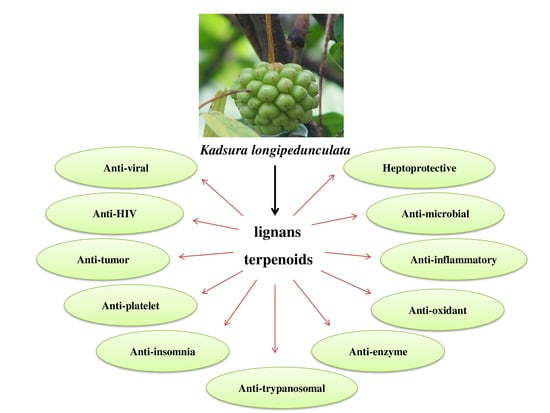
Kadsura longipedunculata Finet & Gagnepain (Schisandraceae): An Overview of Botany, Traditional Uses, Phytochemistry and Pharmacological Activities
Abstract
:1. Introduction
2. Botany
3. Traditional Uses
4. Phytochemistry
4.1. Lignans
4.2. Triterpenoid
5. Pharmacological Activities
5.1. Hepatoprotective Activity
5.2. Cytotoxic Activity
5.3. Anti-Microbial Activity
5.4. Anti-Tumor Activity
5.5. Anti-Inflammatory Activity
5.6. Antioxidant Activity
5.7. Cholesterol Biosynthesis Inhibition Activity
5.8. Anti-Platelet Aggregation Activity
5.9. Anti-Insomnia Activity
5.10. Anti-Trypanosomal Activity
5.11. Anti-Enzyme Activity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Zhengou, W.; Peiping, X. History and Development of Traditional Chinese Medicine; Science Press: Beijing, China, 1999. [Google Scholar]
- Li, Y.; Wang, Y.; Tai, W.; Yang, L.; Chen, Y.; Chen, C.; Liu, C. Challenges and Solutions of Pharmacokinetics for Efficacy and Safety of Traditional Chinese Medicine. Curr. Drug Metab. 2015, 16, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.X.; Xiao, W.L.; Lu, Y.; Li, R.T.; Li, H.B.; Zhang, L.; Li, X.; Zhao, Q.S.; Zheng, Q.T.; Sun, H.D. Kadlongilactones A and B, Two Novel Triterpene Dilactones from Kadsura longipedunculata Possessing a Unique Skeleton. Org. Lett. 2005, 7, 5079–5082. [Google Scholar] [CrossRef]
- Pu, J.X.; Li, R.T.; Xiao, W.L.; Gong, N.B.; Huang, S.X.; Lu, Y.; Zheng, Q.T.; Lou, L.G.; Sun, H.D. Longipedlactones A–I, nine novel triterpene dilactones possessing a unique skeleton from Kadsura longipedunculata. Tetrahedron 2006, 62, 6073–6081. [Google Scholar] [CrossRef]
- Liu, J.; Pandey, P.; Wang, X.; Adams, K.; Qi, X.; Chen, J.; Sun, H.D.; Hou, Q.; Ferreira, D.; Doerksen, R.J.; et al. Hepatoprotective Tetrahydrobenzocyclooctabenzofuranone Lignans from Kadsura longipedunculata. J. Natl. Prod. 2019, 82, 2842–2851. [Google Scholar] [CrossRef]
- Li, L.N.; Xue, H.; Kunio, K.; Akiko, I.; Sadafumi, O. Triterpenoid Acids from Kadsura longipedunculata. Neokadsuranic Acids B and C: Two Novel Triterpenoids with 14 (13–12) abeo-Lanostane Skeletons. Planta Med. 1989, 55, 294–296. [Google Scholar] [CrossRef]
- Song, L.; Ding, J.Y.; Tang, C.; Yin, C.H. Compositions and biological activities of essential oils of Kadsura longepedunculata and Schisandrasphenanthera. Am. J. Chin. Med. 2007, 35, 353–364. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, G.T. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992, 58, 311–313. [Google Scholar] [CrossRef]
- Qi, X.; Liu, J.; Chen, J.; Hou, Q.; Li, S. New seco-dibenzocyclooctadiene lignans with nitric oxide production inhibitory activity from the roots of Kadsura longipedunculata. Chin. Chem. Lett. 2020, 31, 423–426. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Y.; Zhao, R.; Wu, Y.; Zhang, Y.; Wu, D.; Kong, D.; Jin, X.; Zhang, F. High load hepatitis B virus replication inhibits hepatocellular carcinoma cell metastasis through regulation of epithelial-mesenchymal transition. Inter. J. Infect. Dis. 2014, 20, 37–41. [Google Scholar] [CrossRef]
- Sun, Q.Z.; Chen, D.F.; Ding, P.L.; Ma, C.M.; Kakuda, H.; Nakamura, N.; Hattori, M. Three new lignans, longipedunins A-C, from Kadsura longipedunculata and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 2006, 54, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.X.; Gao, X.M.; Lei, C.; Xiao, W.L.; Wang, R.R.; Yang, L.B.; Zhao, Y.; Li, L.M.; Huang, S.X.; Zheng, Y.T.; et al. Three new compounds from Kadsura longipedunculata. Chem. Pharm. Bull. 2008, 56, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.X.; Huang, S.X.; Ren, J.; Xiao, W.L.; Li, L.M.; Li, R.T.; Li, L.B.; Liao, T.G.; Lou, L.G.; Zhu, H.J. Isolation and structure elucidation of kadlongilactones C–F from Kadsura longipedunculata by NMR spectroscopy and DFT computational methods. J. Natl. Prod. 2007, 70, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Finet, A.E.; Gagnepain, E. Bulletin de la Société botanique de France; La Société: Paris, France, 1906; Volume 52, pp. 52–53. Available online: https://www.biodiversitylibrary.org/page/324765#page/52/mode/1up (accessed on 15 July 2021).
- Lan, S.F. Kadsura omeiensis. Acta Sci. Nat. Univ. Sunyatseni 1983, 2, 122. [Google Scholar]
- Rehder, A.; Wilson, E.H. Plantae Wilsonianae: An Enumeration of the Woody Plants Collected in Western China for the Arnold arboretum of Harvard University during the Years 1907, 1908, and 1910; The University Press: Cambridge, UK, 1913; Volume 1, pp. 1–611. [Google Scholar] [CrossRef]
- Xiao, W.L.; Li, R.T.; Huang, S.X.; Pu, J.X.; Sun, H.D. Triterpenoids from the Schisandraceae family. Natl. Prod. Rep. 2008, 25, 871–891. [Google Scholar] [CrossRef]
- de Jussieu, A.L. Suite des observations sur quelques genres de plantes de Loureiro, accompagnées de notes sur ceux qui composent la famille des Anonées. Ann. Mus. Hist. Nat. Paris 1810, 16, 338–340. [Google Scholar]
- Saunders, R.M.K. Species Plantarum: Flora of the World; Australian Biological Resources Study: Canberra, Australia, 2001; Volume 4, pp. 1–62.
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press: Beijing, China, 2008; Volume 7. [Google Scholar]
- Xu, L.J.; Liu, H.T.; Peng, Y.; Xiao, P.G. A preliminary pharmacophylogenetic investigation in Schisandraceae. J. Syst. Evol. 2008, 46, 692–723. [Google Scholar]
- Saunders, R.M.K. Monograph of Kadsura (Schisandraceae). Syst. Bot. Monogr. 1998, 54, 1–106. [Google Scholar] [CrossRef]
- Saunders, R.M.K. Monograph of Schisandra (Schisandraceae). Syst. Bot. Monogr. 2000, 58, 1–146. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Dong, X.; Lin, R.; Fan, J.; Chen, Z. Evaluation of four commonly used DNA barcoding loci for Chinese medicinal plants of the family schisandraceae. PLoS ONE 2015, 10, e0125574. [Google Scholar] [CrossRef]
- Smith, A.C. The Families Illiciaceae and Schisandraceae; Sargentia; Jamaica Plains, Mass, Arnold Arboretum of Harvard University: Boston, MA, USA, 1947; Volume 7, pp. 1–224. [Google Scholar]
- Lin, Q.; Duan, D.; Yao, B.-F. Note on three species of Kadusra Juss. (Schisandraceae). Acta Phytotax. Sin. 2005, 43, 567–570. [Google Scholar] [CrossRef]
- Okada, H. On the monoecism of Kadsura japonica (Thunb.) Dunal. J. Jpn. Bot. 1971, 46, 29–33. [Google Scholar]
- Ueda, K. Sex change in a woody vine species, Schisandra chinensis, a preliminary note. J. Jpn. Bot. 1988, 63, 319–321. [Google Scholar]
- Duan, L.D.; Lin, Q.; Yuan, Q. Floral morphology of Kadsura in relation to its systematic significance. Bull. Bot. Res. 2004, 24, 87–92. [Google Scholar]
- Knudsen, J.T.; Tollsten, L.; Bergström, G. Floralscents—A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 332, 253–280. [Google Scholar] [CrossRef]
- Chen, R.-Y.; Song, W.Q.; Liang, G.L.; Lin, S.H.; Li, X.L.; An, Z.P. Chromosome Atlas of Chinese Principal Economic Plants; Chromosome Atlas of Chinese Fruit Trees and Their Close Wild Relatives; International Academic Publishers: Beijing, China, 1993; Volume 1, pp. 1–619. [Google Scholar]
- Whitaker, T.W. Chromosome number and relationship in the Magnoliales. J. Arnold Arbor. 1933, 14, 376–385. [Google Scholar] [CrossRef]
- Liu, Z.; Hao, G.; Luo, Y.L.; Thien, B.S.; Rosso, W.; Lu, A.; Chen, Z.D. Phylogeny and androecial evolution in Schisandraceae, inferred from sequences of nuclear ribosomal DNA ITS and chloroplast DNA trnL-F regions. Int. J. Plant Sci. 2006, 167, 539–550. [Google Scholar] [CrossRef]
- Wu, C.S.; Wang, R.H.; Qi, Z.C. Characterization of the complete chloroplast genome of the Chinese Kadsura vine Kadsura longipedunculata (Schisandraceae). Mitochond. DNA Part B 2019, 4, 476–477. [Google Scholar]
- Kozo-Poljanski, B.M. The floral mechanism of Woo-we-dzy Schizandrachinesis (Turcz.) Baill. C. R. (Dokl.) Sci. URSS 1946, 53, 749–751. [Google Scholar]
- Willemstein, S.C. An evolutionary Basis for Pollination Ecology: Brill: Leiden, The Netherlands; Leiden University Press: Leiden, The Netherlands, 1987. [Google Scholar]
- Fujita, N. Über die Früche der Schizandra chinensis Bail Und Kadsura japonica Dun. Arch. Pharm. Chem. Life Sci. 1929, 267, 532–540. [Google Scholar] [CrossRef]
- Haensel, R.; Keller, K.; Rimpler, H.; Schneider, G. Hagers Handbuch der Pharmazeutischen Praxis; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Yang, Z.-R.; Lin, Q.; Wen, X.-Y.; Zeng, Q.-W.; Zhou, X.-C. Characters of the Leaf Epidermis in Kadsura (Schisandraceae). Bull. Bot. Res. 2009, 29, 147–163. [Google Scholar]
- National Pharmacopoeia Committee. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2010; p. 316. [Google Scholar]
- State Administration of Traditional Chinese Medicine. Zhong Hua Ben Cao; Shanghai Science and Technology Press: Shanghai, China, 1999. [Google Scholar]
- Guangdong Food and Drug Administration. Chinese Materia Medica Standards of Guangdong Province; Guangdong Science and Technology Press: Guangzhou, China, 2004. [Google Scholar]
- Guangxi Zhuang Autonomous Region Health Department. Chinese Materia Medica Standards of Guangxi Province; Guangxi Science and Technology Press: Nanning, China, 1992. [Google Scholar]
- Fujian Food and Drug Administration. Chinese Materia Medica Standards of Fujian Province; Haifeng Press: Fuzhou, China, 2006. [Google Scholar]
- Liu, H.T.; Qi, Y.D.; Xu, L.J.; Peng, Y.; Zhang, B.G.; Xiao, P.G. Ethno-pharmacological investigation of Schisandraceae plants in China. China J. Chin. Mater. Med. 2012, 37, 1353–1359. [Google Scholar]
- Xie, Q.; Cao, M.R.; Wang, Z.; Peng, C.Y.; Li, B.; Sheng, W.B.; Jian, Y.Q.; Gong, L.M.; Wang, W. Summary on source and pharmacological effects of Tujiaethnomedicine “Blood” drugs. J. Hunan Univ. Chin. Med. 2019, 39, 121–126. [Google Scholar]
- Shehla, N.; Li, B.; Cao, L.; Zhao, J.P.; Jian, Y.Q.; Daniyal, M.; Wahab, A.T.; Khan, I.A.; Liao, D.F.; Rahman, A.U.; et al. Xuetonglactones A-F: Highly oxidized lanostane and cycloartane triterpenoids from Kadsura heteroclite Roxb. Craib. Front. Chem. 2020, 7, 935. [Google Scholar] [CrossRef]
- Hao, D.C.; Gu, X.-J.; Xiao, P.G. Chemotaxonomy. Med. Plants 2015, 1–48. [Google Scholar] [CrossRef]
- Ma, W.; Ma, X.; Huang, H.; Zhou, P.; Chen, D. Dibenzocyclooctane lignans from the stems of Kadsurainduta and their antiviral effect on hepatitis B virus. Chem. Biodivers. 2007, 4, 966–972. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, J.; Feng, X.; Jia, Y.; Huang, J.; Hao, Z.; Zhao, S.; Wang, J. In Silico Analysis and Experimental Validation of Lignan Extracts from Kadsura longipedunculata for Potential 5-HT1AR Agonists. PLoS ONE 2015, 10, e0130055. [Google Scholar] [CrossRef]
- Zaugg, J.; Ebrahimi, S.N.; Smiesko, M.; Baburin, I.; Hering, S.; Hamburger, M. Identification of GABA A receptor modulators in Kadsura longipedunculata and assignment of absolute configurations by quantum-chemical ECD calculations. Phytochemistry 2011, 72, 2385–2395. [Google Scholar] [CrossRef]
- Song, W.Z.; Xiao, P.G. Medicinal uses of Schisandraceae plants in China and their lignan compounds. Chin. Trad. Herb. Drugs 1982, 13, 40–48. (In Chinese) [Google Scholar]
- South China Institute of Botany. A Handbook of Medicinal Plants in Guangdong; South China Institute of Botany: Guangzhou, China, 1982; p. 197. [Google Scholar]
- von ReisAtschul, S. Drugs and Foods from Little-Known Plants; Harvard University Press: Cambridge, MA, USA, 1973. [Google Scholar]
- Wang, W.-T. Iconographia Cormophytorum Sinicorum; Science Press: Beijing, China, 1980; Volume 1. (In Chinese) [Google Scholar]
- Available online: https://kadsura.com/ (accessed on 25 July 2022).
- Pharmacopoeia of the People’s Republic of China; Chinese Pharmacopoeia Commission: Beijing, China, 1977; Volume 1, pp. 396–397.
- Pu, J.X.; Yang, L.M.; Xiao, W.L.; Li, R.T.; Lei, C.; Gao, X.M.; Huang, S.X.; Li, S.H.; Zheng, Y.T.; Huang, H.; et al. Compounds from Kadsuraheteroclite and related anti-HIV activity. Phytochem. 2008, 69, 1266–1272. [Google Scholar] [CrossRef]
- Wang, H.; Deng, X.; Zhou, T.; Wang, C.; Hou, Y.; Jiang, H.; Li, G.L. The in vitro immunomodulatory activity of a polysaccharide isolated from Kadsura marmorata. Carbohydr. Polym. 2013, 97, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Liu, F.Y.; Yu, X. Study on scavenging free radial activity of extracts from Kadsura longipedunculata. Food Ind. 2010, 31, 1–3. [Google Scholar]
- Law, Y.W. Magnoliaceae Trib. Schisandreae DC. In Flora Reipublicae Popularis Sinicae; Law, Y.W., Ed.; Science Press: Beijing, China, 1996; Volume 30, pp. 232–269. (In Chinese) [Google Scholar]
- Xiao, P.G.; Xu, L.J.; Xiao, W.; Peng, Y. Argument on the correct Chinese name of genus Kadsura Kaempf. ex Juss. Yao Xue Xue Bao 2010, 45, 1064–1066. [Google Scholar]
- Tan, R.; Lian-Niang, L.; Qicheng, F. Studies on the Chemical Constituents of Kadsuralongipedunculata: Isolation and Structure Elucidation of Five New Lignans. Planta Med. 1984, 50, 414–417. [Google Scholar] [CrossRef]
- Li, L.N.; Yong, C. Further Dibenzocyclooctadiene Lignans from Roots and Stems of Kadsuralongipedunculata. Planta Med. 1986, 5, 410. [Google Scholar] [CrossRef]
- Amujuri, D.; Siva, B.; Poornima, B.; Sirisha, K.; Sarma, A.V.S.; Nayak, V.L.; Tiwari, A.K.; Purushotham, U.; Babu, K.S. Synthesis and biological evaluation of schizandrin derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2018, 149, 182–192. [Google Scholar] [CrossRef]
- Li, L.N.; Xue, H.; Li, X. Three New Dibenzocyclooctadiene Lignans from Kadsuralongipedunculata. Planta Med. 1991, 57, 169–171. [Google Scholar] [CrossRef]
- Liu, J.S.; Pan, Y.P. Isolation and structures of schisantherin J and schisanlactone F. Acta Chim. Sin. 1991, 49, 308–312. [Google Scholar]
- Li, H.M.; Lei, C.; Luo, Y.M.; Li, X.N.; Li, X.L.; Pu, J.X.; Zhou, S.Y.; Li, R.T.; Sun, H.D. Intermedins A and B: New metabolites from Schisandra propinqua var. intermedia. Arch. Pharmacal. Res. 2008, 31, 684–687. [Google Scholar] [CrossRef]
- Liu, J.S.; Zhou, H.X.; Li, L. Kadsulignans H, I, J and K from Kadsura species. Phytochemistry 1992, 31, 1379–1382. [Google Scholar] [CrossRef]
- Liu, J.S.; Huang, M.F.; Zhou, H.X. Kadsulignan C and D, two novel lignans from Kadsura longipedunculata. Can. J. Chem. 1991, 69, 1403–1407. [Google Scholar] [CrossRef]
- Liu, J.; Pandey, P.; Wang, X.; Qi, X.; Chen, J.; Sun, H.; Zhang, P.; Ding, Y.; Ferreira, D.; Doerksen, R.J.; et al. Hepatoprotective Dibenzocyclooctadiene and Tetrahydrobenzocyclooctabenzofuranone Lignans from Kadsura longipedunculata. J. Natl. Prod. 2018, 81, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.Y.; Qi, X.Z.; Sun, H.; Li, S. Hepatoprotective lignans and triterpenoids from the roots of Kadsura longipedunculata. Fitoterapia 2020, 142, 104487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liu, R.L.; Hsu, H.Y.; Yamamura, S.; Shizuri, Y.; Hirata, Y. The new schizandrin-type lignans, kadsurin and kadsurarin. Bull. Chem. Soc. Jpn. 1977, 50, 1824–1826. [Google Scholar] [CrossRef]
- Kangouri, K.; Miyoshi, T.; Ikeda, A.; Omura, S.; Li, L.N.; Xue, H. Cholesterol Biosynthesis Inhibitory Activities of Novel Triterpenoid Acids from Kadsura heteroclita and K. longipedunculata. Agric. Biol. Chem. 1990, 54, 993–997. [Google Scholar] [CrossRef]
- Chen, J.B.; Liu, J.B.; Cui, B.S.; Li, S. Chemical constituents from roots of Kadsura longipedunculata. Chin. Tradit. Herb. Drugs 2015, 46, 178–184. [Google Scholar]
- Guo, Y.J.; Gao, S.M.; Zhang, B.G.; Liu, H.T. Chemical Constituents from Stems of Kadsuralongipedunculata. Zhong Yao Cai 2016, 39, 1287–1290. [Google Scholar]
- Kuo, Y.H.; Kuo, L.M.; Chen, C.F. Four New C19 Homolignans, Schiarisanrins A, B, and D and Cytotoxic Schiarisanrin C, from Schizandra arisanensis. J. Org. Chem. 1997, 62, 3242–3245. [Google Scholar] [CrossRef]
- Liu, J.S.; Qi, Y.D.; Lai, H.G.; Zhang, J.; Jia, Z.G.; Liu, H.T.; Zhang, B.G.; Xiao, P.G. Genus Kadsura, a good source with considerable characteristic chemical constituents and potential bioactivities. Phytomedicine 2014, 21, 1092–1097. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Pandey, P.; Fronczek, F.R.; Doerksen, R.J.; Chen, J.; Qi, X.; Zhang, P.; Ferreira, D.; Valeriote, F.A.; et al. Computationally Assisted Assignment of the Kadsuraols, a Class of Chemopreventive Agents for the Control of Liver Cancer. Org. Lett. 2018, 20, 5559–5563. [Google Scholar] [CrossRef]
- Tan, R.; Xue, H.; Li, L.N. Kadsulactone and Kudsudilactone, two new Triterpenoid lactones from Kadsura species. Planta Med. 1991, 57, 87–88. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.Z.; Han, J.; Xu, Z.R.; Liu, R.X.; Liu, P.; Ma, X.X.; Guan, S.H.; Guo, D. New triterpenoids from Kadsura heteroclita and their cytotoxic activity. Planta Med. 2006, 72, 450–457. [Google Scholar] [CrossRef] [PubMed]
- You, Z.P.; Liao, M.J.; Shi, Y.H.; Chen, Y.Z. Studies on chemical constituents of Kadsura longepedunculata. Acta Pharm. Sin. 1997, 32, 455–457. [Google Scholar]
- Jia, Z.W.; Lu, Y.; Liao, Z.X.; Chen, D.F. Two new triterpene lactones from the stems of Kadsura polysperma. Helvet. Chim. Acta 2007, 90, 1236–1243. [Google Scholar] [CrossRef]
- Gao, X.M.; Pu, J.X.; Xiao, W.L.; Huang, S.X.; Lou, L.G.; Sun, H.D. Kadcoccilactones K–R, triterpenoids from Kadsura coccinea. Tetrahedron 2008, 64, 11673–11679. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Pandey, P.; Chen, J.; Fronczek, F.R.; Parnham, S.; Qi, X.; Doerksen, R.J.; Ferreira, D.; Sun, H.; et al. Assignment of the absolute configuration of hepatoprotective highly oxygenated triterpenoids using X-ray, ECD, NMR J-based configurational analysis and HSQC overlay experiments. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3089–3095. [Google Scholar] [CrossRef]
- Wang, X.; Hamann, M.T. Integrated X-ray, NMR, ECD, and Computational Approaches to Assign the Stereochemistry of Nortriterpenoids. Natl. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- Do, N.D.; Bui, V.T.; Luu, D.N.A.; Ninh, K.B.; Tran, D.T.; Isiaka, A.O. Composition of Stem Bark Essential Oils of Three Vietnamese Essential Oils of Three Vietnamese Species of Kadsura (Schisandraceae). Rec. Natl. Prod. 2015, 9, 386–393. [Google Scholar]
- Pu, J.X.; Xiao, W.L.; Li, H.M.; Huang, S.X.; Li, S.H.; Sun, H.D. Three new compounds from Kadsura longipedunculata. Helvet. Chim. Acta 2007, 90, 723–729. [Google Scholar] [CrossRef]
- Cen, Y.; Xiao, A.; Chen, X.Q.; Liu, L.L. Isolation of α-Amylase Inhibitors from Kadsura longipedunculata Using a High-Speed Counter-Current Chromatography Target Guided by Centrifugal Ultrafiltration with LC-MS. Molecules 2016, 21, 1190. [Google Scholar] [CrossRef]
- Thanh, B.V.; Ban, N.K. Constituents of essential oil from the root of Kadsura longipedunculata Fin. & Gagnep. collected in Kon Tum province. V. J. Biol. 2011, 33, 57–59. [Google Scholar]
- Erickson, S.K.; Cooper, A.D.; Matsui, S.M.; Gould, R.G. 7-Ketocholesterol. Its effects on hepatic cholesterogenesis and its hepatic metabolism in vivo and in vitro. J. Biol. Chem. 1977, 252, 5186–5193. [Google Scholar] [CrossRef]
- Wu, J.J.; Shemin, D.; Richards, K.E.; Williams, R.C. The Quaternary Structure of δ-Aminolevulinic Acid Dehydratase from Bovine Liver. Proc. Natl. Acad. Sci. USA 1974, 71, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497. [Google Scholar] [CrossRef]
- Liu, J.S.; Huang, M.F. Kadsulignans E−G from Kadsuralongipedunculata. Phytochemistry 1992, 31, 657–960. [Google Scholar]
- Sonoda, Y.; Sato, Y. Hypolipidemic effects of dietary 7-oxo-24,25-dihydrolanosterol. J. Pharm.-Dyn. 1985, 8, 518–524. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De-Martino, L.; Coppola, R.; De-Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Mann, C.M.; Cox, S.D.; Markham, J.L. The outer membrane of Pseudomonas aeruginosa NCTC6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil). Lett. Appl. Microbiol. 2000, 30, 294–297. [Google Scholar] [CrossRef]
- Cakira, A.; Kordalib, S.; Kilicc, H.; Kaya, E. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem. Syst. Ecol. 2005, 33, 245–256. [Google Scholar] [CrossRef]
- Haznedaroglu, M.Z.; Karabay, N.U.; Zeybeka, U. Antibacterial activity of Salvia tomentosa essential oil. Fitoterapia 2001, 72, 829–831. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, D.H. Volatile components and antibacterial effects of pine needle (Pinus densiflora S. and Z.) extracts. Food Microbiol. 2005, 22, 37–45. [Google Scholar] [CrossRef]
- Abraham, K.J.; Pierce, M.L.; Essenberg, M. The phytoalexins desoxyhemigossypol and hemigossypol are elicited by Xanthomonas in Gossypium cotyledons. Phytochemistry 1999, 52, 829–836. [Google Scholar] [CrossRef]
- Davis, G.D.; Essenberg, M. (+)-δ-Cadinene is a product of sesquiterpene cyclase activity in cotton. Phytochemistry 1995, 39, 553. [Google Scholar] [CrossRef]
- Essenberg, M.; Grover, P.B.; Cover, E.C. Accumulation of antibacterial sesquiterpenoids in bacterially inoculated Gossypium leaves and cotyledons. Phytochemistry 1990, 29, 3107. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from lamiaceace and compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberi, B.; Wegand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Chiu, H.F.; Chen, T.Y.; Tzeng, Y.T.; Wang, C.K. Improvement of liver function in humans using a mixture of schisandra fruit extract and sesamin. Phytother. Res. 2013, 27, 368–373. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr.; Monger, D.; Taylor, A.S.; Chamberlain, J.S.; Parish, E.J.; Kisic, A.; Kandutsch, A.A. Inhibitors of sterol synthesis. Hypocholesterolemic action of dietary 5alpha-cholest-8(14)-en-3beta-ol-15-one in rats and mice. Biochem. Biophys. Res. Commun. 1977, 78, 1227–1233. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr.; Parish, E.J.; Kisic, A.; Jackson, E.M.; Farley, C.M.; Mott, G.E. 5 alpha-Cholest-8(14)-en-3 beta-ol-15-one, a potent inhibitor of sterol biosynthesis, lowers serum cholesterol and alters distributions of cholesterol in lipoproteins in baboons. Proc. Natl. Acad. Sci. USA 1982, 79, 3042–3046. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr.; Sherrill, B.C.; Wang, K.S.; Wilson, W.K.; Kisic, A.; Clarkson, T.B. 5 alpha-Cholest-8(14)-en-3 beta-ol-15-one lowers serum cholesterol and induces profound changes in the levels of lipoprotein cholesterol and apoproteins in monkeys fed a diet of moderate cholesterol content. Proc. Natl. Acad. Sci. USA 1984, 81, 6861–6865. [Google Scholar] [CrossRef]
- Miettinen, T.A. Cholesterol metabolism during ketoconazole treatment in man. J. Lipid Res. 1988, 29, 43–51. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Aspirin resistance and promoting blood circulation and removing blood stasis: Current situation and prospectives. Evid. Based Complementary Altern. Med. 2014, 2014, 954863. [Google Scholar] [CrossRef] [PubMed]

| No. | Types | Compounds | References |
|---|---|---|---|
| Dibenzocysclooctadienes | |||
| 1 | R(+)-gomisin M | [64] | |
| 2 | Gomisin M2 | [65] | |
| 3 | Gomisin H | [66] | |
| 4 | Binankadsurin A | [67] | |
| 5 | Acetyl-binankadsurin A | [12,67] | |
| 6 | Isobutyroyalbinankadsurin A | [67] | |
| 7 | Isovaleroylbinankadsurin A | [67] | |
| 8 | Benzoylbinankadsurin A | [12,67] | |
| 9 | Schisantherin | [67] | |
| 10 | Schizandrin | [66] | |
| 11 | Schisantherin J | [68] | |
| 12 | Schizanrin D | [67] | |
| 13 | Angeloylgomisin M | [64] | |
| 14 | Intermedin A | [69] | |
| 15 | Kadsurarin | [64,67,70,71] | |
| 16 | Kadsutherin A | [68] | |
| 17 | Kadsuphilol T | [72] | |
| 18 | Kadsuphilol B | [72] | |
| 19 | Kadsuphilol P | [72] | |
| 20 | Butyrylbinankadsurin A | [67] | |
| 21 | Kadsuralignan A | [12] | |
| 22 | Longipedunculatin D | [4] | |
| 23 | Kadlongilignan G | [73] | |
| 24 | Kadlongilignan E | [73] | |
| 25 | Kadsuralignan B | [12] | |
| 26 | Kadsuralignan C | [13] | |
| 27 | Schiarisanrin D | [72] | |
| 28 | R(+)-schisandrin C | [74] | |
| 29 | Otobaphenol | [13] | |
| 30 | Schisanhenol | [75] | |
| 31 | Schisanhenol A | [65] | |
| 32 | Schisanhenol B | [65] | |
| 33 | Schisandrol | [65] | |
| 34 | Angeloylgomisin H | [65] | |
| 35 | Schisantherin A | [65] | |
| 36 | Schisantherin B | [65] | |
| 37 | Tiglomisin P | [65] | |
| 38 | Kadsulignan C | [67] | |
| 39 | Kadsulignan D | [67] | |
| 40 | Kadsulignan H | [67] | |
| 41 | Angeloylbinankadsurin A | [67] | |
| 42 | Kadsulignan J | [67] | |
| 43 | Kadsulignan K | [67] | |
| 44 | Deoxyschizandrin | [52] | |
| 45 | Kadsutherin D | [75] | |
| 46 | Benzoylgomisin Q | [51] | |
| 47 | Kadsuphilin J | [73] | |
| 48 | (7′S,8R,8′S’)-4,4′,9-trihydroxy-3,3′,5-trimethoxy-9′-O-B-D-xylopryranosyl-2,7′-cyclo-lignan | [76] | |
| 49 | Longipedlignan A | [12,72] | |
| 50 | Longipedlignan B | [12,72] | |
| 51 | Longipedlignan C | [12,72] | |
| 52 | Longipedlignan D | [72] | |
| 53 | Longipedlignan E | [72] | |
| 54 | Longipedlignan K | [5] | |
| 55 | Longipedlignan L | [5] | |
| 56 | Longipedlignan M | [5] | |
| 57 | Longipedlignan N | [5] | |
| 58 | Longipedlignan O | [5] | |
| 59 | Longipedlignan P | [5] | |
| 60 | Longipedlignan Q | [5] | |
| 61 | Longipedlignan R | [5] | |
| 62 | Kadsulignan E | [75] | |
| 63 | Kadsulignan F | [75] | |
| 64 | Kadsulignan G | [75] | |
| 65 | Longipedunin A | [12] | |
| 66 | Longipedunin B | [12] | |
| 67 | Longipedunin C | [12,64] | |
| 68 | Kadsuranin | [12,64] | |
| 69 | Benzoylgomisin Q | [10,12] | |
| 70 | Longipedunin D | [12,77] | |
| 71 | Renchangianin A | [12,77] | |
| 72 | Renchangianin B | [12,77] | |
| 73 | Kadlongilignan A | [10] | |
| 74 | Kadlongilignan B | [10] | |
| 75 | Kadlongilignan C | [10] | |
| 76 | Kadlongilignan D | [10] | |
| 77 | R(+)-wuweizisu C | [64] | |
| 78 | R(+)-angeloylgomisin M1 | [64] | |
| 79 | Angeloylgomisin R | [3,64] | |
| 80 | Angeloylgomisin H | [64] | |
| 81 | Angeloylbinankadsurin R | [67] | |
| 82 | Deacetyldeangeloyl-kadsurarin | [67] | |
| 83 | Isobutyroylbinankadsurin A | [67] | |
| SpirobenzofuranoidDibenzocyclooctadienes | |||
| 84 | Schiarianrin E | [78] | |
| 85 | Schiarisanrin A | [78] | |
| 86 | Isovaleroyloxokadsurane | [67] | |
| 87 | Kadsulignan C | [67] | |
| 88 | Kadsulignan G | [75] | |
| 89 | Heteroclitin D | [72] | |
| 90 | Heteroclitin H | [72] | |
| 91 | HeteroclitinIa | [72] | |
| 92 | Schiarisanrin B | [72,78] | |
| 93 | Heteroclitin J | [5,13] | |
| 94 | Benzoyl-oxo-kadsuraol | [73] | |
| 95 | Propoxyl-oxo kadsuraol | [73] | |
| 96 | Kadsutherin C | [73] | |
| 97 | Heteroclitin E | [73] | |
| 98 | Heteroclitin P | [72,79] | |
| 99 | HeteroclitinIb | [13,72] | |
| Aryltetralins | |||
| 100 | Otobaphenol | [5,13,75] | |
| 101 | Arisantetralone A | [5,52,75] | |
| 102 | Arisantetralone B | [5,52,75] | |
| 103 | Arisantetralone C | [5,52,75] | |
| 104 | Arisantetralone D | [5,52,75] | |
| 105 | Kadsulignan I | [72] | |
| Diarylbutanes | |||
| 106 | Meso-dihydroguaiaretic acid | [52,67,77] | |
| 107 | (+)-anwulignan | [52,75] | |
| 108 | Dihydroguaiaretic acid | [52] | |
| 109 | Monomethyl dihydroguaiaretic acid | [52] | |
| 110 | Saururenin | [52] | |
| 111 | Isoanwulignan | [52] | |
| 112 | 4-[4-(3,4-dime-thoxyphenol)-2,3-dimethyl-butyl]-2-methoxy-phenol | [13] | |
| 113 | 3-methoxy-3′,4′-(methylenedioxy)-9,9′-epoxylignan-4,7′-diol | [55] | |
| 114 | Kadsuphilin J | [73] | |
| Tetrahydrofurans | |||
| 115 | Grandisin | [13] | |
| 116 | Kadlongirin A | [13] | |
| 117 | Kadlongirin B | [13] | |
| 118 | Fragrasin B1 | [13] | |
| 119 | Zuihonin A | [13,52] | |
| 120 | Kadlongilignan F | [73] | |
| 121 | Kadlongilignan G | [73] | |
| 122 | Kadsuraol A | [80] | |
| 123 | Kadsuraol B | [80] | |
| 124 | Kadsuraol C | [80] | |
| 125 | Kadlongilignan I | [80] | |
| 126 | Kadlongilignan J | [80] | |
| 127 | Kadlongilignan H | [80] | |
| 128 | Longipedlignan F | [5,72] | |
| 129 | Longipedlignan G | [5,72] | |
| 130 | Longipedlignan H | [5,72] | |
| 131 | Longipedlignan I | [5,72] | |
| 132 | Longipedlignan J | [5,72] | |
| 133 | Longipedunculatin A | [5] | |
| 134 | Longipedunculatin B | [5] | |
| 135 | Longipedunculatin C | [5] | |
| Other Novel Lignans | |||
| 136 | Neolignan glycosides | 7S,8R-erthro-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignan | [76] |
| 137 | SpirodienoneSesquineolignan | Pinobatol | [76] |
| 138 | Sesquineolignan | Leptolepisol B | [76] |
| 139 | Cyclolignans | Austrobalignans | [76] |
| 140 | vladirol F | [13] | |
| 141 | 4-[4-(3,4-dimethoxyphenyl)-2,3-dimethylbutyl]-2-methoxy-phenol | [13] | |
| 142 | Pregmomisin | [51] | |
| 143 | Tigloylgomisin P | [65] | |
| 144 | Schiarisanrin C | [72] | |
| 145 | Schiarisanrin B | [72] | |
| 146 | Isolariciresinol-9-O-β-D-xyloside | [77] | |
| 147 | (-)-gallocatechi | [77] | |
| 148 | 9-O-benzoyloxokadsuranol | [72] |
| No. | Group | Category | Compounds | References |
|---|---|---|---|---|
| Lanostane | Intact Lanostane | |||
| 149 | Epianwuweizic acid | [67] | ||
| 150 | (24Z)-3-oxo-12α-acyetoxylanosta-8,24-dien-26-oic acid | [6] | ||
| 151 | (24Z)-3-oxo-12α-ahydroxylanosta-8,24-dien-26-oic acid | [6] | ||
| 152 | (24Z)-3-oxo-lanosta-8,24-dien-26-oic acid | [75] | ||
| 14(13–12)-abe-lanostanes | ||||
| 153 | Neokadsuranic acid A | [6] | ||
| 154 | Neokadsuranic acid B | [6] | ||
| 155 | Neokadsuranic acid C | [6] | ||
| 156 | Seco-neokadsuranic acid A | [6] | ||
| 3,4-Seco-lenostane | ||||
| 157 | Schisanlactone F | [68] | ||
| Cycloartane-type triterpenoids | Intact cycloartene | |||
| 158 | Isoschizandrolic acid | [77] | ||
| 159 | Schisandraflorin | [77] | ||
| 160 | 24-methylenecycloartenone | [77] | ||
| 161 | Kadsulactone | [81,82] | ||
| 3,4-Seco-cycloartene | ||||
| 162 | Kadsudilactone | [81] | ||
| 163 | Kadsudilactone A | [12] | ||
| 164 | Kadsulactone acid | [83] | ||
| 165 | Changnanic acid | [67] | ||
| 166 | Nigranoic acid | [27] | ||
| 167 | Schisanlactone E | [14,84] | ||
| 168 | Schisanlactone A | [14] | ||
| 14(13–12)-abeo-cycloartanes | ||||
| 169 | Longipedlactone A | [4,73] | ||
| 170 | Longipedlactone B | [4,73] | ||
| 171 | Longipedlactone C | [4,73] | ||
| 172 | Longipedlactone D | [4] | ||
| 173 | Longipedlactone E | [4] | ||
| 174 | Longipedlactone F | [4] | ||
| 175 | Longipedlactone G | [4] | ||
| 176 | Longipedlactone H | [4] | ||
| 177 | Longipedlactone I | [4] | ||
| Kadlongilactone-type triterpenoids | ||||
| 178 | Kadlongilactone A | [3,14,73,85] | ||
| 179 | Kadlongilactone B | [3,14,73,85] | ||
| 180 | Kadlongilactone C | [14,73] | ||
| 181 | Kadlongilactone D | [14,73,85] | ||
| 182 | Kadlongilactone E | [14,73] | ||
| 183 | Kadlongilactone F | [14,73] | ||
| Schiartane-type triterpenoids | ||||
| 184 | Micrandilactone I | [73,86] | ||
| 185 | Micrandilactone J | [73,86] | ||
| 186 | 22,23-Di-epi-micrandilactone J | [86] | ||
| 187 | Schilancitrilactone A | [87] | ||
| 188 | Schilancitrilactone B | [87] | ||
| 189 | Schilancitrilactone C | [87] | ||
| 190 | Wuweizidilactone P | [87] | ||
| 191 | Arisanlactone A | [87] | ||
| 192 | Propindilactone H | [87] | ||
| 193 | Micandilactone H | [87] | ||
| 194 | Lanocolides A | [87] | ||
| 195 | Lanocolides B | [87] | ||
| 196 | Lanocolides C | [87] | ||
| 197 | Lanocolides D | [87] | ||
| 198 | Preschisanartanin O | [87] | ||
| 199 | Lancifornin A | [87] | ||
| 200 | Schicagenin A | [87] | ||
| 201 | Rubrifloradilactone C | [87] | ||
| 202 | Wilsonianadilacone A | [87] |
| No. | Compounds | References |
|---|---|---|
| 203 | α-muurolol | [88] |
| 204 | α-cadinol | [88] |
| 205 | β-caryophyllene | [88] |
| 206 | α-Terpineol | [88] |
| 207 | α-Copaene | [88] |
| 208 | β-Elemene | [88] |
| 209 | cis-α-Bergamotene | [88] |
| 210 | β-Caryophylene | [88] |
| 211 | trans-α-Bergamotene | [88] |
| 212 | cis-Muurola-3,5-diene | [88] |
| 213 | α-Humulene | [88] |
| 214 | cis-Muurola-4(14),5-diene | [88] |
| 215 | δ-Muurolene | [88] |
| 216 | Germacrene D | [88] |
| 217 | β-Selinene | [88] |
| 218 | Viridiflorene | [88] |
| 219 | cis-Cadina-1,4-diene | [88] |
| 220 | β-Bisabolene | [88] |
| 221 | δ-Cadinene | [88] |
| 222 | Zonarene | [88] |
| 223 | trans-Cadina-1(6), 4-diene | [88] |
| 224 | Caryophyllenyl alcohol | [88] |
| 225 | Caryophyllene oxide | [88] |
| 226 | Spathulenol | [88] |
| 227 | Humulene Epoxide II | [88] |
| 228 | Caryophyllene oxide | [88] |
| 229 | epi-Cubenol | [88] |
| 230 | 10-diepi-Cubenol | [88] |
| 231 | Caryophylla-3(15), (7(14)-dien-6-ol e | [88] |
| 232 | Cadalene | [88] |
| 233 | Eudesma-4(15), 7-dien-1-ol | [88] |
| 234 | epi-α-Bisabolol | [88] |
| 235 | 1,2-Benzenedicarboxylic acid | [88] |
| 236 | 9-(b-d-glucopyranosyloxy)-3′-methoxy-3,4-(methylenedioxy)-7,9′-epoxylignan-4′-ol | [88] |
| 237 | 3-methoxy-3′,4′-(methylenedioxy)-9,9′-epoxylignan-4,7′-diol | |
| 238 | Bsitosterol | [89] |
| 239 | Daucosterol | [89] |
| 240 | Schizandronic acid | [89] |
| 241 | Parkeol | [89] |
| 242 | Licarin A | [89] |
| 243 | Mangiferolic acid | [89] |
| 244 | Schizandriside | [89] |
| 245 | Quercetin-3-O-rhamnoside | [90] |
| 246 | Protocatechuic acid | [90] |
| 247 | 2, 7-di-hyrdroxy-11,12-dehydrocalamenene | [90] |
| 248 | α-pinen | [90] |
| 249 | Camphen | [91] |
| 250 | P-cymen | [91] |
| 251 | Limonen | [91] |
| 252 | Borneol | [91] |
| 253 | Terpenolin-4-ol | [91] |
| 254 | Bornyl axetat | [91] |
| 255 | α-cubeben | [91] |
| 256 | α-Copaen | [91] |
| 257 | β-cubeben | [91] |
| 258 | α-bergamoten | [91] |
| 259 | Aromadendren | [91] |
| 260 | α-amorphen | [91] |
| 261 | Epi-bicyclophellandren | [91] |
| 262 | β-gurjunen | [91] |
| 263 | Caryophyllenoxit | [91] |
| 264 | Acorenol B | [91] |
| 265 | Tricycline | [8] |
| 266 | α-Thujene | [8] |
| 267 | α-Pinene | [8] |
| 268 | Camphene | [8] |
| 269 | β-Pinene | [8] |
| 270 | β-Myrcene | [8] |
| 271 | α-Terpinene | [8] |
| 272 | P-Cymene | [8] |
| 273 | Limonene | [8] |
| 274 | δ-Terpinene | [8] |
| 275 | Terpinolene | [8] |
| 276 | 1,8-Cineole | [8] |
| 277 | Camphor | [8] |
| 278 | Borneol | [8] |
| 279 | Terpinen-4-ol | [8] |
| 280 | α-Tepineo | [8] |
| 281 | Bornyl acetate | [8] |
| 282 | δ-Elemene | [8] |
| 283 | α-Copaene | [8] |
| 284 | β-Elemene | [8] |
| 285 | α-Gurjunene | [8] |
| 286 | β-Caryophyllene | [8] |
| 287 | β-Copaene | [8] |
| 288 | +)-Aromadendrene | [8] |
| 289 | α-Humulene | [8] |
| 290 | allo-Aromadendrene | [8] |
| 291 | β-Chamigrene | [8] |
| 292 | Germacrene D | [8] |
| 293 | δ-Muurolene | [8] |
| 294 | β-Selinene | [8] |
| 295 | epi-Bicyclosesquiphellandrene | [8] |
| 296 | Viridiflorene | [8] |
| 297 | Calamenene | [8] |
| 298 | δ-Cadinene | [8] |
| 299 | α-Calacorene | [8] |
| 300 | Cadina-1,4-diene | [8] |
| 301 | Cadala-1(10),3,8-triene | [8] |
| 302 | α-Calacorene | [8] |
| 303 | Spathulenol | [8] |
| 304 | Viridiflorol | [8] |
| 305 | trans-Nerolidol | [8] |
| 306 | β-Caryophyllene oxide | [8] |
| 307 | Cubenol | [8] |
| 308 | α-Bisabolol | [8] |
| 309 | Cadalene | [8] |
| 310 | γ-Muurolol | [8] |
| 311 | δ-Cadinol | [8] |
| 312 | γ-Cadinol | [8] |
| 313 | Bicyclol | [8] |
| 314 | 7-ketocholesterol | [92,93] |
| 315 | 5ct-cholest-8(14)-en-3f3-01-l5one | [92,93] |
| Properties | Compounds | Observations | Ref. |
|---|---|---|---|
| Hepatoprotective effects | Longipedlignans F and G | N-acetyl-p-aminophenol-induced toxicity in HepG2 cells with cell survival rates at 10 μM of 52.2% and 50.2% | [5] |
| Hepatoprotective effects | Micrandilactone I | APAP-induced toxicity in HepG2 cells, with cell survival rates of 53.04% | [73] |
| Hepatoprotective effects | 22,23-di-epi-micrandilactone J and micrandilactone I | APAP-induced toxicity in HepG2 cells, with cell survival rates of 53.0% and 50.2% | [86] |
| Hepatoprotective effects | Kadsurol C | N-acetyl-paminophenol (APAP)-induced toxicity in HepG2 cells, with a cell survival rate of 48.22 % | [80] |
| NO production | Longipedunculatin B, M and R | In vitroinhibitory effects on nitric oxide production assays, with inhibition rates of 55.1%, 74.9%, and 89.8% | [5] |
| Hepatoprotective effects | Longipedlignan M | N-acetyl-p-aminophenol induced toxicity in HepG2 cells, with a cell survival rate of 50.8% | [5] |
| Cytototoxic effects | Kadlongilactone A and E | HT-29, A549, and K562 cell lines, with IC50 values of 0.49–3.61 μ Minvitro | [14] |
| Cytototoxic effects | Kadlongilactone A and B | Human tumor K562 cells, with IC50 values of 1.40 and 1.71 µg/mL | [3] |
| Cytototoxic effects | Camphene and Borneol | HepG-2, MIAPaCa-2, and SW-480 cell lines with IC50 values of 133.53, 136.96, and 136.62 µg/mL | [8] |
| Cytototoxic effects | Essential oils | HepG2 cells lines, with an IC50 value of 147 μg/mL | [7] |
| Anti-microbial effects | Essential oils | Gram-positive bacteria (S. aureus and B. subtilis) with inhibition zones of 28.91 mm | [7] |
| Anti-microbial effects | Camphene and Borneol | Antimicrobial effect with inhibition zones in the range of 6.7–11.0 mm | [8] |
| Anti-viral effects | Longipedunin A and Schisanlactone A | HIV-1 protease, with IC50 values of 50 and 20 µM | [12] |
| Anti-viral effects | Kadlongirin A and 2,7-dihydroxy-11,12-dehydrocalamenene | HIV-1 in C8166 cells, EC50 2.03 µg/mL and CC50 1146.08 µg/mL | [13] |
| Anti-viral effects | Kadlongirin B | HIV-1 activity, with an EC50 value of 16.0 µg/mL, and therapeutic index (TI) value of 6.7 | [13] |
| Anti-tumor effects | Bicyclol | Enhance impaired liver function, prevent HBV replication in chronic hepatitis B patients, and induce differentiation of human hepatocarcinoma cells (HepG2 and Bel-7402 cells) with no side effects | [94] |
| Anti-tumor effects | Kadsulignan H, I, and J | Leukemia P-388 cells in vitro, with IC50 values of 40, 10, and 10 µg/mL | [95] |
| NO production effects | Kadlongilignan C and D | Nitric oxide (NO) production of lipo-polysaccharide (LPS)-induced murine macrophages, with the inhibition rates of 36.3% and 26.9%, respectively | [10] |
| Anti-inflammatory effects | Essential oils | 5-lipoxygenase activity, with an IC50 value of 38.58 ± 3.8 mg/mL and inhibited prostaglandin E2 formation by 28.82%. | [8] |
| Anti-inflammatory effects | Borneol and Camphene | 5-lipoxygenase activity, with an IC50 value of 39.72 ± 2.16 and 69.22 ± 3.66 µg/mL and inhibited prostaglandin E2 formation by 33.74% and 45.78% | [8] |
| Antioxidant effects | Sal | Prevented iron-induced lipid peroxidation of polyunsaturated fatty acids (PUFA) of liver microsomes | [9] |
| Antioxidant effects | Essential oils | Reduce the purple-colored DPPH radical to the yellow-colored diphenylpicrylhydrazine with an IC50 of 3.06 ± 0.79 µg/mL | [8] |
| Antioxidant effects | Essential oils | Inhibited lipid peroxidation in the rat liver, with the IC50 values of 2.4 µg/mL, which were similar to the IC50 (1.2 mg/mL) value of ascorbic acid (1.2 µg/mL) | [7] |
| Cholesterol biosynthesis inhibition effects | 3-oxo-LA | 82% inhibits cholesterol biosynthesis in rat liver cells | [75] |
| Cholesterol biosynthesis inhibition effects | 7-oxo-24,25-dihydrolanosterol | Lower the total serum cholesterol in rats fed a cholesterol diet | [96] |
| Antiplatelet aggregation effects | Petroleum ether extract | Increased the GABA-induced chloride current (IGABA) by 122.5 ± 0.3% (n = 2) when measured at 100 µg/mL in Xenopus laevis oocytes expressing GABA A receptors | [52] |
| Anti-insomnia effects | Administration of lignin extracts | Reduced FST and TST immobility time in the PCPA-induced 5HT-depleted insomnia rat model and suggesting that extracts had 5-HT1AR agonist-like effects | [51] |
| Anti-trypanosomaleffects | Essential oils | Moderate trypandosomal activity, with an IC50 value of 50.52 ± 0.029 µg/mL | [8] |
| Anti-trypanosomaleffects | Camphene and Borneol | Significant trypandosomal activity, with an IC50 value of 80.66 ± 0.87 and 70.00 ± 1.28 µg/mL. | [8] |
| Anti-enzyme effects | Quercetin-3-O-rhamnoside and Protocatechuic acid | Inhibitory activity on α-amylase, with IC50 values of 28.8 and 12.5 µmol/L | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, M.; Zhang, Z.; Yaseen, A.; Jiao, Y.; Zheng, X. Kadsura longipedunculata Finet & Gagnepain (Schisandraceae): An Overview of Botany, Traditional Uses, Phytochemistry and Pharmacological Activities. Forests 2022, 13, 1281. https://doi.org/10.3390/f13081281
Idrees M, Zhang Z, Yaseen A, Jiao Y, Zheng X. Kadsura longipedunculata Finet & Gagnepain (Schisandraceae): An Overview of Botany, Traditional Uses, Phytochemistry and Pharmacological Activities. Forests. 2022; 13(8):1281. https://doi.org/10.3390/f13081281
Chicago/Turabian StyleIdrees, Muhammad, Zhiyong Zhang, Aftab Yaseen, Yongqing Jiao, and Xu Zheng. 2022. "Kadsura longipedunculata Finet & Gagnepain (Schisandraceae): An Overview of Botany, Traditional Uses, Phytochemistry and Pharmacological Activities" Forests 13, no. 8: 1281. https://doi.org/10.3390/f13081281
APA StyleIdrees, M., Zhang, Z., Yaseen, A., Jiao, Y., & Zheng, X. (2022). Kadsura longipedunculata Finet & Gagnepain (Schisandraceae): An Overview of Botany, Traditional Uses, Phytochemistry and Pharmacological Activities. Forests, 13(8), 1281. https://doi.org/10.3390/f13081281