Effects of Exogenous Antioxidant Melatonin on Physiological and Biochemical Characteristics of Populus cathayana × canadansis ‘Xin Lin 1’ under Salt and Alkaline Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Physiological Indicators
2.2. Statistical Analysis
3. Results
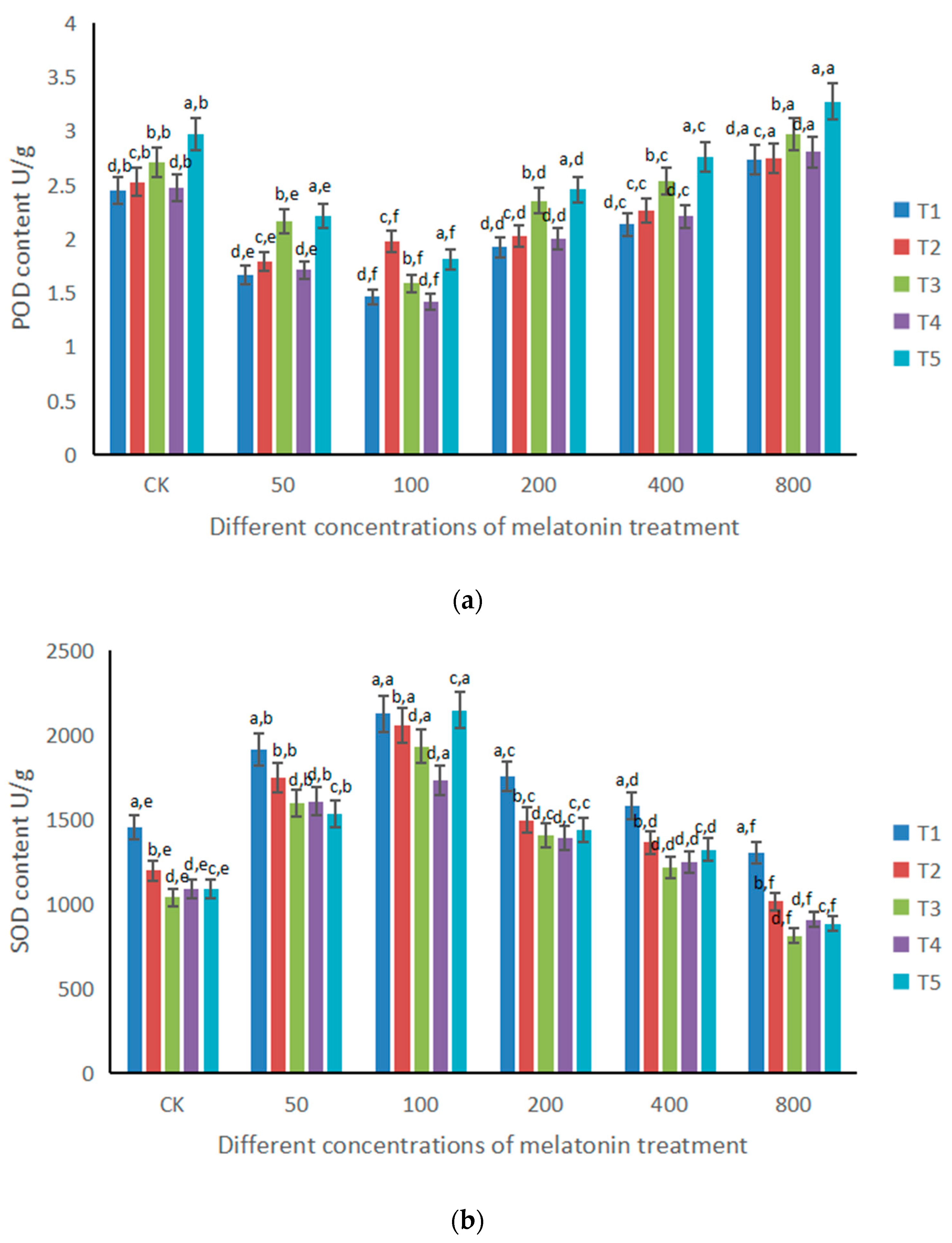
3.1. Changes of Antioxidant Enzymes under the Influence of Different Doses of Melatonin
3.2. Changes of Osmotic Regulators under the Influence of Different Doses of Melatonin
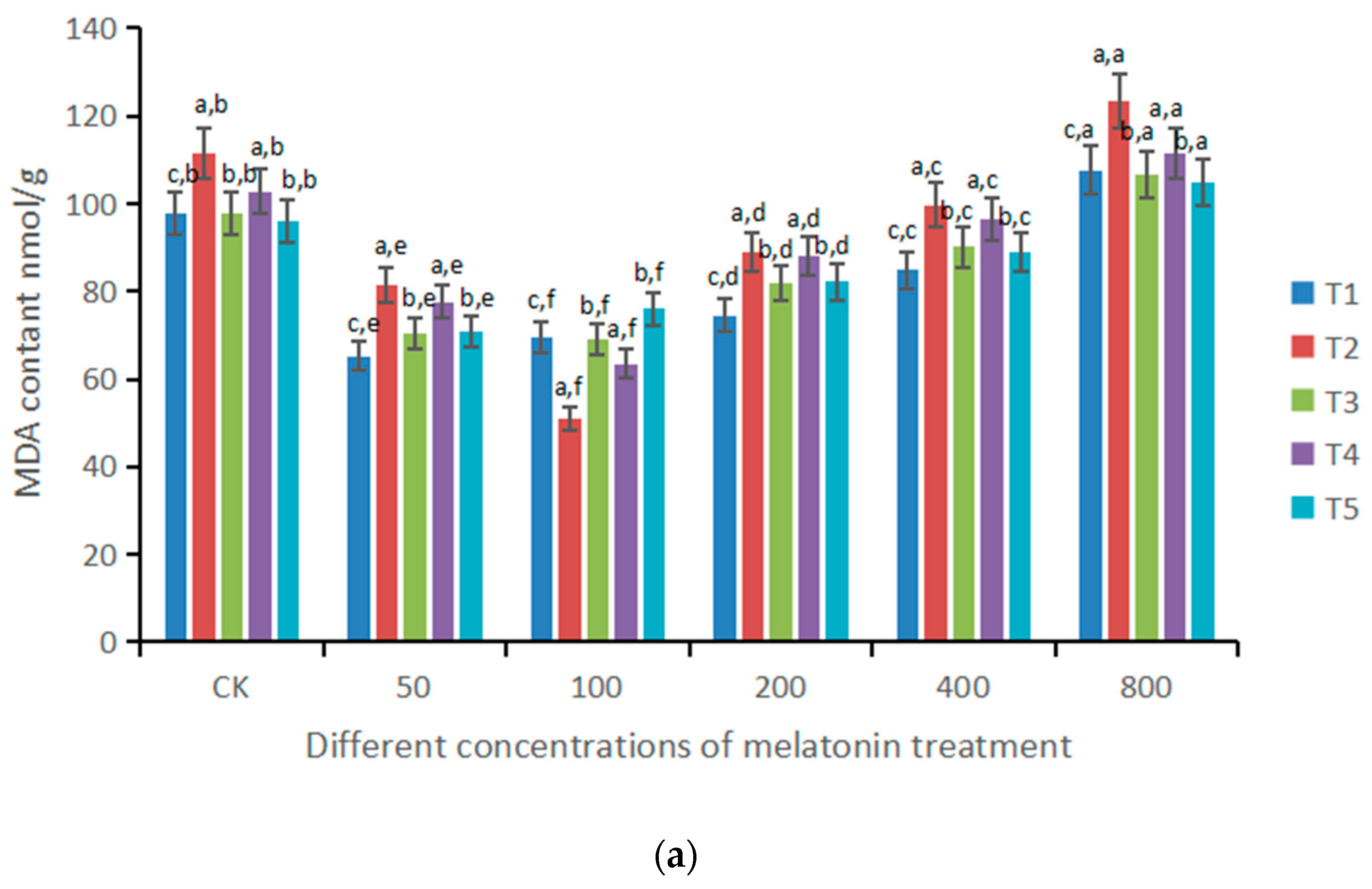
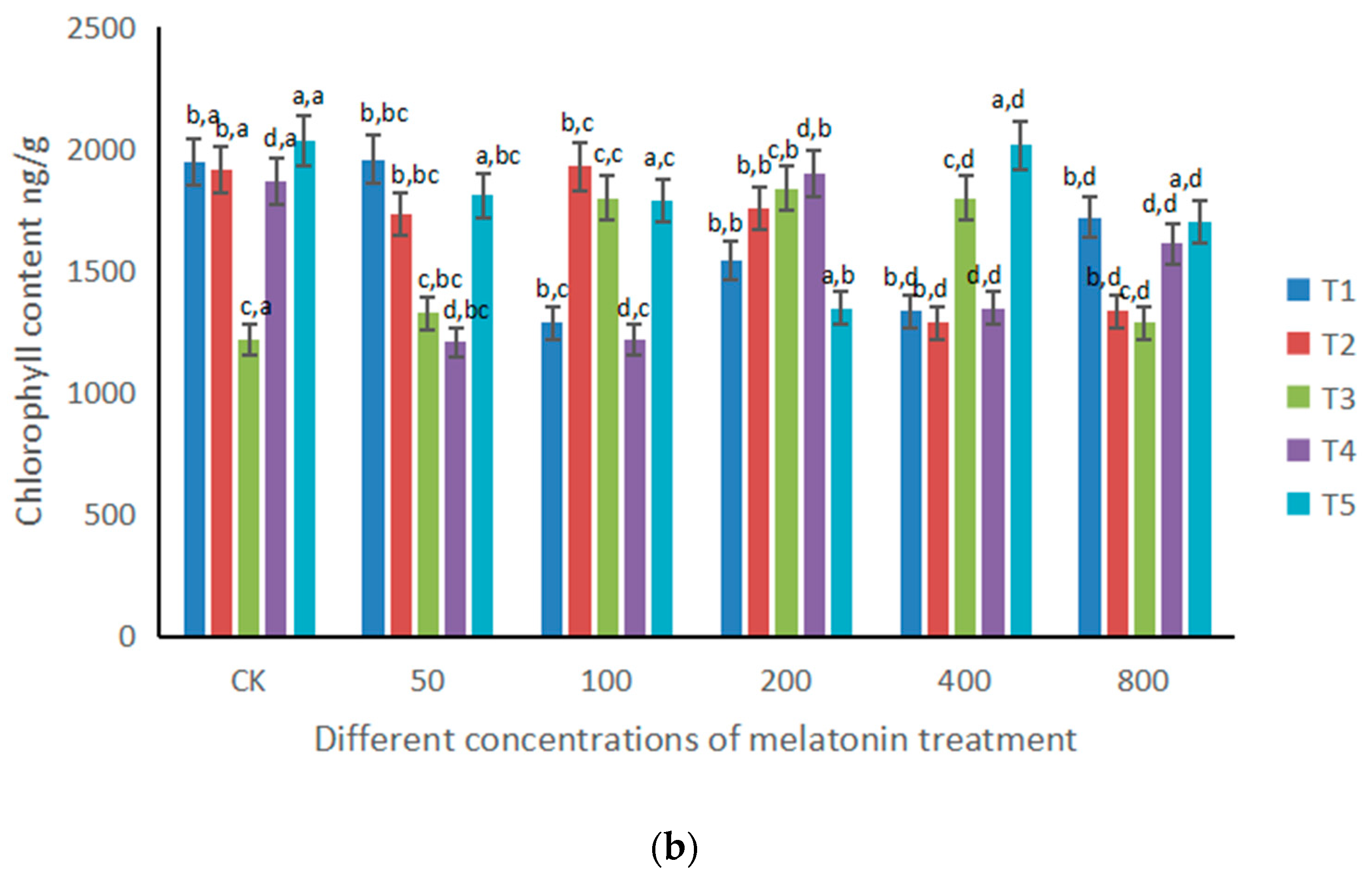
3.3. Changes of MDA and Chlorophyll under the Influence of Different Doses of Melatonin
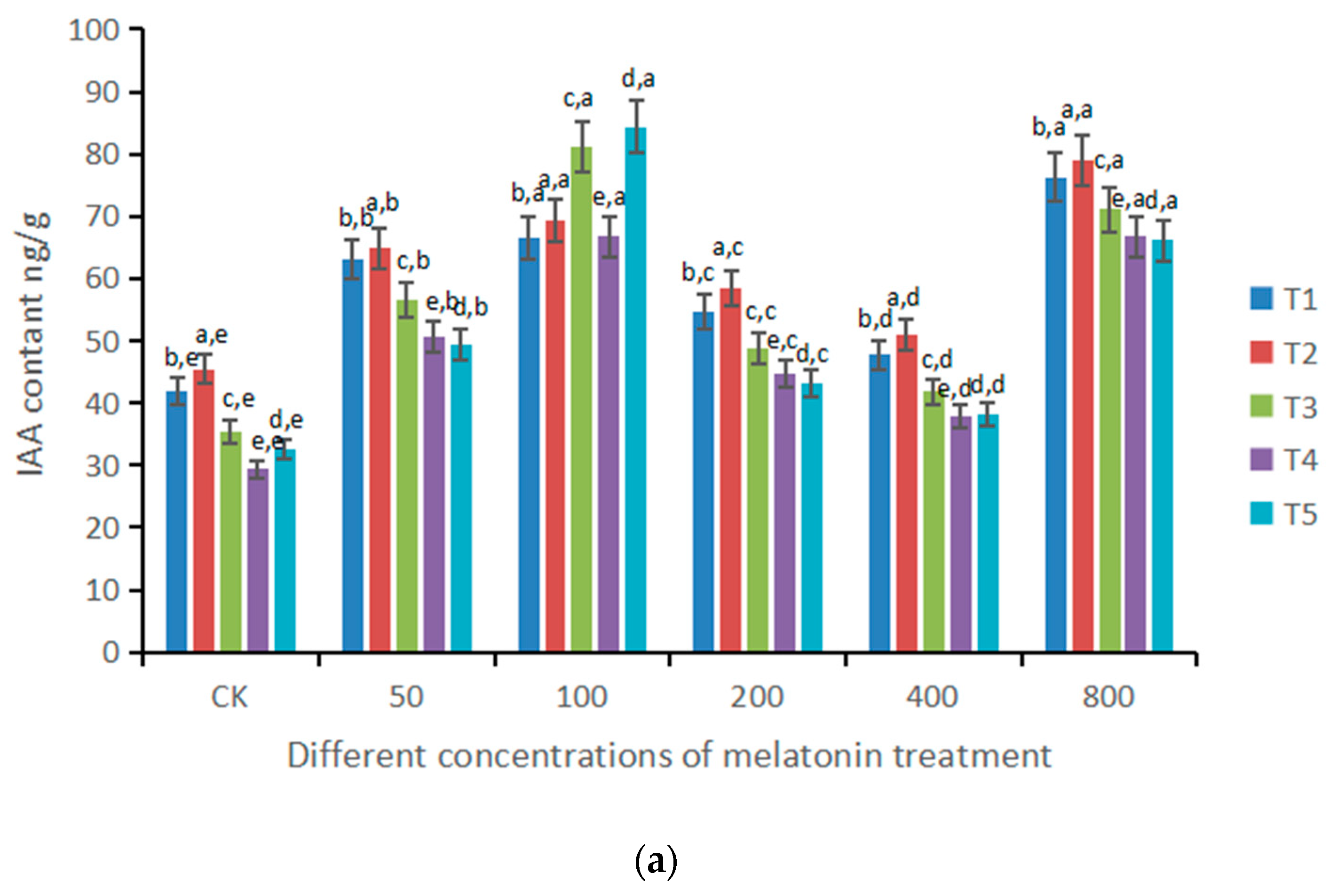
3.4. Effects of Different Doses of Melatonin on Plant Growth-Stimulating Hormones
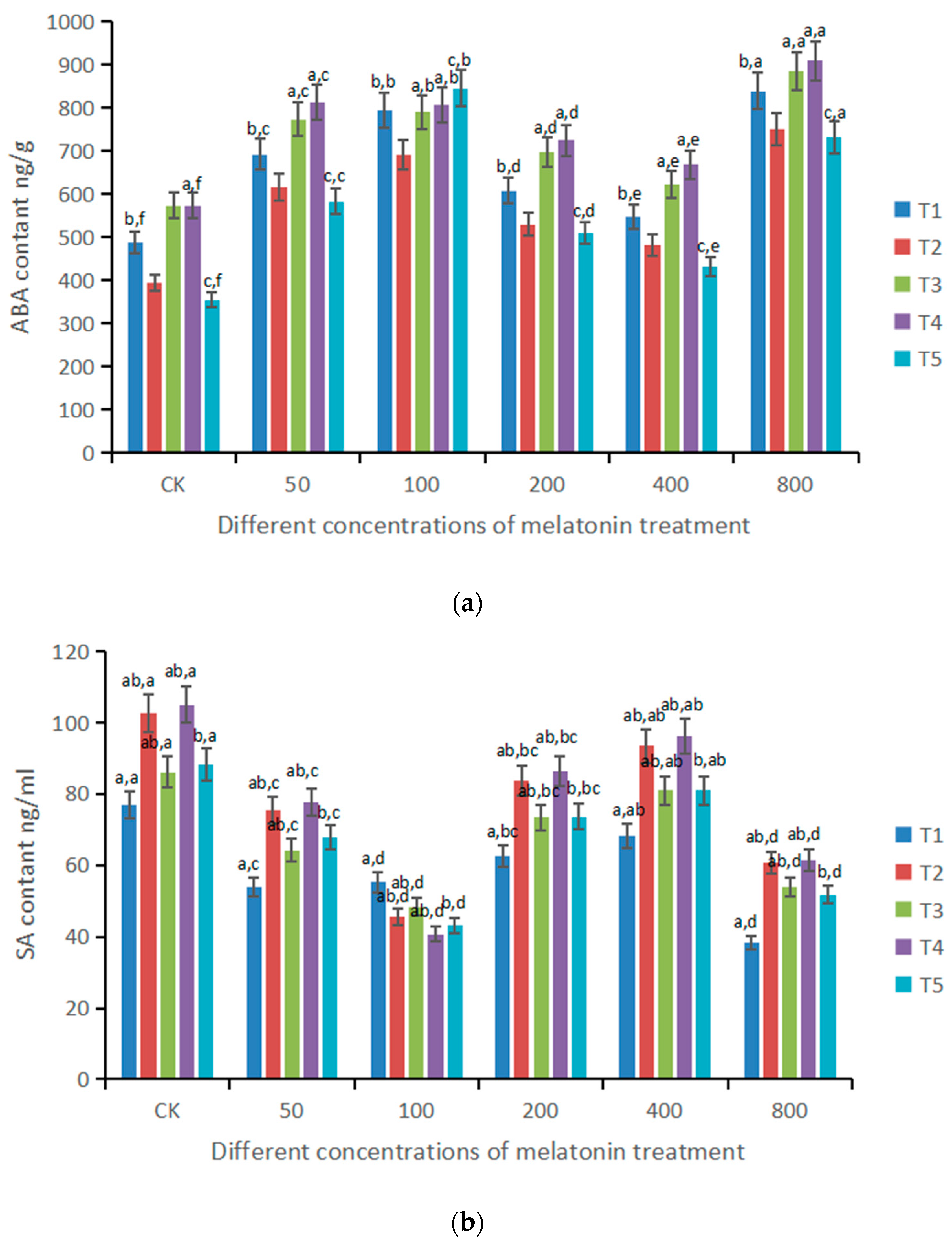
3.5. Effects of Different Doses of Melatonin on Plant Irritability Hormones
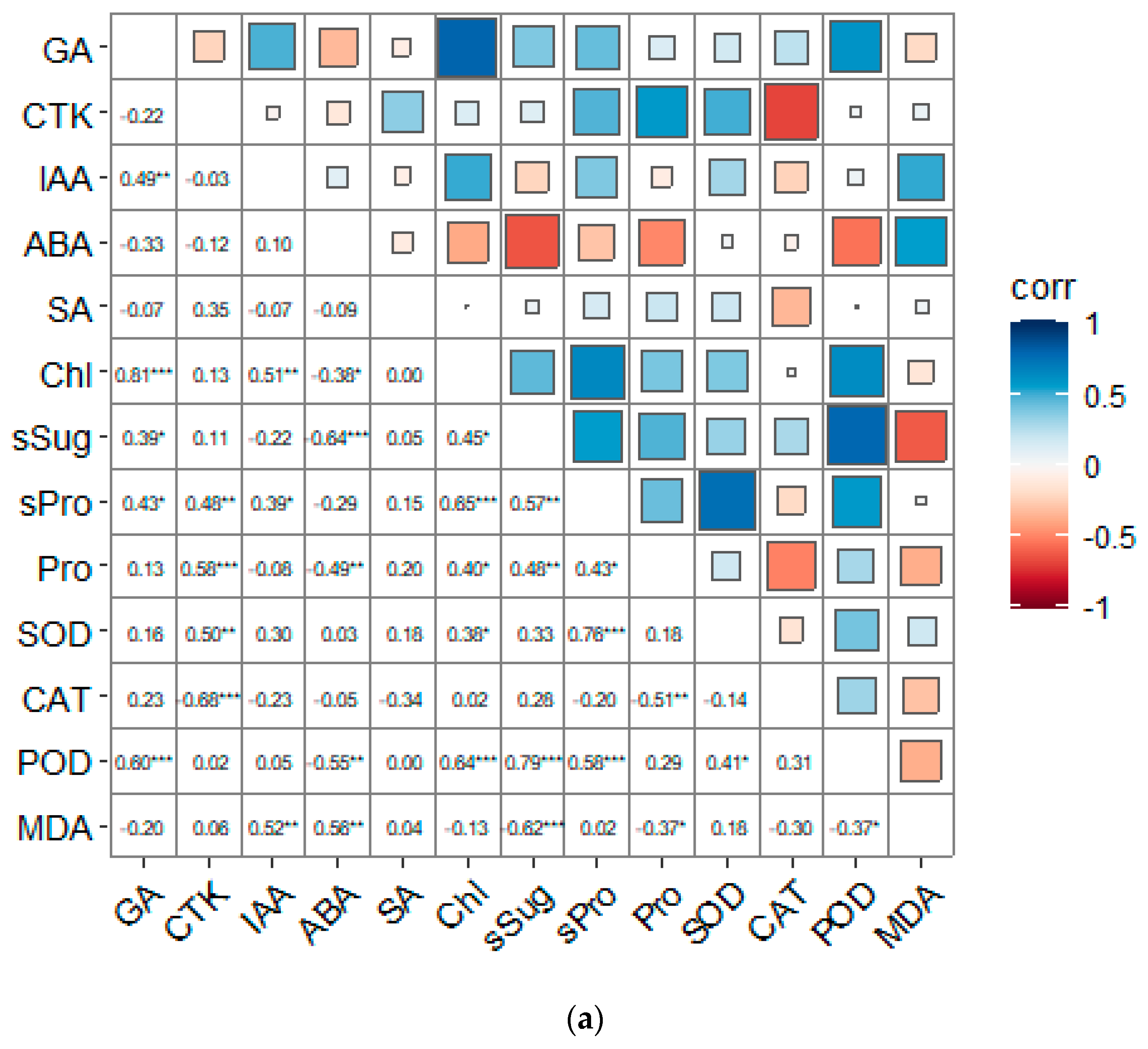
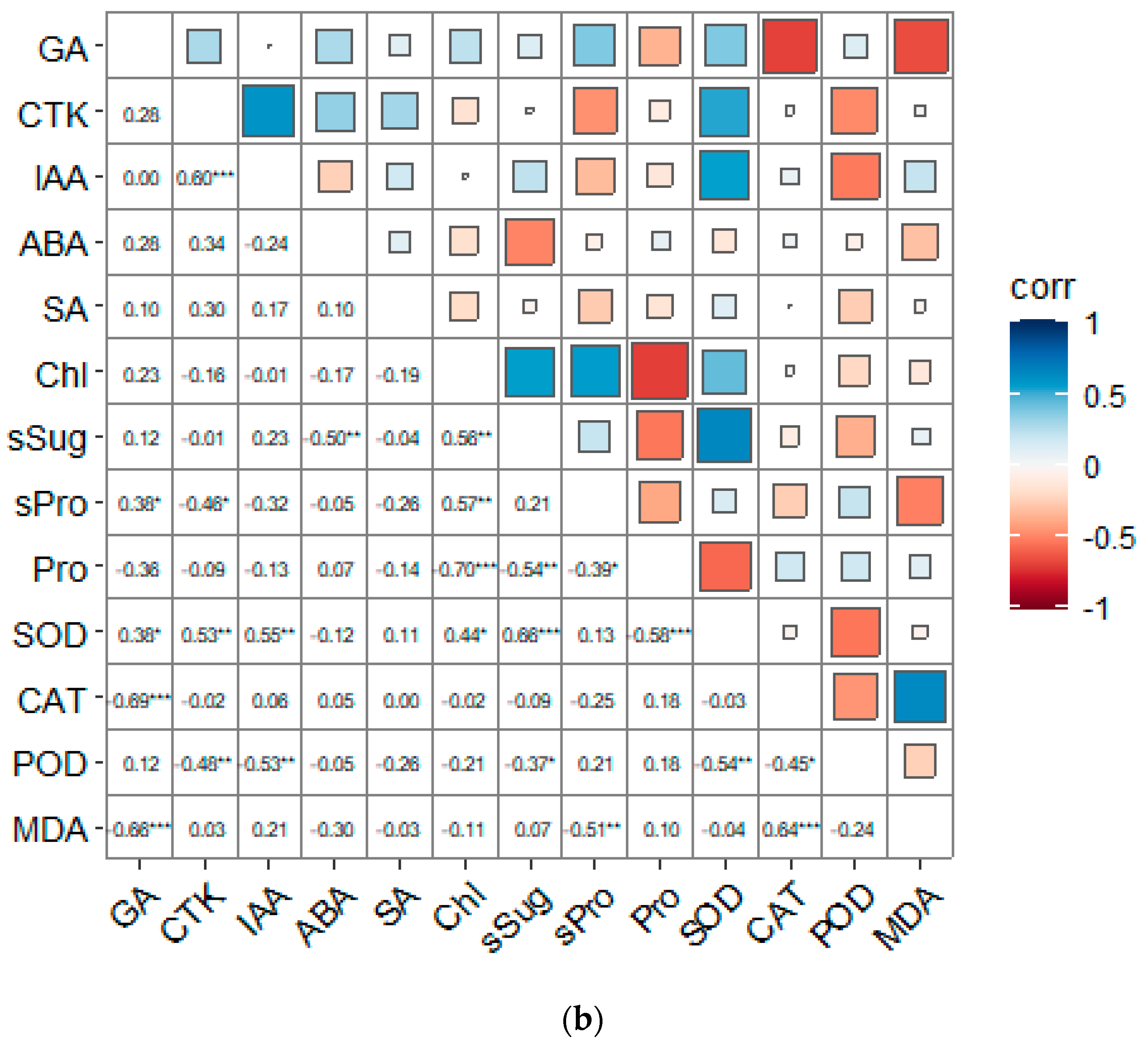
3.6. Correlation Analysis of Each Indicator
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xiao, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in Understanding the Physiological and Molecular Responses of Populus to Salt Stress. Int. J. Mol. Sci. 2019, 20, 1312. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, B.T.; Denkena, J.; Colomé-Tatché, M.; Shahryary, Y.; Hazarika, R.; Grimwood, J.; Mamidi, S.; Jenkins, J.; Grabowski, P.P.; Sreedasyam, A.; et al. A genome assembly and the somatic genetic and epigenetic mutation rate in a wild long-lived perennial Populus trichocarpa. Genome Biol. 2020, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, T.; Xing, C.; Dong, H.; Qi, K.; Gao, J.; Tao, S.; Wu, J.; Wu, J.; Zhang, S.; et al. The β-amylase PbrBAM3 from pear (Pyrus betulaefolia) regulates soluble sugar accumulation and ROS homeostasis in response to cold stress. Plant Sci. Int. J. Exp. Plant Biol. 2019, 287, 110184. [Google Scholar] [CrossRef]
- Qi, W.; Wang, F.; Ma, L.; Qi, Z.; Liu, S.; Chen, C.; Wu, J.; Wang, P.; Yang, C.; Wu, Y.; et al. Physiological and Biochemical Mechanisms and Cytology of Cold Tolerance in Brassica napus. Front. Plant Sci. 2020, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, L.; Song, J.; Li, C.; Zhang, Y.; Wang, Y.; Pang, Y.; Zhang, B. Physiological and transcriptome analyses highlight multiple pathways involved in drought stress in Medicago falcata. PLoS ONE 2022, 17, e0266542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Z.; Jiang, X.; Qin, Y. Physiological changes and transcript identification in Coreopsis tinctoria Nutt. in early stages of salt stress. PeerJ 2021, 9, e11888. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef]
- Yang, N.; Sun, K.; Wang, X.; Wang, K.; Kong, X.; Gao, J.; Wen, D. Melatonin Participates in Selenium-Enhanced Cold Tolerance of Cucumber Seedlings. Front. Plant Sci. 2021, 12, 786043. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. PPB 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, X.; Ma, C.; Wang, Y.; Zhao, J. Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol. Biochem. PPB 2021, 167, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Wang, T.; Gong, L.; Liu, F.; Brestic, M.; Liu, S.; Song, F.; Li, X. Melatonin reduces nanoplastic uptake, translocation, and toxicity in wheat. J. Pineal Res. 2021, 71, e12761. [Google Scholar] [CrossRef]
- Wang, H.; Ren, C.; Cao, L.; Jin, X.; Wang, M.; Zhang, M.; Zhao, Q.; Li, H.; Zhang, Y.; Yu, G. The mechanisms underlying melatonin improved soybean seedling growth at different nitrogen levels. Funct. Plant Biol. FPB 2021, 48, 1225–1240. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Campbell, K.R.; Lun, A.T.; Wills, Q.F. Scater: Pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 2017, 33, 1179–1186. [Google Scholar] [CrossRef]
- Gu, Z.; Hübschmann, D. Make Interactive Complex Heatmaps in R. Bioinformatics 2021, 38, 1460–1462. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Gene regulation at transcriptional and post-transcriptional levels to combat salt stress in plants. Physiol. Plant. 2021, 173, 1556–1572. [Google Scholar] [CrossRef]
- Kaiwen, G.; Zisong, X.; Yuze, H.; Qi, S.; Yue, W.; Yanhui, C.; Jiechen, W.; Wei, L.; Huihui, Z. Effects of salt concentration, pH, and their interaction on plant growth, nutrient uptake, and photochemistry of alfalfa (Medicago sativa) leaves. Plant Signal. Behav. 2020, 15, 1832373. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2020, 15, e0244365. [Google Scholar] [CrossRef]
- Wang, X.-s.; Ren, H.l.; Wei, Z.-w.; Wang, Y.-w.; Ren, W.-b. Effects of neutral salt and alkali on ion distributions in the roots, shoots, and leaves of two alfalfa cultivars with differing degrees of salt tolerance. J. Integr. Agric. 2017, 16, 1800–1807. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Wang, N.; Tan, D.X.; Ma, F. Melatonin enhances the occurrence of autophagy induced by oxidative stress in Arabidopsis seedlings. J. Pineal Res. 2015, 58, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs. oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. PPB 2021, 163, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, M.; Zhang, W.; Liu, F.; Huang, K.; Lin, K. Insight into the tolerance, biochemical and antioxidative response in three moss species on exposure to BDE-47 and BDE-209. Ecotoxicol. Environ. Saf. 2019, 181, 445–454. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J. Sci. Food Agric. 2013, 93, 1699–1705. [Google Scholar] [CrossRef]
- Feng, N.; Yu, M.; Li, Y.; Jin, D.; Zheng, D. Prohexadione-calcium alleviates saline-alkali stress in soybean seedlings by improving the photosynthesis and up-regulating antioxidant defense. Ecotoxicol. Environ. Saf. 2021, 220, 112369. [Google Scholar] [CrossRef]
- Guan, Q.; Liao, X.; He, M.; Li, X.; Wang, Z.; Ma, H.; Yu, S.; Liu, S. Tolerance analysis of chloroplast OsCu/Zn-SOD overexpressing rice under NaCl and NaHCO3 stress. PLoS ONE 2017, 12, e0186052. [Google Scholar] [CrossRef]
- Kaouthar, F.; Ameny, F.K.; Yosra, K.; Walid, S.; Ali, G.; Faiçal, B. Responses of transgenic Arabidopsis plants and recombinant yeast cells expressing a novel durum wheat manganese superoxide dismutase TdMnSOD to various abiotic stresses. J. Plant Physiol. 2016, 198, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. PPB 2013, 70, 396–402. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Y.; Xu, Z.; Zhang, W.; Jiang, K. Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Chen, L.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. PeerJ 2020, 8, e10486. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Ding, X.; Hu, Y.; Tian, S.; Yan, D.; Shao, S.; Gao, Y.; Liu, R.; Yang, Y. Effects of Saline and Alkaline Stress on Germination, Seedling Growth, and Ion Balance in Wheat. Agric. Res. 2010, 102, 1252–1260. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.G.; Pang, Q.Y. Physiological evaluation of the responses of Larix olgensis families to drought stress and proteomic analysis of the superior family. Genet. Mol. Res. GMR 2015, 14, 15577–15586. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, C.; Huang, D.; Li, H.; Wang, C. The Papain-like Cysteine Protease HpXBCP3 from Haematococcus pluvialis Involved in the Regulation of Growth, Salt Stress Tolerance and Chlorophyll Synthesis in Microalgae. Int. J. Mol. Sci. 2021, 22, 11539. [Google Scholar] [CrossRef]
- Park, H.S.; Kazerooni, E.A.; Kang, S.M.; Al-Sadi, A.M.; Lee, I.J. Melatonin Enhances the Tolerance and Recovery Mechanisms in Brassica juncea (L.) Czern. Under Saline Conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.J.; Ma, H.Y.; Wang, Z.J.; Wang, Z.Y.; Bu, Q.Y.; Liu, S.K. A rice LSD1-like-type ZFP gene OsLOL5 enhances saline-alkaline tolerance in transgenic Arabidopsis thaliana, yeast and rice. BMC Genom. 2016, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, A.; Zhang, W.H. Higher endogenous bioactive gibberellins and α-amylase activity confer greater tolerance of rice seed germination to saline-alkaline stress. Environ. Exp. Bot. 2019, 162, 357–363. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wang, J.; Lin, J. Exogenous Abscisic Acid Alleviates Harmful Effect of Salt and Alkali Stresses on Wheat Seedlings. Int. J. Environ. Res. Public Health 2020, 17, 3770. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Ritonga, F.N.; Yu, H.; Ding, C.; Zhao, X. Effects of Exogenous Antioxidant Melatonin on Physiological and Biochemical Characteristics of Populus cathayana × canadansis ‘Xin Lin 1’ under Salt and Alkaline Stress. Forests 2022, 13, 1283. https://doi.org/10.3390/f13081283
Song R, Ritonga FN, Yu H, Ding C, Zhao X. Effects of Exogenous Antioxidant Melatonin on Physiological and Biochemical Characteristics of Populus cathayana × canadansis ‘Xin Lin 1’ under Salt and Alkaline Stress. Forests. 2022; 13(8):1283. https://doi.org/10.3390/f13081283
Chicago/Turabian StyleSong, Runxian, Faujiah Nurhasanah Ritonga, Haiyang Yu, Changjun Ding, and Xiyang Zhao. 2022. "Effects of Exogenous Antioxidant Melatonin on Physiological and Biochemical Characteristics of Populus cathayana × canadansis ‘Xin Lin 1’ under Salt and Alkaline Stress" Forests 13, no. 8: 1283. https://doi.org/10.3390/f13081283





