao, R.; Huang, G.; Chen, X.; Hu, J.; Liu, Q.; Yang, X.; Zhang, L. Forest Resources in China—The 9th National Forest Inventory; National Forestry and Grassland Administration: Beijing, China, 2019. [Google Scholar]
- Cai, Z. Greenhouse gas budget for terrestrial ecosystems in China. Sci. China Earth Sci. 2012, 55, 173–182. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Yang, G.; Zhu, D.; Tian, J.; Tian, L.; Kang, X.; et al. Soil methane uptake by grasslands and forests in China. Soil Biol. Biochem. 2014, 74, 70–81. [Google Scholar] [CrossRef]
- Ni, X.; Groffman, P.M. Declines in methane uptake in forest soils. Proc. Natl. Acad. Sci. USA 2018, 115, 8587–8590. [Google Scholar] [CrossRef] [Green Version]
- Gatica, G.; Fernández, M.E.; Juliarena, M.P.; Gyenge, J. Environmental and anthropogenic drivers of soil methane fluxes in forests: Global patterns and among-biomes differences. Glob. Change Biol. 2020, 26, 6604–6615. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, X.; Xing, W.; Liu, G. Soil-atmosphere exchange of CH4 in response to nitrogen addition in diverse upland and wetland ecosystems: A meta-analysis. Soil Biol. Biochem. 2022, 164, 108467. [Google Scholar] [CrossRef]
- China Vegetation Editorial Committee. Vegetation Zoning Data of China. 1980. Available online: https://www.resdc.cn/data.aspx?DATAID=133 (accessed on 5 May 2022).
- Xie, P.; Arkin, P.A. Global Precipitation: A 17-Year Monthly Analysis Based on Gauge Observations, Satellite Estimates, and Numerical Model Outputs. Bull. Am. Meteorol. Soc. 1997, 78, 2539–2558. [Google Scholar] [CrossRef]
- Fan, Y.; van den Dool, H. A global monthly land surface air temperature analysis for 1948–present. J. Geophys. Res. Atmos. 2008, 113, D01103. [Google Scholar] [CrossRef]
- Van den Dool, H.; Huang, J.; Fan, Y. Performance and analysis of the constructed analogue method applied to U.S. soil moisture over 1981–2001. J. Geophys. Res. Atmos. 2003, 108, 8617. [Google Scholar] [CrossRef]
- Wei, S.; Yongjiu, D.; Baoyuan, L.; Axing, Z.; Qingyun, D.; Lizong, W.; Duoying, J.; Ye, A.; Yuan, H.; Zhang, Q.; et al. A China data set of soil properties for land surface modeling. J. Adv. Model. Earth Syst. 2013, 5, 212–224. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Q.; Zhu, J.; Chen, Z.; He, N.; Yu, G. A spatial and temporal dataset of atmospheric inorganic nitrogen wet deposition in China (1996–2015). China Sci. Data 2019, 4, 76–83. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; van de Wiel, M.; Zeileis, A. A Lego system for conditional inference. Am. Stat. 2006, 60, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Muggeo, V.M.R. Segmented: An R Package to Fit Regression Models with Broken-Line Relationships. R News 2008, 8, 20–25. [Google Scholar]
- Muggeo, V.M.R. Interval estimation for the breakpoint in segmented regression: A smoothed score-based approach. Aust. N. Z. J. Stat. 2017, 59, 311–322. [Google Scholar] [CrossRef]
- Murphy, M.A.; Evans, J.S.; Storfer, A. Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 2010, 91, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Evans, J.S.; Murphy, M.A. rfUtilities: R Package Version 2.1-3. 2018. Available online: https://cran.r-project.org/package=rfUtilities (accessed on 2 April 2022).
- Kim, S. ppcor: Partial and Semi-Partial (Part) Correlation. R Package Version 1.1. 2015. Available online: https://CRAN.R-project.org/package=ppcor (accessed on 28 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 April 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.1.6. 2017. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 2 April 2022).
- Urbanek, S.; Horner, J. Cairo: R Graphics Device Using Cairo Graphics Library for Creating High-Quality Bitmap (PNG, JPEG, TIFF), Vector (PDF, SVG, PostScript) and Display (X11 and Win32) Output. R Package Version 1.5-15. 2022. Available online: https://www.rforge.net/Cairo/ (accessed on 2 April 2022).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 0.7.5. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 2 April 2022).
- Bivand, R.; Lewin-Koh, N. maptools: Tools for Handling Spatial Objects. R Package Version 1.1-4. 2022. Available online: https://CRAN.R-project.org/package=maptools (accessed on 2 April 2022).
- Pebesma, E.J.; Bivand, R.S. Classes and methods for spatial data in R. R News 2005, 5, 9–13. [Google Scholar]
- Bivand, R.; Keitt, T.; Rowlingson, B. rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library. R Package Version 1.5-31. 2022. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 2 April 2022).
- Megonigal, J.P.; Guenther, A.B. Methane emissions from upland forest soils and vegetation. Tree Physiol. 2008, 28, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Xu, W.; Li, Q.; Han, M.; Tang, A.; Zhang, Y.; Luo, X.; Shen, J.; Wang, W.; Li, K.; et al. Changes of nitrogen deposition in China from 1980 to 2018. Environ. Int. 2020, 144, 106022. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yuan, F.; Bai, J.; Duan, H.; Gu, X.; Hou, L.; Huang, Y.; Yang, M.; He, J.-S.; Zhang, Z.; et al. Phosphorus alleviation of nitrogen-suppressed methane sink in global grasslands. Ecol. Lett. 2020, 23, 821–830. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Xu, K. Response of soil CH4 fluxes to stimulated nitrogen deposition in a temperate deciduous forest in northern China: A 5-year nitrogen addition experiment. Eur. J. Soil Biol. 2017, 82, 43–49. [Google Scholar] [CrossRef]
- Carlsen, H.N.; Joergensen, L.; Degn, H. Inhibition by ammonia of methane utilization in Methylococcus capsulatus (Bath). Appl. Microbiol. Biotechnol. 1991, 35, 124–127. [Google Scholar] [CrossRef]
- Nyerges, G.; Stein, L.Y. Ammonia cometabolism and product inhibition vary considerably among species of methanotrophic bacteria. FEMS Microbiol. Lett. 2009, 297, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zeng, R.J.; Keller, J.; Lant, P.A.; Yuan, Z. Effect of nitrate and nitrite on the selection of microorganisms in the denitrifying anaerobic methane oxidation process. Environ. Microbiol. Rep. 2011, 3, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Luo, R.; McConkey, B.G.; Ziadi, N. Effects of nitrogen deposition and litter layer management on soil CO2, N2O, and CH4 emissions in a subtropical pine forestland. Sci. Rep. 2020, 10, 8959. [Google Scholar] [CrossRef] [PubMed]
- Walkiewicz, A.; Rafalska, A.; Bulak, P.; Bieganowski, A.; Osborne, B. How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini-Review. Forests 2021, 12, 1276. [Google Scholar]
- Dijkstra, F.A.; Morgan, J.A.; von Fischer, J.C.; Follett, R.F. Elevated CO2 and warming effects on CH4 uptake in a semiarid grassland below optimum soil moisture. J. Geophys. Res. Biogeosci. 2011, 116, G01007. [Google Scholar] [CrossRef]
- Khokhar, N.H.; Park, J.-W. Precipitation Decreases Methane Uptake in a Temperate Deciduous Forest. J. Soil Groundw. Environ. 2019, 24, 24–34. [Google Scholar]
- Yue, P.; Zuo, X.; Li, K.; Li, X.; Wang, S.; Misselbrook, T. Precipitation changes regulate the annual methane uptake in a temperate desert steppe. Sci. Total Environ. 2022, 804, 150172. [Google Scholar] [CrossRef] [PubMed]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Von Fischer, J.C.; Butters, G.; Duchateau, P.C.; Thelwell, R.J.; Siller, R. In situ measures of methanotroph activity in upland soils: A reaction-diffusion model and field observation of water stress. J. Geophys. Res. Biogeosci. 2009, 114, G01015. [Google Scholar] [CrossRef] [Green Version]
- Ran, Y.; Li, X.; Cheng, G.; Zhang, T.; Wu, Q.; Jin, H.; Jin, R. Distribution of Permafrost in China: An Overview of Existing Permafrost Maps. Permafr. Periglac. Process. 2012, 23, 322–333. [Google Scholar] [CrossRef]
- Hu, J.; Guan, W.; Chen, H. Methane Emissions from Surface of Mangrove River on Hainan Island, China. Atmosphere 2021, 12, 1126. [Google Scholar] [CrossRef]
- Song, C.; Yan, B.; Wang, Y.; Wang, Y.; Lou, Y.; Zhao, Z. Fluxes of carbon dioxide and methane from swamp and impact factors in Sanjiang Plain, China. Chin. Sci. Bull. 2003, 48, 2749–2753. [Google Scholar] [CrossRef]
- Yang, M.; Geng, X.; Grace, J.; Lu, C.; Zhu, Y.; Zhou, Y.; Lei, G. Spatial and Seasonal CH4 Flux in the Littoral Zone of Miyun Reservoir near Beijing: The Effects of Water Level and Its Fluctuation. PLoS ONE 2014, 9, e94275. [Google Scholar] [CrossRef]
- Tang, W.; Xu, Y.J.; Ma, Y.; Maher, D.T.; Li, S. Hot spot of CH4 production and diffusive flux in rivers with high urbanization. Water Res. 2021, 204, 117624. [Google Scholar] [CrossRef]
- Schmitt, C.B.; Burgess, N.D.; Coad, L.; Belokurov, A.; Besançon, C.; Boisrobert, L.; Campbell, A.; Fish, L.; Gliddon, D.; Humphries, K.; et al. Global analysis of the protection status of the world’s forests. Biol. Conserv. 2009, 142, 2122–2130. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 2021, 14, 225–230. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Dong, J.; Quan, Q.; Liu, J. Magnitudes and environmental drivers of greenhouse gas emissions from natural wetlands in China based on unbiased data. Environ. Sci. Pollut. Res. 2021, 28, 44973–44986. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Li, Q.; Peng, C.; Zhang, J.; Li, Y.; Song, X. Nitrogen addition decreases methane uptake caused by methanotroph and methanogen imbalances in a Moso bamboo forest. Sci. Rep. 2021, 11, 5578. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Fang, H.; Yu, G.; Xu, M.; Dang, X.; Li, L.; Wang, L. Simulated nitrogen deposition reduces CH4 uptake and increases N2O emission from a subtropical plantation forest soil in southern China. PLoS ONE 2014, 9, e93571. [Google Scholar] [CrossRef] [PubMed]
- Steudler, P.; Bowden, R.; Melillo, J.; Aber, J. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 1989, 341, 314–316. [Google Scholar] [CrossRef]
- Xinhua News Agency. Outline of the 14th Five-Year Plan (2021–2025) for National Economic and Social Development and Vision 2035 of the People’s Republic of China. 2021. Available online: http://www.gov.cn/xinwen/2021-03/13/content_5592681.htm (accessed on 2 June 2022).
- Conrad, R. Microbial Ecology of Methanogens and Methanotrophs. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 1–63. [Google Scholar]
- Serrano-Silva, N.; Sarria-Guzman, Y.; Dendooven, L.; Luna-Guido, M. Methanogenesis and Methanotrophy in Soil: A Review. Pedosphere 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Hempel, S.; Saha, C.K.; Fiedler, M.; Berg, W.; Hansen, C.; Amon, B.; Amon, T. Non-linear temperature dependency of ammonia and methane emissions from a naturally ventilated dairy barn. Biosyst. Eng. 2016, 145, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Van den Pol-van Dasselaar, A.; van Beusichem, M.L.; Oenema, O. Effects of soil moisture content and temperature on methane uptake by grasslands on sandy soils. Plant Soil 2004, 204, 213–222. [Google Scholar] [CrossRef]
- Castaldi, S.; Bertolini, T.; Nicolini, G.; Valentini, R. Soil Is a Net Source of Methane in Tropical African Forests. Forests 2020, 11, 1157. [Google Scholar] [CrossRef]
- China Meteorological Administration. Blue Book on Climate Change in China 2021; Science Press: Beijing, China, 2021. [Google Scholar]




| Environmental Factors | Correlation Coefficients | p-Value |
|---|---|---|
| MAP | −0.14 | 0.80 |
| MAT | 0.37 | 0.50 |
| SWC | −0.26 | 0.66 |
| TN | −0.49 | 0.36 |
| AN | −0.94 * | 0.02 |
| AP | −0.54 | 0.30 |
| AK | −0.37 | 0.50 |
| PH | 0.60 | 0.24 |
| BD | 0.01 | 0.92 |
| POR | −0.31 | 0.56 |
| DIN | 0.26 | 0.66 |
| NH4 | 0.26 | 0.66 |
| NO3 | 0.14 | 0.80 |
| Parameters | All Data | Vegetation Zones | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean ± SE | CTc (Mean ± SE) | Tm (Mean ± SE) | Tg (Mean ± SE) | WTd (Mean ± SE) | STe (Mean ± SE) | Tr (Mean ± SE) | |
| MAP (mm) | 2363.4 | 231.5 | 1095.4 ± 35.4 | 427.8 ± 15.3 | 668.3 ± 24.4 | 358.3 ± 54.9 | 571.5 ± 38.3 | 1398.3 ± 36.9 | 1711.5 ± 61.0 |
| MAT (°C) | 25.1 | −6.2 | 11.8 ± 0.6 | −5.2 ± 0.2 | 2.2 ± 0.2 | 5.0 ± 1.0 | 11.7 ± 0.6 | 17.7 ± 0.3 | 23.4 ± 0.2 |
| SWC (mm) | 599.9 | 124.3 | 404.1 ± 6.5 | 270.0 ± 5.0 | 376.9 ± 11.4 | 185.9 ± 23.4 | 283.2 ± 6.7 | 477 ± 5.2 | 435.7 ± 11 |
| SOM (g/100 g) | 22.7 | 0.7 | 4.4 ± 0.3 | 7.9 ± 0.4 | 5.0 ± 0.2 | 1.4 ± 0.4 | 2.7 ± 0.5 | 2.6 ± 0.1 | 2.0 ± 0.1 |
| TN (g/100 g) | 0.7 | 0.0 | 0.2 ± 0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| AN (mg/kg) | 340.3 | 32.6 | 156.4 ± 5.6 | 279.4 ± 11.4 | 241.5 ± 9.2 | 84.1 ± 19.2 | 77.2 ± 6.4 | 109 ± 2.3 | 85.3 ± 4.4 |
| TP (g/100 g) | 0.2 | 0.0 | 0.1 ± 0 | 0.10 ± 0.00 | 0.08 ± 0.00 | 0.06 ± 0.01 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 |
| AP (mg/kg) | 11.7 | 1.8 | 5.6 ± 0.2 | 9.9 ± 0.4 | 7.8 ± 0.2 | 3.1 ± 0.8 | 4.9 ± 0.1 | 3.9 ± 0.1 | 3.2 ± 0.4 |
| TK (g/100 g) | 3.2 | 0.6 | 1.7 ± 0 | 2.2 ± 0.0 | 1.9 ± 0.0 | 1.9 ± 0.1 | 2.0 ± 0.0 | 1.5 ± 0.0 | 1.0 ± 0.0 |
| AK (mg/kg) | 196.2 | 33.2 | 104 ± 3 | 182.3 ± 4.6 | 127.8 ± 3.5 | 125.5 ± 21.4 | 94.5 ± 6.3 | 80.7 ± 2.3 | 57.4 ± 7.3 |
| pH | 8.3 | 4.2 | 5.8 ± 0.1 | 5.6 ± 0.1 | 6.0 ± 0.1 | 8.3 ± 0.0 | 7.2 ± 0.3 | 5.7 ± 0.1 | 5.2 ± 0.0 |
| BD (g/cm3) | 1.4 | 0.8 | 1.3 ± 0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.1 | 1.4 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 |
| POR (cm3/100 cm3) | 58.0 | 43.3 | 51.9 ± 0.2 | 51.8 ± 0.2 | 54.4 ± 0.3 | 52.6 ± 2.3 | 47.9 ± 0.6 | 52.1 ± 0.2 | 49.8 ± 0.3 |
| GRAV (g/100 g) | 25.6 | 0.8 | 10.6 ± 0.4 | 12.9 ± 0.7 | 20.6 ± 0.8 | 4.5 ± 0.9 | 11.3 ± 1.6 | 8.3 ± 0.5 | 9.4 ± 0.3 |
| DIN (kg N ha−1 yr−1) | 27.2 | 3.0 | 15.8 ± 0.5 | 6.6 ± 0.4 | 10.9 ± 0.2 | 13.4 ± 0.2 | 20.6 ± 0.9 | 20.3 ± 0.4 | 10.2 ± 0.8 |
| NH4 (kg N ha−1 yr−1) | 18.9 | 1.1 | 9.4 ± 0.3 | 3.7 ± 0.4 | 5.8 ± 0.4 | 9.7 ± 0.4 | 12.1 ± 0.9 | 12.2 ± 0.3 | 5.9 ± 0.4 |
| NO3 (kg N ha−1 yr−1) | 15.3 | 1.5 | 6.4 ± 0.2 | 2.9 ± 0.1 | 5 ± 0.2 | 3.7 ± 0.2 | 8.6 ± 0.2 | 8.1 ± 0.2 | 4.3 ± 0.4 |
| CH4 uptake (g m−2 yr−1) | 1.79 | −0.83 | 0.24 ± 0.02 | 0.22 ± 0.05 | 0.23 ± 0.06 | 0.29 ± 0.05 | 0.46 ± 0.10 | 0.22 ± 0.03 | 0.26 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M. Increases in the Methane Uptake of Upland Forest Soil in China Could Significantly Contribute to Climate Change Mitigation. Forests 2022, 13, 1270. https://doi.org/10.3390/f13081270
Yang M. Increases in the Methane Uptake of Upland Forest Soil in China Could Significantly Contribute to Climate Change Mitigation. Forests. 2022; 13(8):1270. https://doi.org/10.3390/f13081270
Chicago/Turabian StyleYang, Meng. 2022. "Increases in the Methane Uptake of Upland Forest Soil in China Could Significantly Contribute to Climate Change Mitigation" Forests 13, no. 8: 1270. https://doi.org/10.3390/f13081270
APA StyleYang, M. (2022). Increases in the Methane Uptake of Upland Forest Soil in China Could Significantly Contribute to Climate Change Mitigation. Forests, 13(8), 1270. https://doi.org/10.3390/f13081270





