Similar Pattern of Potential Distribution of Pinus yunnanensis Franch and Tomicusyunnanensis Kirkendall under Climate Change in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data and Environmental Factors
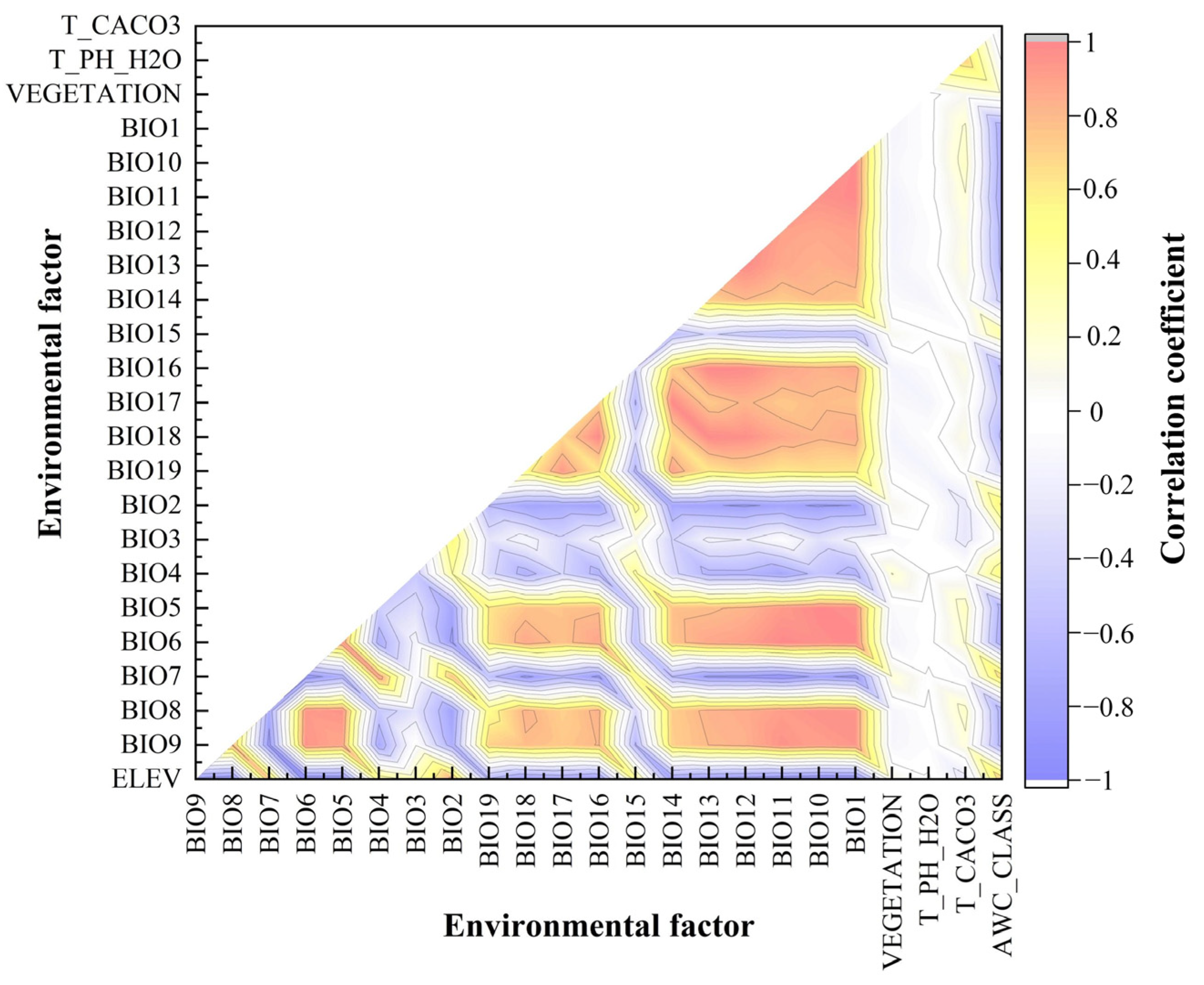
2.2. Environmental Factor Pre-Process
2.3. MaxEnt Model Construction and Result Evaluation
2.4. Changes in the Potential Distribution of P. yunnanensis and T. yunnanensis
3. Results
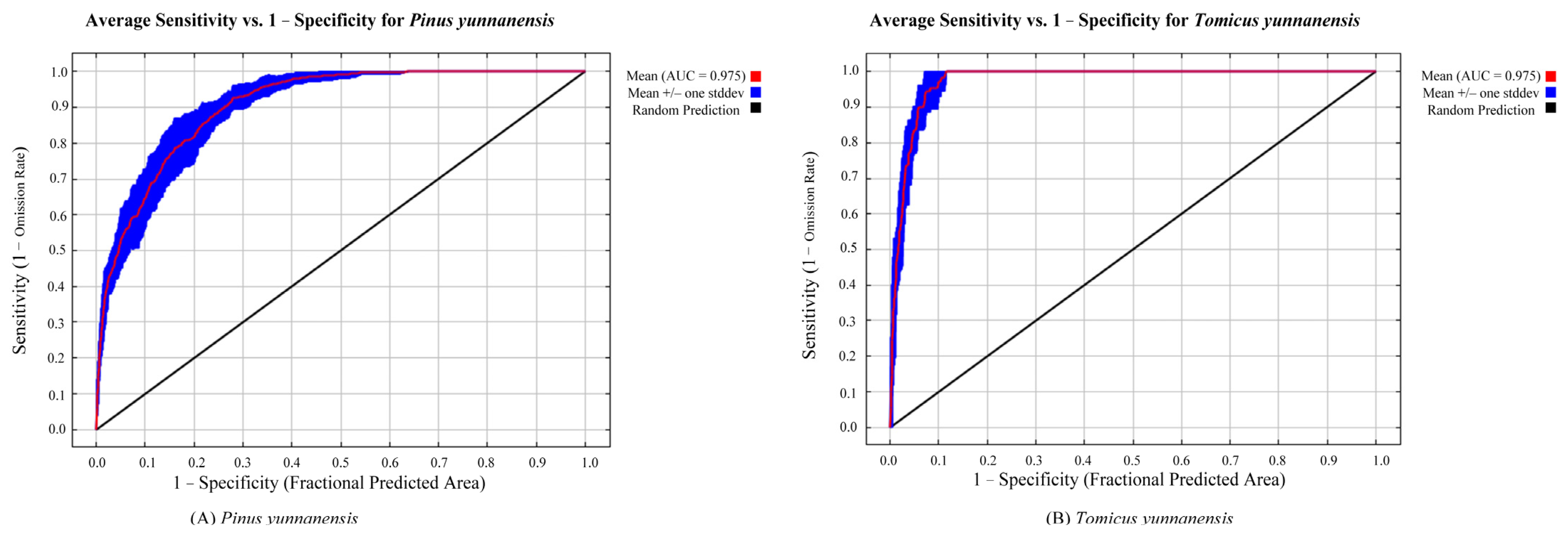
3.1. Model Accuracy Evaluation
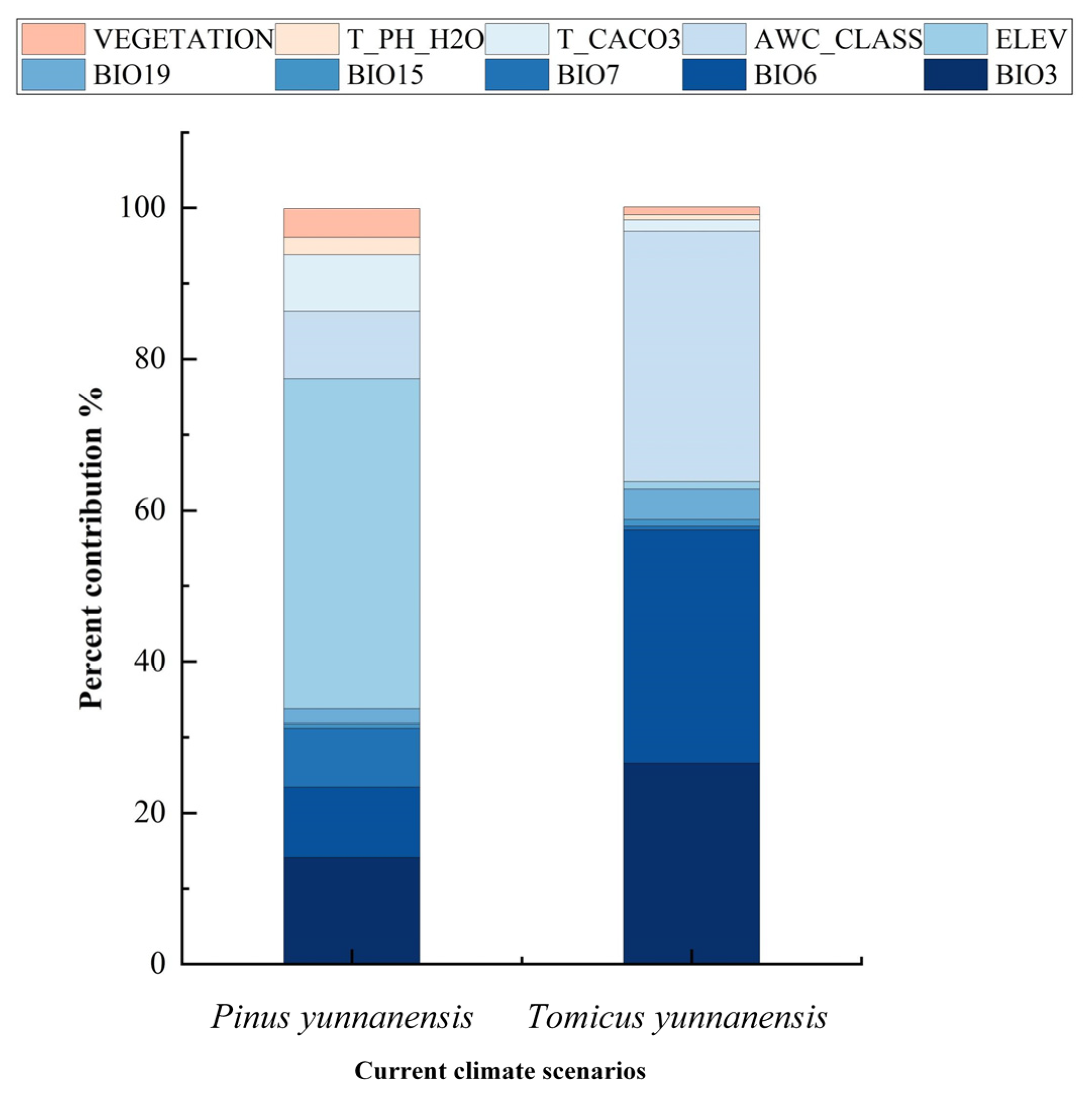
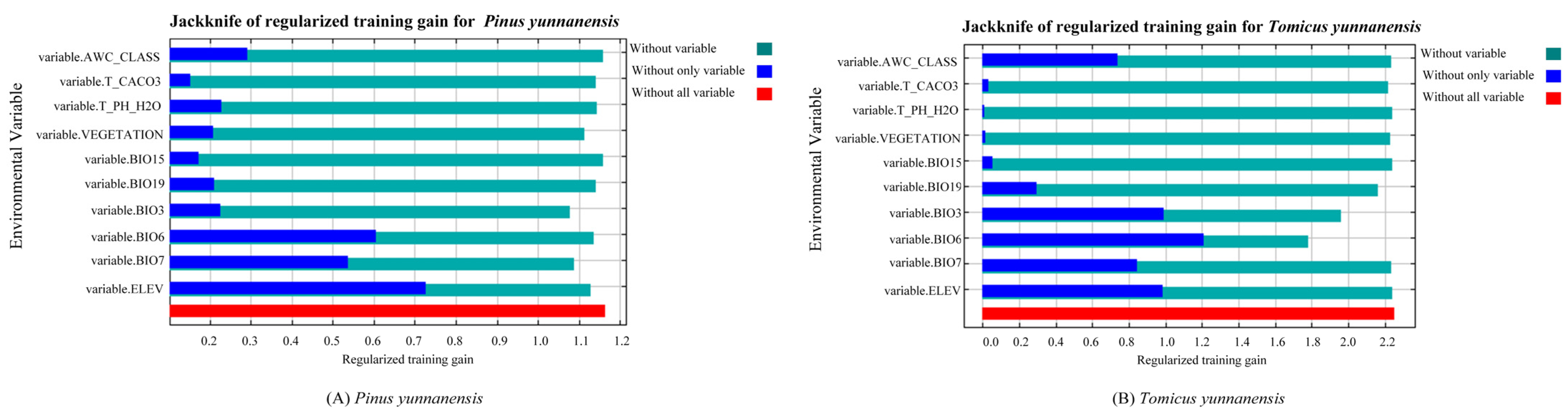
3.2. Environmental Factors Affecting the Distribution of P. yunnanensis and T. yunnanensis
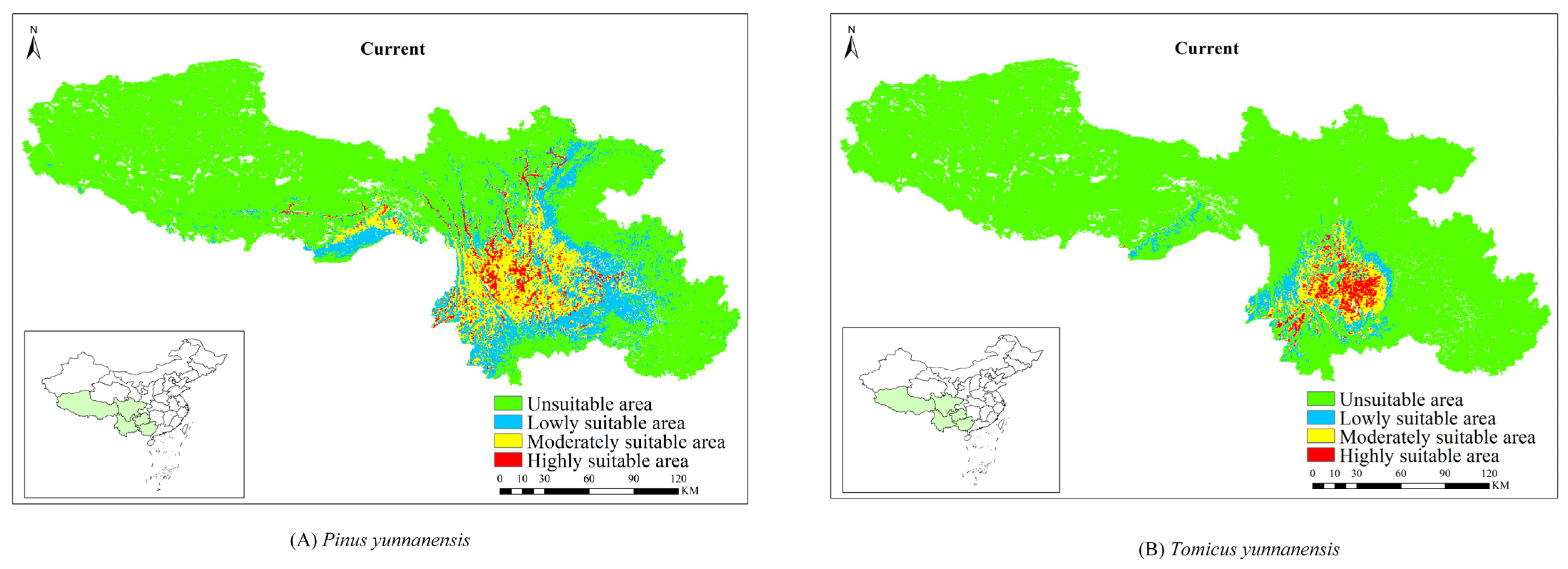
3.3. The Current Potential Distribution of P. yunnanensis and T. yunnanensis
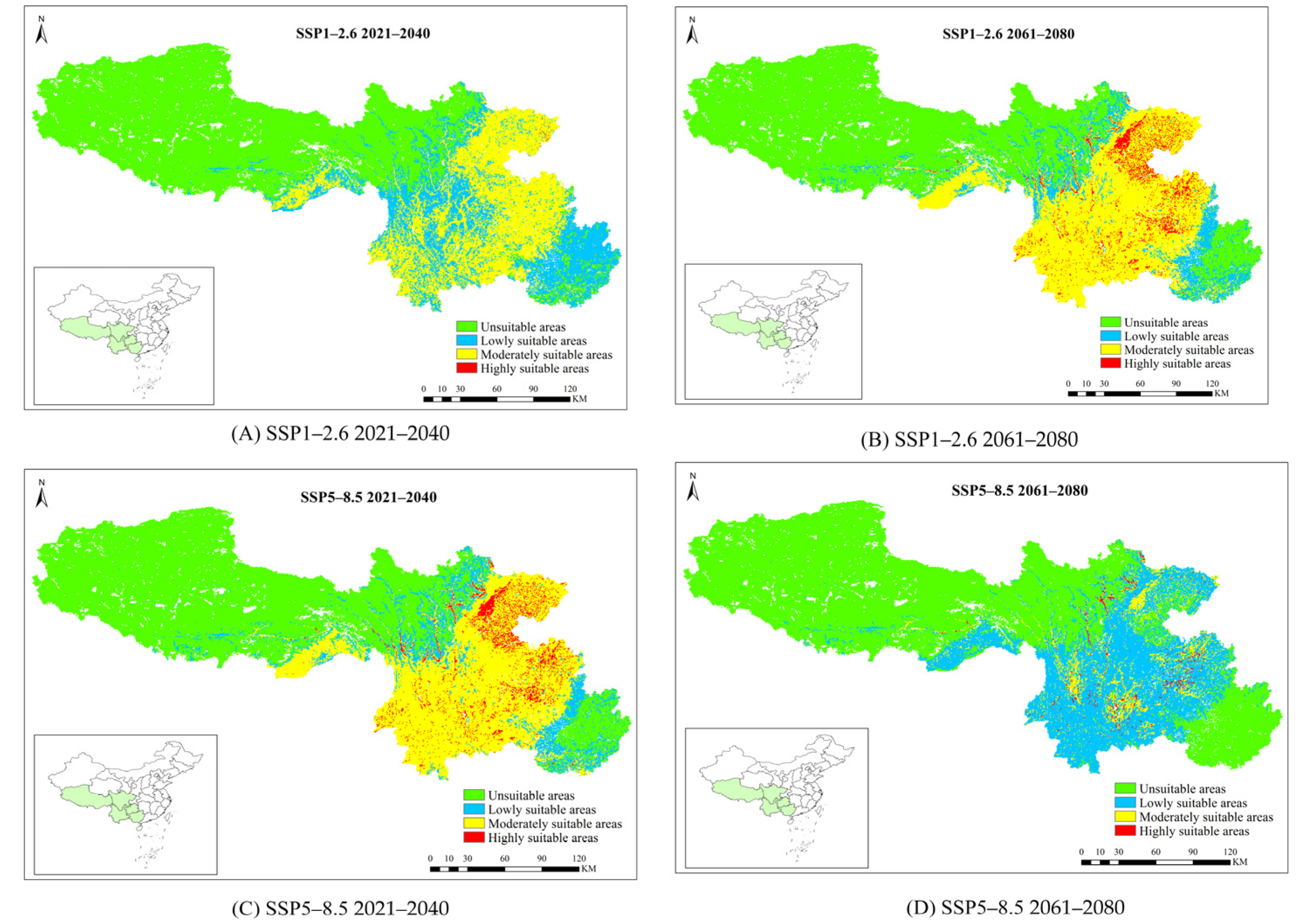
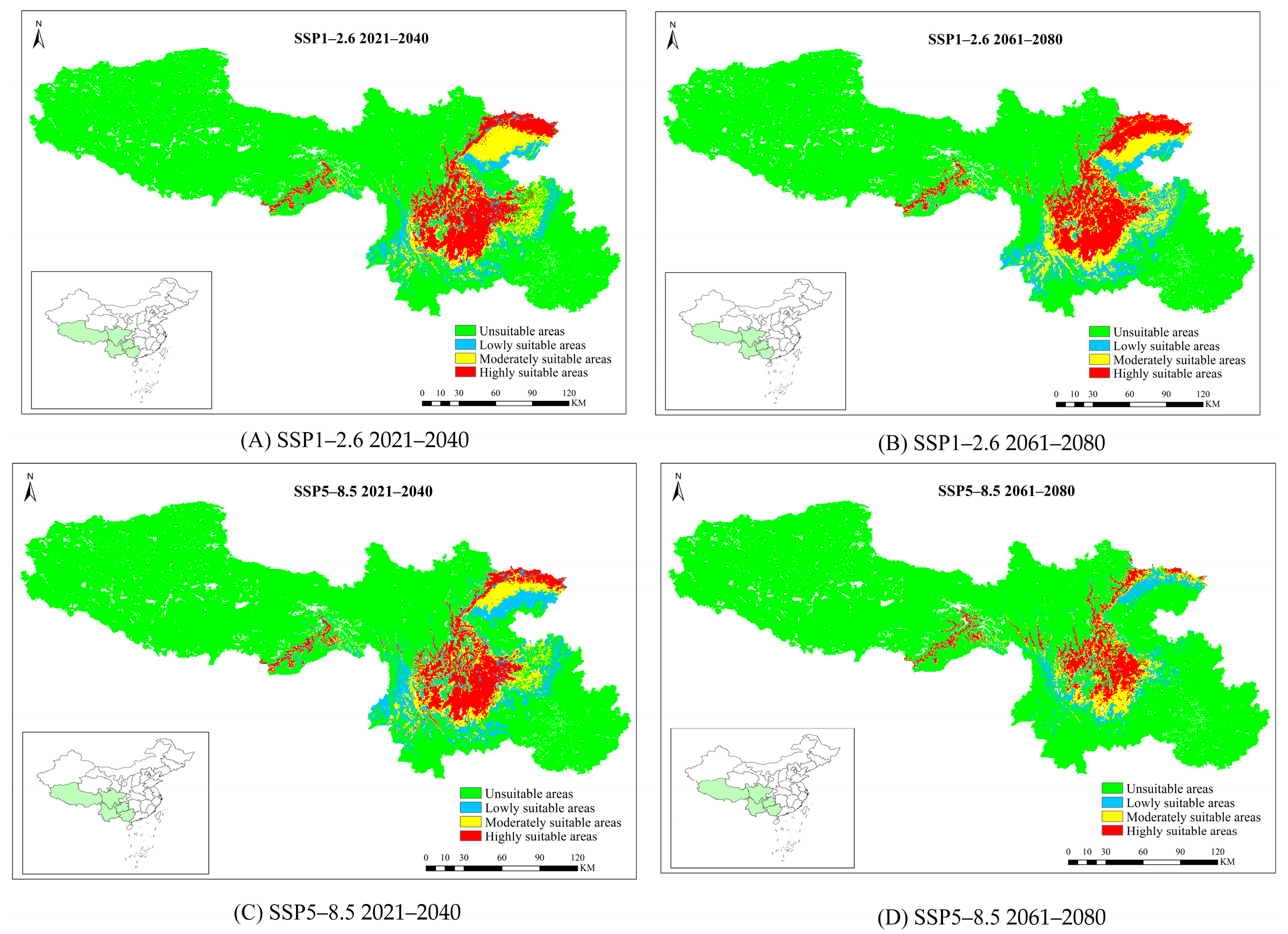
3.4. The Future Potential Distribution of P. yunnanensis and T. yunnanensis
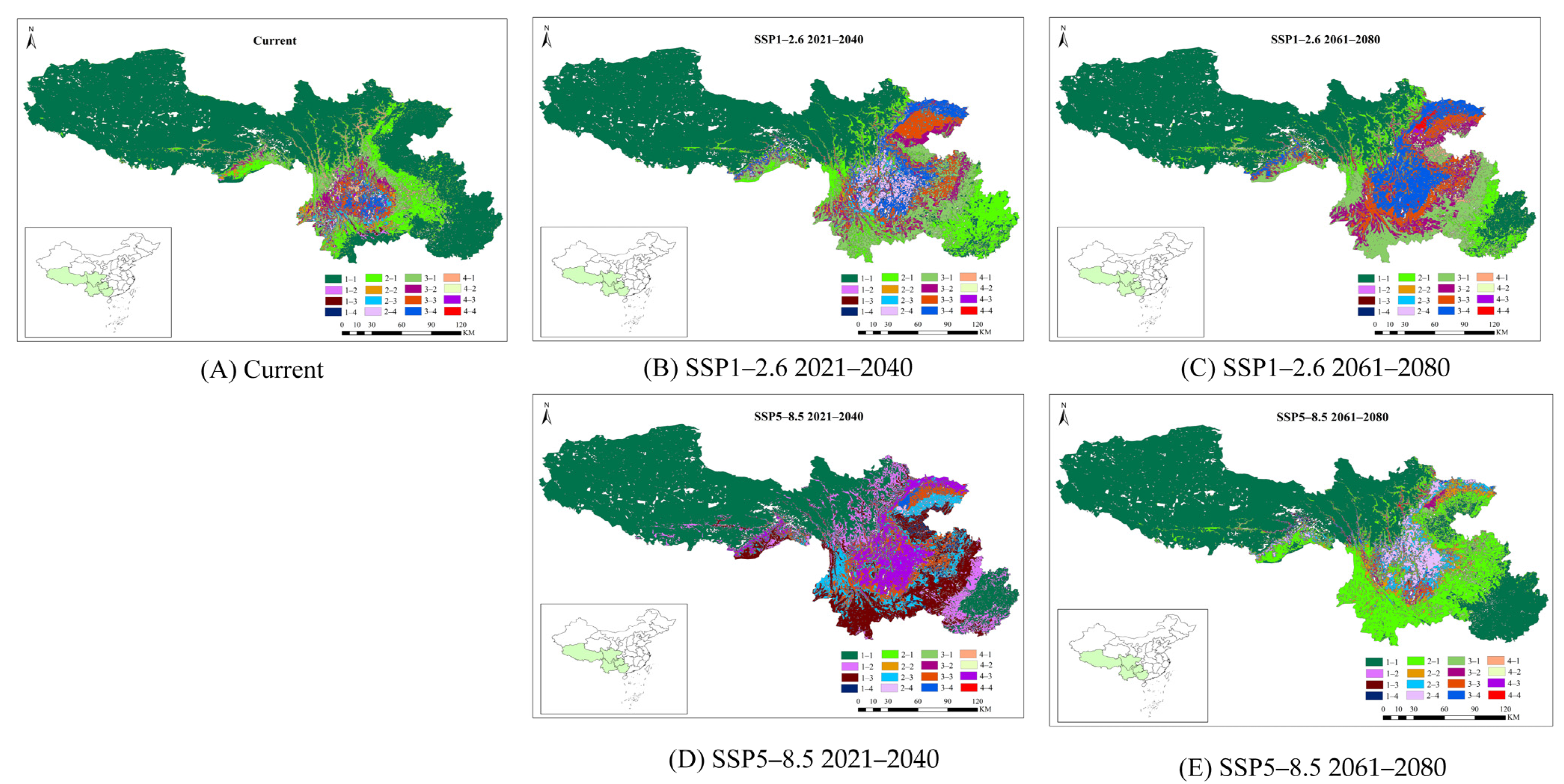
3.5. Changes in the Potential Distribution of P. yunnanensis and T. yunnanensis
4. Discussion
4.1. Potential Current Distribution of Suitable Areas for P. yunnanensis and T. yunnanensis
4.2. Potential Future Distribution and Relationship between P. yunnanensis and T. yunnanensis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the SixthAssessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Ying, Z. Projections of 2.0 °C Warming over the Globe and China under RCP4.5. Atmos. Ocean. Sci. Lett. 2012, 5, 514–520. [Google Scholar] [CrossRef]
- Zhao, D.S.; Gao, X.; Wu, S.G.; Zheng, D. Trend of climate variation in China from 1960 to 2018 based on natural regionalization. Adv. Earth Sci. 2020, 35, 750–760. [Google Scholar]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, W.; Zhao, X.; Zhu, H.; Ma, L.; Qian, Z.; Zhang, Z. Modeling the Potential Distribution of Three Taxa of Akebia Decne. Under Climate Change Scenarios in China. Forests 2021, 12, 1710. [Google Scholar]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond Predictions: Biodiversity Conservation in a Changing Climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Yin, Y.; He, Q.; Pan, X.; Liu, Q.; Wu, Y.; Li, X. Predicting Current Potential Distribution and the Range Dynamics of Pomacea canaliculata in China under Global Climate Change. Biology 2022, 11, 110. [Google Scholar] [CrossRef]
- Chen, Y.G.; Yue, X.G.; Chen, Y.H.; Cheng, W.X.; Du, G.J.; Zhong, Q.L.; Cheng, Q.L. Identification of potential distribution area of Cunninghamia lanceolata in China under climate change based on the MaxEnt model. Chin. J. Appl. Ecol. 2022, 33, 1207–1214. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Bálint, M.; Domisch, S.; Engelhardt, C.H.M.; Haase, P.; Lehrian, S.; Sauer, J.; Theissinger, K.; Pauls, S.U.; Nowak, C. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Chang. 2011, 1, 313–318. [Google Scholar] [CrossRef]
- Oliver, T.; Hill, J.K.; Thomas, C.D.; Brereton, T.; Roy, D.B. Changes in habitat specificity of species at their climatic range boundaries. Ecol. Lett. 2009, 12, 1091–1102. [Google Scholar] [CrossRef]
- Peterson, M.L.; Doak, D.F.; Morris, W.F. Incorporating local adaptation into forecasts of species’ distribution and abundance under climate change. Glob. Chang. Biol. 2019, 25, 775–793. [Google Scholar] [PubMed]
- Anderson, R.P. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Qiao, H.J.; Hu, J.H.; Huang, J.H. Theoretical basis, future directions, and challenges for ecological niche models. Sci. Sin. 2013, 43, 915–927. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized Maxent Model Predictions of Climate Change Impacts on the Suitable Distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Dang, A.T.N.; Kumar, L.; Reid, M. Modelling the Potential Impacts of Climate Change on Rice Cultivation in Mekong Delta, Vietnam. Sustainability 2020, 12, 9608. [Google Scholar] [CrossRef]
- Kogo, B.K.; Kumar, L.; Koech, R.; Kariyawasam, C.S. Modelling Climate Suitability for Rainfed Maize Cultivation in Kenya Using a Maximum Entropy (MaxENT) Approach. Agronomy 2019, 9, 727. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, X.H.; Wang, J.H.; Xing, L.G.; Fu, Y.Y. Maxent modelling for predicting climate change effects on the potential planting area of tuber mustard in China. J. Agric. Sci. 2019, 157, 375–381. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Li, K.; Wang, C.; Guna, A.; Zhang, J. Prediction of Potential Geographical Distribution Patterns of Actinidia arguta under Different Climate Scenarios. Sustainability 2021, 13, 3526. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt Modeling for Predicting the Current and Future Potential Geographical Distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, C. Study on Taiwania cryptomerioides under climate change: MaxEnt modeling for predicting the potential geographical distribution. Glob. Ecol. Conserv. 2020, 24, e01313. [Google Scholar] [CrossRef]
- Koo, K.S.; Park, D.; Oh, H.S. Analyzing habitat characteristics and predicting present and future suitable habitats of Sibynophis chinensis based on a climate change scenario. J. Asia-Pac. Biodivers. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Saeedi, H.; Costello, M.J.; Warren, D.; Brandt, A. Latitudinal and bathymetrical species richness patterns in the NW Pacific and adjacent Arctic Ocean. Sci. Rep. 2019, 9, 9303. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, H.; Basher, Z.; Costello, M.J. Modelling present and future global distributions of razor clams (Bivalvia: Solenidae). Helgol. Mar. Res. 2016, 70, 235. [Google Scholar] [CrossRef]
- Jones, M.C.; Dye, S.R.; Pinnegar, J.K.; Warren, R.; Cheung, W.W.L. Modelling commercial fish distributions: Prediction and assessment using different approaches. Ecol. Model. 2012, 225, 133–145. [Google Scholar] [CrossRef]
- Yi, Y.-j.; Cheng, X.; Yang, Z.-F.; Zhang, S.-H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Du, Z.; He, Y.; Wang, H.; Wang, C.; Duan, Y. Potential geographical distribution and habitat shift of the genus Ammopiptanthus in China under current and future climate change based on the MaxEnt model. J. Arid. Environ. 2021, 184, 104328. [Google Scholar] [CrossRef]
- Anand, V.; Oinam, B.; Singh, I.H. Predicting the current and future potential spatial distribution of endangered Rucervus eldii eldii (Sangai) using MaxEnt model. Environ. Monit. Assess. 2021, 193, 147. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef]
- Ning, H.; Tang, M.; Chen, H. Impact of Climate Change on Potential Distribution of Chinese White Pine Beetle Dendroctonus armandi in China. Forests 2021, 12, 544. [Google Scholar] [CrossRef]
- Negrini, M.; Fidelis, E.G.; Picanco, M.C.; Ramos, R.S. Mapping of the Steneotarsonemus spinki invasion risk in suitable areas for rice (Oryza sativa) cultivation using MaxEnt. Exp. Appl. Acarol. 2020, 80, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kumar, S.; Neven, L.G. Codling Moth (Lepidoptera: Tortricidae) Establishment in China: Stages of Invasion and Potential Future Distribution. J. Insect. Sci. 2017, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, H.; Luo, W.; Wang, M.; Lu, X.; Huang, T.; Zhao, J.; Li, Q. Predicting the potential distribution of the Asian citrus psyllid, Diaphorina citri (Kuwayama), in China using the MaxEnt model. PeerJ 2019, 7, e7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Liu, J.; Jiao, J.; Lu, J.; Lv, D.; Mao, J.; Zhao, Y.; Zhang, Y. Applications of machine learning in pine nuts classification. Sci. Rep. 2022, 12, 8799. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.S.; Luo, T.; Zheng, W.; Yang, W.Z.; Li, J.W.; Wang, Y.B.; Wang, S. Distribution patterns of Pinus yunnanensis and P. yunnanensis var.pygmaea and related key ecological factors. J. Northeast. For. Univ. 2021, 49, 8–14. [Google Scholar]
- Wang, X.W.; Chen, P.; Wang, Y.X.; Yuan, R.L.; Feng, D.; Li, L.S.; Ye, H.; Pan, Y.; Lv, J.; Zhou, Y.F.; et al. Population Structure and Succession Law of Tomicus Species in Yunnan. For. Res. 2018, 31, 167–172. [Google Scholar]
- Yan, G.; Zhang, M.D.; Qian, L.B.; Ze, S.X.; Yang, B.; Li, Z.B. Electrophysiological and behavioral responses of Tominus yunnanensis to plant volatiles from primarily infected pinus yunnanensis in Yunnan, Southwest China. J. Environ. Entomol. 2021, 43, 1389–1397. [Google Scholar]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Liu, X. The Beijing Climate Center Climate System Model (BCC-CSM): Main Progress from CMIP5 to CMIP6. Geosci. Model Dev. Discuss. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, Y.; Zhang, J.; Yang, W.; Zhang, L.; Hull, V.; Wang, Z.; Zheng, H.; Liu, J.; Polasky, S.; et al. Strengthening protected areas for biodiversity and ecosystem services in China. Proc. Natl. Acad. Sci. USA 2017, 114, 1601–1606. [Google Scholar] [CrossRef]
- Alcala-Canto, Y.; Alberti-Navarro, A.; Figueroa-Castillo, J.A.; Ibarra-Velarde, F.; Vera-Montenegro, Y.; Cervantes-Valencia, M.E. Maximum Entropy Ecological Niche Prediction of the Current Potential Geographical Distribution of Eimeria Species of Cattle, Sheep and Goats in Mexico. Open J. Anim. Sci. 2019, 09, 234–248. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Zhang, L.; Wang, C.; Xu, Y. Predicting Possible Distribution of Tea (Camellia sinensis L.) under Climate Change Scenarios Using MaxEnt Model in China. Agriculture 2021, 11, 1122. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Processes 2020, 9, 6. [Google Scholar] [CrossRef]
- Swets, J. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.F.; Chen, Y.H.; Zhou, S.Y.; Yin, X.J. Responses of Pinus species to climate change in southwestern China. J. For. Environ. 2020, 40, 466–477. [Google Scholar]
- Yang, R.Q.; Fan, Z.X.; Li, Z.S.; Wen, Q.Z. Radial growth of Pinus yunnanensis at different elevations and their responses to climatic factors in the Yulong Snow Mountain, Northwest Yunnan, China. Acta Ecol. Sin. 2018, 38, 8983–8991. [Google Scholar]
- Regniere, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar] [CrossRef]
- Wang, Q. Effect of Extreme Climate on the Occurrence Tendency of Three Forest Pests. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, July 2016. [Google Scholar]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Thomas, C.D.; Gillingham, P.K. The performance of protected areas for biodiversity under climate change. Biol. J. Linn. Soc. 2015, 115, 718–730. [Google Scholar] [CrossRef] [Green Version]
- Rew, L.J.; McDougall, K.L.; Alexander, J.M.; Daehler, C.C.; Essl, F.; Haider, S.; Kueffer, C.; Lenoir, J.; Milbau, A.; Nuñez, M.A.; et al. Moving up and over: Redistribution of plants in alpine, Arctic, and Antarctic ecosystems under global change. Arct. Antarct. Alp. Res. 2020, 52, 651–665. [Google Scholar] [CrossRef]
- Hu, R.; Gu, Y.; Luo, M.; Lu, Z.; Wei, M.; Zhong, J. Shifts in bird ranges and conservation priorities in China under climate change. PLoS ONE 2020, 15, e0240225. [Google Scholar] [CrossRef]
- Weber, T.C. Maximum entropy modeling of mature hardwood forest distribution in four U.S. states. For. Ecol. Manag. 2011, 261, 779–788. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lv, Y.W.; Yin, X.J. Predicting habitat suitability of 12 coniferous forest tree species in southwest China based on climate change. J. Nanjing For. Univ. (Nat. Sci. Ed.). 2019, 43, 113–120. [Google Scholar]
- Li, X.; Ma, B.; Lu, C.; Yang, H.; Sun, M. Spatial Pattern and Development of Protected Areas in the North-south Transitional Zone of China. Chin. Geogr. Sci. 2021, 31, 149–166. [Google Scholar] [CrossRef]
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Klein Goldewijk, K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
| Type | Environmental Factors | Description of Environmental Factors |
|---|---|---|
| Temperature | BIO1 | Annual Mean Temperature |
| BIO2 | Mean Diurnal Range | |
| BIO3 * | Isothermality | |
| BIO4 | Temperature Seasonality | |
| BIO5 | Maximum Temperature of Warmest Month | |
| BIO6 * | Minimum Temperature of Coldest month | |
| BIO7 * | Temperature Annual Range | |
| BIO8 | Mean Temperature of Wettest Quarter | |
| BIO9 | Mean Temperature of Driest Quarter | |
| BIO10 | Mean Temperature of Warmest Quarter | |
| BIO11 | Mean Temperature of Coldest Quarter | |
| Precipitation | BIO12 | Annual Precipitation |
| BIO13 | Precipitation of Wettest Month | |
| BIO14 | Precipitation of Driest Month | |
| BIO15 * | Precipitation Seasonality | |
| BIO16 | Precipitation of Wettest Quarter | |
| BIO17 | Precipitation of Driest Quarter | |
| BIO18 | Precipitation of Warmest Quarter | |
| BIO19 * | Precipitation of Coldest Quarter | |
| Terrain | ELEV * | Elevation |
| Soil | AWC_CLASS * | Soil’s available water content |
| T_CACO3 * | Soil’s carbonate or lime content | |
| T_PH_H2O * | Soil’s pH | |
| Vegetation | VEGETATION * | Vegetation type |
| Species | Climate Scenarios | Total Suitable Area | Lowly Suitable Area | Moderately Suitable Area | Highly Suitable Area | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | |||
| P. yunnanensis | Current | 1970–2000 | 63.42 | 33.40 | 24.09 | 5.92 | ||||
| SSP1-2.6 | 2021–2040 | 111.12 | 75.21 | 47.97 | 43.62 | 63.08 | 161.85 | 0.08 | −98.64 | |
| 2061–2080 | 111.72 | 76.15 | 22.43 | −32.84 | 81.43 | 238.02 | 7.86 | 32.77 | ||
| SSP5-8.5 | 2021–2040 | 111.28 | 75.47 | 21.67 | −35.11 | 81.32 | 237.67 | 8.29 | 40.03 | |
| 2061–2080 | 90.78 | 43.14 | 75.62 | 126.40 | 13.03 | −45.91 | 1.86 | −68.58 | ||
| T. yunnanensis | Current | 1970–2000 | 22.81 | 9.36 | 9.14 | 4.31 | ||||
| SSP1-2.6 | 2021–2040 | 54.78 | 140.15 | 14.15 | 51.12 | 18.25 | 99.67 | 22.38 | 419.26 | |
| 2061–2080 | 59.47 | 160.71 | 17.07 | 82.31 | 19.04 | 108.32 | 23.35 | 441.76 | ||
| SSP5-8.5 | 2021–2040 | 53.94 | 136.48 | 19.63 | 109.72 | 15.73 | 72.10 | 18.57 | 330.85 | |
| 2061–2080 | 35.12 | 53.97 | 11.68 | 24.79 | 11.65 | 27.46 | 12.18 | 182.60 | ||
| Current | SSP1-2.6 | SSP5-8.5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2021–2040 | 2061–2080 | 2021–2040 | 2061–2080 | ||||||
| Change | Aera (×104 km2) | Change | Aera (×104 km2) | Change | Aera (×104 km2) | Change | Aera (×104 km2) | Change | Aera (×104 km2) |
| 1–1 | 187.78 | 1–1 | 140.78 | 1–1 | 140.61 | 1–1 | 141.02 | 1–1 | 160.71 |
| 3–2 | 4.33 | 3–2 | 10.65 | 3–2 | 14.68 | 3–2 | 0.30 | 3–2 | 2.20 |
| 3–3 | 4.76 | 3–3 | 14.08 | 3–3 | 16.56 | 3–3 | 13.32 | 3–3 | 2.24 |
| 3–4 | 2.93 | 3–4 | 13.92 | 3–4 | 21.25 | 3–4 | 2.13 | 3–4 | 1.70 |
| 4–1 | 2.85 | 4–1 | 0.00 | 4–1 | 2.12 | 4–1 | 0.0 | 4–1 | 0.64 |
| 4–2 | 1.37 | 4–2 | 0.01 | 4–2 | 1.79 | 4–2 | 0.21 | 4–2 | 0.36 |
| 4–3 | 1.38 | 4–3 | 0.03 | 4–3 | 2.05 | 4–3 | 17.47 | 4–3 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Mao, J.; Zhao, Y.; Sun, Y.; Cao, Y.; Xiong, Z. Similar Pattern of Potential Distribution of Pinus yunnanensis Franch and Tomicusyunnanensis Kirkendall under Climate Change in China. Forests 2022, 13, 1379. https://doi.org/10.3390/f13091379
Huang B, Mao J, Zhao Y, Sun Y, Cao Y, Xiong Z. Similar Pattern of Potential Distribution of Pinus yunnanensis Franch and Tomicusyunnanensis Kirkendall under Climate Change in China. Forests. 2022; 13(9):1379. https://doi.org/10.3390/f13091379
Chicago/Turabian StyleHuang, Biaosheng, Jiawei Mao, Youjie Zhao, Yongke Sun, Yong Cao, and Zhi Xiong. 2022. "Similar Pattern of Potential Distribution of Pinus yunnanensis Franch and Tomicusyunnanensis Kirkendall under Climate Change in China" Forests 13, no. 9: 1379. https://doi.org/10.3390/f13091379




