Abstract
Triterpenes are natural products of plants that can defend against microorganisms and various stresses. Oxidosqualene cyclase (OSC), the key rate-limiting enzyme of the triterpene biosynthetic pathway, catalyzes 2,3-oxidosqualene into sterols and triterpenes with different skeletons through the chair–boat–chair (CBC) conformation or chair–chair–chair (CCC) conformation. They were expanded in plants mainly by tandem duplication and are distributed in many plant lineages. They have multiple biological activities, including as functional foods and drugs. Here, we summarize the current characterized forest OSCs and their potential functions, especially for pharmacological applications. The study of triterpene-catalyzed enzyme OSC has an important scientific role and potential economic value. This paper summarizes the research advances of the main members of the OSC family in plants, their structure and function, the biosynthesis of triterpenes, and the molecular evolution of OSC.
1. Introduction
The long evolutionary process of plants over approximately 470 million years has been a gradual development process from aquatic to terrestrial [1]. Different from animals or microorganisms, plants have evolved a set of “weapons” to conquer various challenges from the surrounding environment. Plant secondary metabolites (“weapons”) are small molecular organic compounds that are unnecessary for cell life activities or plant growth and development but help plants cope with various challenges. The known plant secondary metabolites mainly include nitrogen-containing compounds, phenylpropanoids, flavonoids, and terpenes [2]. Terpenes are the most abundant secondary metabolites in plants, with more than 80,000 structures reported [3]. Based on the number of isoprene units, they can be divided into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesquiterpenes (C25), triterpenes (C30), and polyterpenes [4]. Triterpenes are a class of compounds, and more than 20,000 different triterpenes have been reported. Generally, triterpene skeletons widely exist in nature in the form of glycosides (triterpene saponins) or esters [5]. According to its structure, the plant triterpene skeleton (non-sugar part) mainly includes cucurbitanes, dammaranes, lupanes, ursanes, and friedelanes [6].
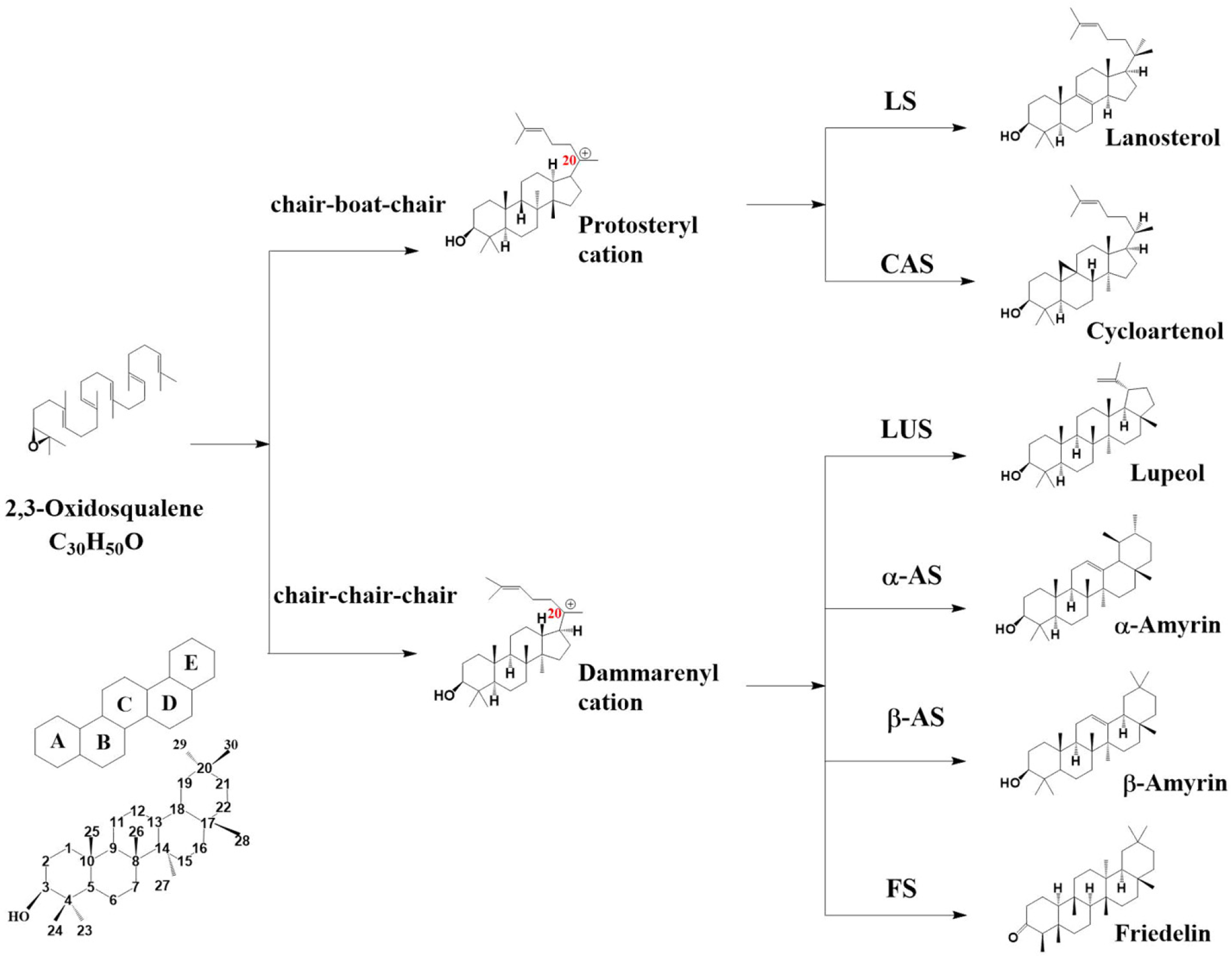
Oxidosqualene cyclase (OSC) can catalyze the cyclization of 2,3-oxidosqualene to sterols and triterpenes with different skeletons [7,8]. It is a pivotal enzyme and promotes triterpene scaffold diversification by a set of programs including protonation, cyclization, rearrangement, and deprotonation [9]. According to the properties of its catalytic intermediates, two groups of intermediates were named: protosteryl cation and dammarenyl cation. In sterol biosynthesis, protosteryl cations are cyclized from 2,3-oxidosqualene through the “chair–boat–chair” conformation (CBC), including cycloartenol, lanosterol, and cucurbitadienol (Figure 1). In triterpene biosynthesis, the dammarenyl cation is cyclized from 2,3-oxidosqualene through the “chair–chair–chair” conformation (CCC) into products such as lupeol, α-amyrin, β-amyrin, and friedelin.
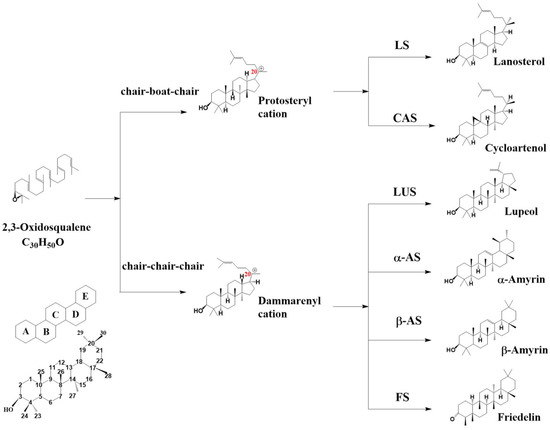
Figure 1.
OSC catalyzes 2,3-oxidosqualene to produce different skeletons through two conformations. LS, lanosterol synthase; CAS, cycloartenol synthase; LUS, lupeol synthase; α-AS, α-amyrin synthase; β-AS, β-amyrin synthase; FS, friedelin synthase.
More than 150 OSC genes in more than 75 plants have been functionally characterized through the heterologous expression of related OSC genes in yeast or tobacco [10]. The largest numbers are of β-amyrin synthase (β-AS), cycloartenol synthase (CAS), and lupeol synthase (LUS), which account for 23.3%, 20.7%, and 14% of the total identified OSCs, respectively [10]. Others, such as friedelin synthase (FS), α-amyrin synthase (α-AS), cucurbitadienol synthase (CPQ), and lanosterol synthase (LS), have also been identified in various plant species.
These compounds have multiple biological activities. For example, OsOSC12 catalyzes the substrate to generate poaceatapetol, and the mutation of OsOSC12 causes the rapid dehydration of pollen grains, showing a humidity-sensitive genic male sterility phenotype [11]. β-amyrin-derived triterpene glycosides from Barbarea vulgaris show certain resistance to flea beetle larvae (Phyllotreta nemorum) [12]. In Artemisia annua, OSC2 produces triterpenoids for the cuticle of aerial organs and probably plays a role against biotic and abiotic stress [13].
The forest is a huge treasure, home to millions of secondary metabolites, but only tip of the iceberg has been discovered. Secondary metabolites from forests play important roles in improving plant resistance to biotic or abiotic stresses. In addition, they are valuable sources of medicinal ingredients that cannot be ignored, as triterpenes and glycosidic products have a wide range of medicinal functions. The study on the catalytic mechanisms of OSC can reveal the synthetic pathways of the medicinal components of plants in forests and thus advance the exploration of the medicinal value of these plants.
2. The OSC Main Members and the Characterized OSCs from Forests
2.1. Lanosterol Synthase (LS)
LS can catalyze 2,3-oxidosqualene to lanosterol. Through the CBC cyclization, it takes the protosterol cation as the intermediate and transfers the positive charge of the cation from C-20 to C-8. After deprotonation, a double bond forms between C-8 and C-9 to produce lanosterol (Figure 1). Many lanosterol synthases have been heterologously characterized from Arabidopsis thaliana, Panax ginseng, and Lotus japonicus [14,15,16]. Lanosterol has been excluded as an intermediate in phytosterol synthesis, as no relative product has yet been detected in plants.
2.2. Cycloartenol Synthase (CAS)
Cycloartenol synthase can also catalyze 2,3-oxidosqualene to cycloartenol through the CBC conformation using protosteryl cations (Figure 1). Cycloartenol is one of the key precursors for the biosynthesis of phytosterols such as campesterol, stigmasterol, and sitosterol. Sterols, as essential components of the plasma membrane of all eukaryotic cells and cell viability, are widely distributed in various tissues and organs.
More than 15 different types of CAS have been identified by heterologous expression [13,15,17,18,19,20,21,22,23,24,25]. CAS1 (At2g07050) from A. thaliana was the first characterized CAS in plants and it is a tetracyclic triterpene synthase [26]. ASCS1 (AJ311790), which is mainly expressed in root tip cells from Avena strigosa, was characterized using a yeast expression system [27]. Interestingly, two cycloartenol synthase genes, BPX1 (AB055509) and BPX2 (AB055510), were identified in Betula platyphylla, and the study also proposed that LUS and β-amyrin synthase were evolved from cycloartenol synthase [28]. A study on Costus speciosus proved that CsOSC1 (AB058507) encodes cycloartenol synthase, the first reported cycloartenol synthase in monocots [29].
2.3. Lupeol Synthase (LUS)
Taking the dammarenyl cation as the intermediate, the lupyl cation is generated through E-ring expansion through CCC conformation (Figure 1). The lupyl cation is quenched by eliminating methyl protons, resulting in the formation of lupeol. Lupeol, one of the pentacyclic triterpenes, is extensively studied due to its pharmacological activities. Matsuda’s team found that LUP1 (At1g78970) has 57% homology with A. thaliana cycloartenol synthase, and its product is mainly lupeol [30]. In Bruguiera gymnorrhiza, the authors verified that the product of BgLUS (AB289586) is lupeol by using a yeast system [31]. Ebizuka’s group identified BPW (AB055511) as LUS from the suspension cell culture line of B. platyphylla [28]. KdLUS (HM623871) from Kalanchoe daigremontiana was heterologously expressed in yeast, and the mixed product contained 94% lupeol. RT-qPCR analysis showed that KdLUS was only expressed in the epidermal cells [32]. OSC3 (AB181245) in L. japonicus has 81% homology with BPW, and the product is also lupeol, mainly accumulated in plant roots [33]. OEW (AB025343) from Olea europaea was expressed in yeast to produce lupeol. The OEW product was unexpected, as oleanolic acid is the main triterpene of O. europaea leaves; however, no lupeol skeleton product was discovered in leaves. In Ricinus communis, RcLUS (DQ268869) was found to make lupeol in the cuticular region, which in turn is responsible for the formation of epicuticular wax crystals on the stem and hypocotyl surfaces, resulting in its glaucous phenotype [20]. In Malus × domestica, the lupeol, which is mainly accumulated in the peel, was the catalytic product of MdOSC5 (KT383436) [34].
2.4. α-Amyrin Synthase (α-AS)
The catalytic mechanism of α-amyrin synthase is to take the dammarenyl cation as the intermediate and form the ursanyl cation through E-ring expansion (Figure 1). The homology of MdOSC1 (FJ032006) and MdOSC3 (FJ032008) in Malus × domestica is nearly 99%, and α-amyrin accounted for 85% of the mixed product [34]. In Pisum sativum, α-amyrin synthase produced up to 50% of the total product [35]. Interestingly, among the identified α-amyrin synthase species, most genes were from the Rosaceae family, implying common α-amyrin synthases in this plant lineage.
2.5. β-Amyrin Synthase (β-AS)
β-amyrin synthase is a common OSC that has been found in more than 30 plants, including in A. thaliana, Glycyrrhiza glabra, L. japonicus, Nigella sativa, and P. ginseng [18,33,36,37,38]. The catalytic mechanism of β-amyrin synthase also involves the dammarenyl cation as the intermediate; the ring is expanded on the basis of the lupyl cation to obtain the oleanolyl secondary cation. Furthermore, 1,2-hydride displacement and deprotonation at C-12 form β-amyrin [7] (Figure 1). Phylogenetic analysis showed that AsbAS1 (AJ311789) from A. strigosa and lanosterol synthase from animals and fungi were more related than cycloartenol synthase from plants. AsbAS1 is the only single-functional β-amyrin synthase found in monocotyledonous [27]. The first gene reported to be involved in the triterpene synthesis pathway in Euphorbiaceae plants was the β-amyrin synthase coded by EtAS (AB206469) in Euphorbia tirucalli [39]. β-amyrin synthase is encoded by AsOXA1 (AY836006), whose transcripts were found to accumulate strongly in leaves but weakly in roots in Aster sedifolius. The results showed a higher level of triterpenes in roots, which may be due to the transport of triterpenes from leaves to roots [40].
2.6. Friedelin Synthase (FS)
Through the CCC conformation cyclization, the friedelin synthase takes the dammarenyl cation as the intermediate and undergoes 10 positive charge shifts from C-20 to C-2 (Figure 1). It crosses the largest range of skeleton modification of the dammarane cyclization pathway and is one of the most complex pentacyclic triterpenes [32]. Through RNAi and tissue expression pattern analysis, Zhou determined that TwOSC1 (QHT64456) and TwOSC3 (QHT64454) in Tripterygium wilfordii were responsible for the biosynthesis of the medicinal compound celastrol, thereby proving that friedelin was a precursor of celastrol [41], laying the foundation for the biosynthesis of celastrol in engineering yeast in the future. Friedelin is also a leptin sensitizer, which is expected to be utilized in the treatment of obesity [42]. Friedelin synthases were also identified in K. daigremontiana, Maytenus ilicifolia, Populus davidiana, and some other species [32,43,44].
2.7. The Characterized OSCs from Forests
Ilex asprella is a medicinal plant that is used extensively in southern China. It contains ursane-type triterpenoids and triterpenoid saponins with well-known pharmacological activities. The ursane triterpene α-amyrin is catalyzed by the key gene laAS1(AIS39793) [45]. The leaves of loquat (Eriobotrya japonica) possess high medicinal value due to the high amount of ursolic acid, which is one of the most effective active compounds. Similarly, α-amyrin, the precursor of ursolic acid, was catalyzed by EjAS (JX173279) [46]. P. ginseng is one of the most universally used herbal medicines, and most of the biological activities of ginseng are derived from its main constituents, ginsenosides. Oleanolic acid-type ginsenosides are one type of ginsenoside molecules, and β-amyrin, the precursor of oleanolic acid-type ginsenosides, was catalyzed by PNY (AB009030) [18]. Glycyrrhizic acid in G. glabra is an oleanolane-type triterpene, and β-amyrin was catalyzed by the key gene GgbAS1 (AB037203). Yu found that glycyrrhizic acid was a non-toxic, broad-spectrum, anti-coronavirus molecule in vitro, especially to SARS-CoV-2 [47]. The medicinal value of forest trees enriches the source of medicinal ingredients, rendering forest trees as invaluable human medical resources.
Interestingly, some specific triterpenes are accumulated in the wax layers of K. daigremontiana, Ligustrum vulgare, and Macaranga ant plants and are believed to be involved in defense against herbivores or insects [48,49,50]. However, the molecular mechanism of biosynthesis of these chemical compounds is still unclear. Characterizations of these OSCs would advance their contribution to forest plants.
3. Molecular Evolution of OSC
The ancestral lanosterol synthase-like (ALSL) and ancestral cycloartenol synthase-like (ACS) appeared before the differentiation of dicotyledons and monocotyledonous plants about 140 million years ago [51,52]. LS and CAS have been found in bacteria, indicating that OSC in bacteria may be the ancestors of the sterol pathway [53,54,55]. After the differentiation of monocotyledons and dicotyledons, the LS gene was replicated multiple times, resulting in the expansion of the OSC gene in dicotyledons. The duplication of the ACS gene led to the amplification of the OSC gene in monocotyledons. The amplification of the OSC gene in the genomes of dicotyledons and monocotyledons was mainly due to tandem duplication [56]. Tandem duplication may contribute to plant defense against biological and abiotic stresses [57,58,59,60,61,62,63]. Thus, OSC gene families derived from tandem duplication often respond to external stimuli. Xue showed that local tandem duplication is crucial in expanding the OSC family [56]. Ober also reported that gene families involved in secondary metabolism are generated through gene duplication and form gene clusters on chromosomes [64].
After duplication and expansion, OSCs were widely distributed among multiple plant lineages. LUS, β-amyrin synthase, and cycloartenol synthase account for a large proportion of the OSC family and are mainly distributed in asterids and rosids. LUS is mainly distributed in Contortae, Fabales, Asterales, Solanales, Brassicales, and Rosales. β-amyrin synthase is mainly distributed in Gentianales, Apiales, Fabales, Solanales, and Ranunculales. Cycloartenol synthase is mainly distributed in Sapindales, Cucurbitales, Fabales, Brassicales, Saxifragales, Solanacea, Solanales, Apiales, and Asterales. Interestingly, cucurbitadienol synthase is only found in Cucurbitaceae plants [10].
4. Research Progress on OSC Structure and Function
The catalytic activity of an enzyme is primarily determined by a few key amino acids located in the active center. Thus, it is important to understand the relationship of these amino acids with enzyme structure and function.
DCTAE and QW are conserved motifs in OSC proteins [65]. DCTAE is related to substrate binding. The QW motif is generally a negative non-tandem repeat with a length of 16 amino acids, namely, Arg/Lys Gly/Ala X2-3 Tyr/Phe/Trp Leu X3 Gln X2-5 Gly X Trp [66]. The QW motif, which is located on the exterior of the enzyme, can stabilize carbon cations during cyclization [67]. When Trp259 in PNY (β-amyrin synthase) is mutated into Leu, a product ratio of 2:1 (lupeol and β-amyrin) is found. When Leu256 in OEW (lupeol synthase) is mutated into Trp, this results in β-amyrin as a major product with very few lupeols. This finding indicates that Trp259 is a key amino acid to determine the formation of β-amyrin synthase, which is speculated to occur through the stabilization of the oleanyl cation [68].
Research on the β-amyrin synthase of E. tirucalli has shown that steric hindrance is another effect on cyclization. The substitution of aliphatic amino groups significantly reduces the yield of bicyclic compounds. This result shows that the appropriate steric size is critical to the cyclization reaction [69].
The conformation of amino acid residues in the active center also affects triterpene skeleton formation. OSC2 has a unique catalytic domain (FLALA and LMVLA) and a special amino acid residue, Y531. Site-directed mutagenesis showed that a catalytic triad, Y-LL, was responsible for the migration and deprotonation of C-29 methyl [70]. This key active site was found to be important for the plasticity of the catalytic mechanism of OSC.
5. Biosynthesis of Triterpenes in Yeast
Various strategies were employed for the heterologous synthesis of high-value triterpenes, such as ginsenosides [71,72,73]. By increasing the optimizing module, regulating metabolic flow, engineering organelles, assembling enzymes, and expanding carbon sources, the target triterpene products can be optimized in engineered yeast.
The yeast strain GY-1 can produce 17.2 mg/L protopanaxadiol by overexpressing 3-hydroxy-3-methylglutaryl-CoA reductase, squalene synthase, and 2,3-oxidosqualene synthase genes [74]. By manipulating 3-hydroxy-3-methylglutaryl-CoA reductase, the yield of β-amyrin yield increases by 50% [75]. Glycerol and xylose can serve as a carbon source to improve the acetyl-CoA supply and thus enhance the yield of target products [76,77]. In addition, inhibition of the expression of sterol can also induce more substrate (2,3-oxidosqualene) to flow through the triterpene metabolic pathway [78]. Kim expanded the endoplasmic reticulum space to increase the synthesis and folding capacity, and the yield of protopanaxadiol increased by 72-fold [79].
Yuan increased the supply of substrate 2,3-oxidosqualene so the yield of α-amyrin would be 11.97 ± 0.61 mg/L [80]. In addition, OSC activity can be improved by site-directed mutagenesis of key amino acids. The triple-mutant MdOSC1N11T/P250H/P373A was employed to increase the yield of α-amyrin by 11-fold compared with the control group. Interestingly, the intracellular storage capacity was expanded by the overexpression of diacylglycerol acyltransferase to yield 106-fold α-amyrin (1107.9 ± 76.8 mg/L) in the aAM12 yeast strain [81].
Adding methylated β-cyclodextrin promoted the transport of the nonvolatile hydrophobic terpenes from yeast cells into the culture medium, thus greatly improving productivity [82].
6. Conclusions and Prospects
In general, while secondary metabolites do not participate directly in plant growth and development, they are believed to be involved in plant defense against various stresses [83,84].
In addition, many active medicinal ingredients are derived from triterpene skeletons [85]. Much progress has been achieved in biological research on characterizing OSCs and elucidating amino acids that are important for enzyme activity and product specificity [68,70]. Researchers have introduced reasonable design approaches such as the regulation of metabolic flow, compartmentalization engineering, and other methods to improve the chassis of triterpene biosynthesis [79,81]. However, due to the complex structure of triterpenes and the wide variety of modifying enzymes, the specific catalytic mechanism of triterpenes is not yet clear.
This paper reviews the progress of research on the main members of OSCs and expounds their biosynthesis, molecular evolution, structure, and function. We also summarized characterized OSCs from forests and their potential pharmacological roles. We expect that extensive studies on the catalytic mechanism and further functional characterization of numerous OSCs, especially from forests, can provide the foundation for the application of synthetic biological strategies to produce high-value terpenes from forests in the future.
Author Contributions
L.F. and G.W. conceived and designed the project. P.W. drafted the manuscript. L.F. and G.W. modified the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (grant numbers 31972454, 32171861, 32002076), the National Key R&D Program of China (grant number 2018YFD1000400), the Jiangsu Provincial Natural Science Foundation (grant number SBK2020043530), the Jiangsu Provincial Natural Science Research Project of Universities (grant number 20KJB210004), and the Jiangsu Provincial Agricultural Science and Technology Independent Innovation Fund (grant numbers CX(20)3023, CX(20)3026), and supported by the high-end talent support program of Yangzhou University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kenrick, P.; Crane, P.R. The origin and early evolution of plants on land. Nature 1997, 389, 33–39. [Google Scholar] [CrossRef]
- D’Auria, J.; Gershenzon, J. The secondary metabolism of Arabidopsis thaliana: Growing like a weed. Curr. Opin. Plant Biol. 2005, 8, 308–316. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Abe, I. Enzymatic synthesis of cyclic triterpenes. Nat. Prod. Rep. 2007, 24, 1311–1331. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002, 75, 31–49. [Google Scholar] [CrossRef]
- Shibuya, M.; Zhang, H.; Endo, A.; Shishikura, K.; Kushiro, T.; Ebizuka, Y. Two branches of the lupeol synthase gene in the molecular evolution of plant oxidosqualene cyclases. Eur. J. Biochem. 1999, 266, 302–307. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Yin, X.; Wang, X.; Qi, X.; Xue, Z. Diverse triterpene skeletons are derived from the expansion and divergent evolution of 2,3-oxidosqualene cyclases in plants. Crit. Rev. Biochem. Mol. Biol. 2021, 57, 113–132. [Google Scholar] [CrossRef]
- Xue, Z.; Xu, X.; Zhou, Y.; Wang, X.; Zhang, Y.; Liu, D.; Zhao, B.; Duan, L.; Qi, X. Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice. Nat. Commun. 2018, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Kuzina, V.; Ekstrøm, C.T.; Andersen, S.B.; Nielsen, J.K.; Olsen, C.E.; Bak, S. Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol. 2009, 151, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Shen, Q.; Soetaert, S.; Reed, J.; Erffelinck, M.L.; Van Nieuwerburgh, F.C.; Vanden Bossche, R.; Osbourn, A.; Thevelein, J.M.; et al. OSC2 and CYP716A14v2 catalyze the biosynthesis of triterpenoids for the cuticle of aerial organs of Artemisia annua. Plant Cell 2015, 27, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, M.D.; Xiong, Q.; Lodeiro, S.; Hua, L.; Matsuda, S.P. Lanosterol biosynthesis in plants. Arch. Biochem. Biophys. 2006, 447, 87–95. [Google Scholar] [CrossRef]
- Sawai, S.; Akashi, T.; Sakurai, N.; Suzuki, H.; Shibata, D.; Ayabe, S.; Aoki, T. Plant lanosterol synthase: Divergence of the sterol and triterpene biosynthetic pathways in eukaryotes. Plant Cell Physiol. 2006, 47, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Xiang, T.; Ohyama, K.; Seki, H.; Saito, K.; Muranaka, T.; Hayashi, H.; Katsube, Y.; Kushiro, T.; Shibuya, M.; et al. Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol. 2006, 47, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Shibuya, M.; Lee, M.-S.; Sankawa, U.; Ebizuka, Y. Molecular Cloning of Pea cDNA Encoding Cycloartenol Synthase and Its Functional Expression in Yeast. Biol. Pharm. Bull. 1997, 20, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. Beta-amyrin synthase--cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef]
- Hayashi, H.; Huang, P.; Takada, S.; Obinata, M.; Inoue, K.; Shibuya, M.; Ebizuka, Y. Differential Expression of Three Oxidosqualene Cyclase mRNAs in Glycyrrhiza glabra. Biol. Pharm. Bull. 2004, 27, 1086–1092. [Google Scholar] [CrossRef]
- Guhling, O.; Hobl, B.; Yeats, T.; Jetter, R. Cloning and characterization of a lupeol synthase involved in the synthesis of epicuticular wax crystals on stem and hypocotyl surfaces of Ricinus communis. Arch. Biochem. Biophys. 2006, 448, 60–72. [Google Scholar] [CrossRef]
- Basyuni, M.; Oku, H.; Tsujimoto, E.; Baba, S. Cloning and functional expression of cycloartenol synthases from mangrove species Rhizophora stylosa Griff. and Kandelia candel (L.) Druce. Biosci. Biotechnol. Biochem. 2007, 71, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, J.; Shibuya, M.; Masuda, K.; Ebizuka, Y. Squalene cyclase and oxidosqualene cyclase from a fern. FEBS Lett. 2008, 582, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Mori, K.; Hashimoto, I.; Nakano, C.; Sato, T.; Hoshino, T. Triterpene cyclases from Oryza sativa L.: Cycloartenol, parkeol and achilleol B synthases. Org. Lett. 2011, 13, 2678–2681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guhling, O.; Yao, R.; Li, F.; Yeats, T.H.; Rose, J.K.; Jetter, R. Two oxidosqualene cyclases responsible for biosynthesis of tomato fruit cuticular triterpenoids. Plant. Physiol. 2011, 155, 540–552. [Google Scholar] [CrossRef]
- Souza-Moreira, T.M.; Alves, T.B.; Pinheiro, K.A.; Felippe, L.G.; De Lima, G.M.; Watanabe, T.F.; Barbosa, C.C.; Santos, V.A.; Lopes, N.P.; Valentini, S.R.; et al. Friedelin Synthase from Maytenus ilicifolia: Leucine 482 Plays an Essential Role in the Production of the Most Rearranged Pentacyclic Triterpene. Sci. Rep. 2016, 6, 36858. [Google Scholar] [CrossRef]
- Corey, E.J.; Matsuda, S.P.; Bartel, B. Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen. Proc. Natl. Acad. Sci. USA 1993, 90, 11628–11632. [Google Scholar] [CrossRef]
- Haralampidis, K.; Bryan, G.; Qi, X.; Papadopoulou, K.; Bakht, S.; Melton, R.; Osbourn, A. A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc. Natl. Acad. Sci. USA 2001, 98, 13431–13436. [Google Scholar] [CrossRef]
- Zhang, H.; Shibuya, M.; Yokota, S.; Ebizuka, Y. Oxidosqualene cyclases from cell suspension cultures of Betula platyphylla var. japonica: Molecular evolution of oxidosqualene cyclases in higher plants. Biol. Pharm. Bull. 2003, 26, 642–650. [Google Scholar] [CrossRef]
- Kawano, N.; Ichinose, K.; Ebizuka, Y. Molecular cloning and functional expression of cDNAs encoding oxidosqualene cyclases from Costus speciosus. Biol. Pharm. Bull. 2002, 25, 477–482. [Google Scholar] [CrossRef]
- Herrera, J.B.; Bartel, B.; Wilson, W.K.; Matsuda, S.P. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry 1998, 49, 1905–1911. [Google Scholar] [CrossRef]
- Basyuni, M.; Oku, H.; Tsujimoto, E.; Kinjo, K.; Baba, S.; Takara, K. Triterpene synthases from the Okinawan mangrove tribe, Rhizophoraceae. Febs J. 2007, 274, 5028–5042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yeats, T.; Han, H.; Jetter, R. Cloning and characterization of oxidosqualene cyclases from Kalanchoe daigremontiana: Enzymes catalyzing up to 10 rearrangement steps yielding friedelin and other triterpenoids. J. Biol. Chem. 2010, 285, 29703–29712. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Shindo, T.; Sato, S.; Kaneko, T.; Tabata, S.; Ayabe, S.-i.; Aoki, T. Functional and structural analysis of genes encoding oxidosqualene cyclases of Lotus japonicus. Plant Sci. 2006, 170, 247–257. [Google Scholar] [CrossRef]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.F.; Larondelle, Y.; et al. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar] [CrossRef]
- Morita, M.; Shibuya, M.; Kushiro, T.; Masuda, K.; Ebizuka, Y. Molecular cloning and functional expression of triterpene synthases from pea (Pisum sativum) new alpha-amyrin-producing enzyme is a multifunctional triterpene synthase. Eur. J. Biochem. 2000, 267, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Katsube, Y.; Otsuka, M.; Zhang, H.; Tansakul, P.; Xiang, T.; Ebizuka, Y. Identification of a product specific beta-amyrin synthase from Arabidopsis thaliana. Plant. Physiol. Biochem. 2009, 47, 26–30. [Google Scholar] [CrossRef]
- Hayashi, H.; Huang, P.; Kirakosyan, A.; Inoue, K.; Hiraoka, N.; Ikeshiro, Y.; Kushiro, T.; Shibuya, M.; Ebizuka, Y. Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosyntheses in licorice. Biol. Pharm. Bull. 2001, 24, 912–916. [Google Scholar] [CrossRef]
- Scholz, M.; Lipinski, M.; Leupold, M.; Luftmann, H.; Harig, L.; Ofir, R.; Fischer, R.; Prüfer, D.; Müller, K.J. Methyl jasmonate induced accumulation of kalopanaxsaponin I in Nigella sativa. Phytochemistry 2009, 70, 517–522. [Google Scholar] [CrossRef]
- Kajikawa, M.; Yamato, K.T.; Fukuzawa, H.; Sakai, Y.; Uchida, H.; Ohyama, K. Cloning and characterization of a cDNA encoding beta-amyrin synthase from petroleum plant Euphorbia tirucalli L. Phytochemistry 2005, 66, 1759–1766. [Google Scholar] [CrossRef]
- Cammareri, M.; Consiglio, M.F.; Pecchia, P.; Corea, G.; Lanzotti, V.; Ibeas, J.I.; Tava, A.; Conicella, C. Molecular characterization of β-amyrin synthase from Aster sedifolius L. and triterpenoid saponin analysis. Plant Sci. 2008, 175, 255–261. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, T.; Gao, L.; Su, P.; Zhang, Y.; Zhao, Y.; Chen, S.; Tu, L.; Song, Y.; Wang, X.; et al. Friedelane-type triterpene cyclase in celastrol biosynthesis from Tripterygium wilfordii and its application for triterpenes biosynthesis in yeast. New Phytol. 2019, 223, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.B.; Souza-Moreira, T.M.; Valentini, S.R.; Zanelli, C.F.; Furlan, M. Friedelin in Maytenus ilicifolia Is Produced by Friedelin Synthase Isoforms. Molecules 2018, 23, 700. [Google Scholar] [CrossRef]
- Han, J.Y.; Ahn, C.H.; Adhikari, P.B.; Kondeti, S.; Choi, Y.E. Functional characterization of an oxidosqualene cyclase (PdFRS) encoding a monofunctional friedelin synthase in Populus davidiana. Planta 2019, 249, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Luo, X.; Ye, G.; Chen, Y.; Ji, X.; Wen, L.; Xu, Y.; Xu, H.; Zhan, R.; Chen, W. Characterisation of two oxidosqualene cyclases responsible for triterpenoid biosynthesis in Ilex asprella. Int. J. Mol. Sci. 2015, 16, 3564–3578. [Google Scholar] [CrossRef] [PubMed]
- Hui-hua, L. Clone of Amyrin Synthase Gene Conservative District from Eriobotrya japonica ‘Jie Fang Zhong’. Subtrop. Plant Sci. 2013, 42, 1–4. [Google Scholar]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Sodhi, R.N.S. Chemical composition and microstructure of waxy plant surfaces: Triterpenoids and fatty acid derivatives on leaves of Kalanchoe daigremontiana. Surf. Interface Anal. 2011, 43, 326–330. [Google Scholar] [CrossRef]
- Buschhaus, C.; Herz, H.; Jetter, R. Chemical composition of the epicuticular and intracuticular wax layers on the adaxial side of Ligustrum vulgare leaves. New Phytol. 2007, 176, 311–316. [Google Scholar] [CrossRef]
- Markstädter, C.; Federle, W.; Jetter, R.; Riederer, M.; Hölldobler, B. Chemical composition of the slippery epicuticular wax blooms on Macaranga (Euphorbiaceae) ant-plants. Chemoecology 2000, 10, 33–40. [Google Scholar] [CrossRef]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Bell, C.D.; Soltis, P.S.; Soltis, D.E. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proc. Natl. Acad. Sci. USA 2007, 104, 19363–19368. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Jackson, C.J.; Warrilow, A.G.S.; Manning, N.J.; Kelly, D.E.; Kelly, S.L. Lanosterol Biosynthesis in the Prokaryote Methylococcus Capsulatus: Insight into the Evolution of Sterol Biosynthesis. Mol. Biol. Evol. 2007, 24, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Budin, M.; Brocks, J.J. Phylogenetic and biochemical evidence for sterol synthesis in the bacterium Gemmata obscuriglobus. Proc. Natl. Acad. Sci. USA 2003, 100, 15352–15357. [Google Scholar] [CrossRef]
- Nakano, C.; Motegi, A.; Sato, T.; Onodera, M.; Hoshino, T. Sterol biosynthesis by a prokaryote: First in vitro identification of the genes encoding squalene epoxidase and lanosterol synthase from Methylococcus capsulatus. Biosci. Biotechnol. Biochem. 2007, 71, 2543–2550. [Google Scholar] [CrossRef][Green Version]
- Xue, Z.; Duan, L.; Liu, D.; Guo, J.; Ge, S.; Dicks, J.; ÓMáille, P.; Osbourn, A.; Qi, X. Divergent evolution of oxidosqualene cyclases in plants. New Phytol. 2012, 193, 1022–1038. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Kovalchuk, O.; Kalck, V.; Boyko, V.; Filkowski, J.; Heinlein, M.; Hohn, B. Pathogen-induced systemic plant signal triggers DNA rearrangements. Nature 2003, 423, 760–762. [Google Scholar] [CrossRef]
- Leister, D. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance gene. Trends Genet. 2004, 20, 116–122. [Google Scholar] [CrossRef]
- Lucht, J.M.; Mauch-Mani, B.; Steiner, H.Y.; Metraux, J.P.; Ryals, J.; Hohn, B. Pathogen stress increases somatic recombination frequency in Arabidopsis. Nat. Genet. 2002, 30, 311–314. [Google Scholar] [CrossRef]
- Maere, S.; De Bodt, S.; Raes, J.; Casneuf, T.; Van Montagu, M.; Kuiper, M.; Van de Peer, Y. Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. USA 2005, 102, 5454–5459. [Google Scholar] [CrossRef]
- Michelmore, R.W.; Meyers, B.C. Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 1998, 8, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M.; Hammond-Kosack, K.E.; Golstein, C.; Thomas, C.M.; Jones, D.A.; Harrison, K.; Wulff, B.B.; Jones, J.D. Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell 1997, 91, 821–832. [Google Scholar] [CrossRef]
- Rizzon, C.; Ponger, L.; Gaut, B.S. Striking similarities in the genomic distribution of tandemly arrayed genes in Arabidopsis and rice. PLoS Comput. Biol. 2006, 2, e115. [Google Scholar] [CrossRef] [PubMed]
- Ober, D. Seeing double: Gene duplication and diversification in plant secondary metabolism. Trends Plant. Sci. 2005, 10, 444–449. [Google Scholar] [CrossRef]
- Siedenburg, G.; Jendrossek, D. Squalene-hopene cyclases. Appl. Environ. Microbiol. 2011, 77, 3905–3915. [Google Scholar] [CrossRef]
- Poralla, K. The possible role of a repetitive amino acid motif in evolution of triterpenoid cyclases. Bioorganic Med. Chem. Lett. 1994, 4, 285–290. [Google Scholar] [CrossRef]
- Racolta, S.; Juhl, P.B.; Sirim, D.; Pleiss, J. The triterpene cyclase protein family: A systematic analysis. Proteins 2012, 80, 2009–2019. [Google Scholar] [CrossRef]
- Kushiro, T.; Shibuya, M.; Masuda, K.; Ebizuka, Y. Mutational Studies on Triterpene Synthases: Engineering Lupeol Synthase into β-Amyrin Synthase. J. Am. Chem. Soc. 2000, 122, 6816–6824. [Google Scholar] [CrossRef]
- Ito, R.; Masukawa, Y.; Nakada, C.; Amari, K.; Nakano, C.; Hoshino, T. β-Amyrin synthase from Euphorbia tirucalli. Steric bulk, not the π-electrons of Phe, at position 474 has a key role in affording the correct folding of the substrate to complete the normal polycyclization cascade. Org. Biomol. Chem. 2014, 12, 3836–3846. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, F.; Xiong, W.; Molnár, I.; Liang, J.; Ji, A.; Wang, C.; Wang, S.; Liu, Z.; Wu, R.; et al. An Unexpected Oxidosqualene Cyclase Active Site Architecture in the Iris tectorum Multifunctional α-Amyrin Synthase. ACS Catal. 2020, 10, 9515–9520. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Zhang, X.; Shi, M.; Wang, B.; Wang, D.; Huang, L.; Zhang, X. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab. Eng. 2013, 20, 146–156. [Google Scholar] [CrossRef]
- Wei, W.; Wang, P.; Wei, Y.; Liu, Q.; Yang, C.; Zhao, G.; Yue, J.; Yan, X.; Zhou, Z. Characterization of Panax ginseng UDP-Glycosyltransferases Catalyzing Protopanaxatriol and Biosyntheses of Bioactive Ginsenosides F1 and Rh1 in Metabolically Engineered Yeasts. Mol. Plant. 2015, 8, 1412–1424. [Google Scholar] [CrossRef]
- Yan, X.; Fan, Y.; Wei, W.; Wang, P.; Liu, Q.; Wei, Y.; Zhang, L.; Zhao, G.; Yue, J.; Zhou, Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014, 24, 770–773. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing aglycons of ginsenosides in bakers’ yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar] [CrossRef]
- Kirby, J.; Romanini, D.W.; Paradise, E.M.; Keasling, J.D. Engineering triterpene production in Saccharomyces cerevisiae-beta-amyrin synthase from Artemisia annua. FEBS J. 2008, 275, 1852–1859. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, S.; Gao, X.; Li, M.; Li, D.; Lu, W. Enhanced protopanaxadiol production from xylose by engineered Yarrowia lipolytica. Microb. Cell Fact. 2019, 18, 83. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.; Nan, W.; Li, D.; Ke, D.; Lu, W. Glycerol improves heterologous biosynthesis of betulinic acid in engineered Yarrowia lipolytica. Chem. Eng. Sci. 2019, 196, 82–90. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, S.; Zhang, X. Antisense Suppression of Cycloartenol Synthase Results in Elevated Ginsenoside Levels in Panax ginseng Hairy Roots. Plant Mol. Biol. Rep. 2009, 27, 298–304. [Google Scholar] [CrossRef]
- Kim, J.E.; Jang, I.S.; Son, S.H.; Ko, Y.J.; Cho, B.K.; Kim, S.C.; Lee, J.Y. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab. Eng. 2019, 56, 50–59. [Google Scholar] [CrossRef]
- Yu, Y.; Chang, P.; Yu, H.; Ren, H.; Hong, D.; Li, Z.; Wang, Y.; Song, H.; Huo, Y.; Li, C. Productive Amyrin Synthases for Efficient alpha-Amyrin Synthesis in Engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2391–2402. [Google Scholar] [CrossRef]
- Yu, Y.; Rasool, A.; Liu, H.; Lv, B.; Chang, P.; Song, H.; Wang, Y.; Li, C. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab. Eng. 2020, 62, 72–83. [Google Scholar] [CrossRef]
- Moses, T.; Pollier, J.; Almagro, L.; Buyst, D.; Montagu, M.V.; Pedreño, M.A.; Martins, J.C.; Thevelein, J.M.; Goossens, A. Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α; hydroxylase from Bupleurum falcatum. Proc. Natl. Acad. Sci. USA 2014, 111, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Field, B.; Fiston-Lavier, A.-S.; Kemen, A.; Geisler, K.; Quesneville, H.; Osbourn, A.E. Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proc. Natl. Acad. Sci. USA 2011, 108, 16116–16121. [Google Scholar] [CrossRef] [PubMed]
- Field, B.; Osbourn, A.E. Metabolic diversification--independent assembly of operon-like gene clusters in different plants. Science 2008, 320, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).