Changes in Chemical Composition, Crystallizability, and Microstructure of Decayed Wood-Fiber-Mat-Reinforced Composite Treated with Copper Triazole Preservative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WFMRCs
2.3. Fungal Durability Experiment
2.4. Chemical Analysis
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6. X-ray Diffraction (XRD) Analysis
2.7. Scanning Electron Microscopy (SEM) Analysis
2.8. Statistical Analysis
3. Results and Discussion
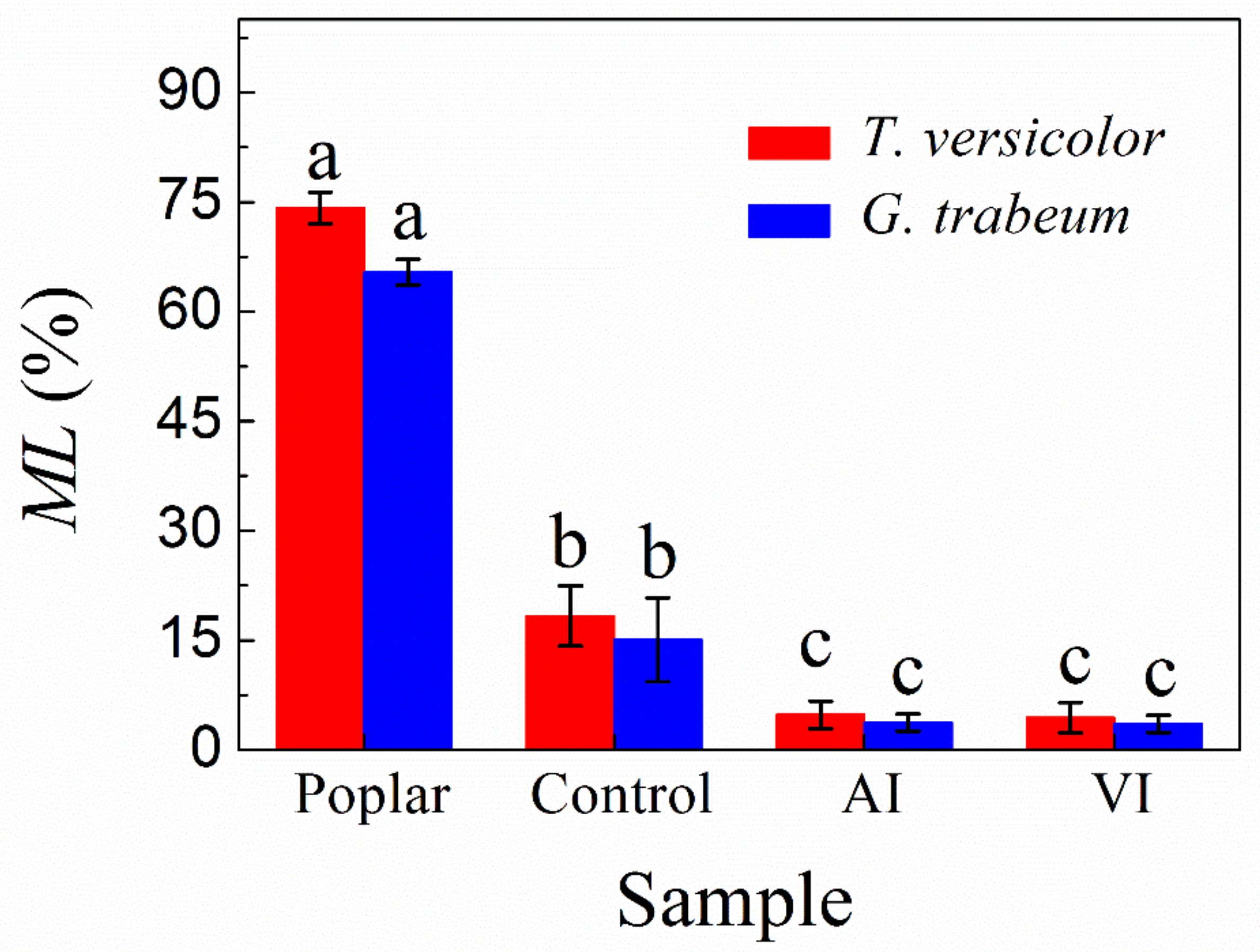
3.1. Mass Loss Analysis
3.2. Chemical Analysis
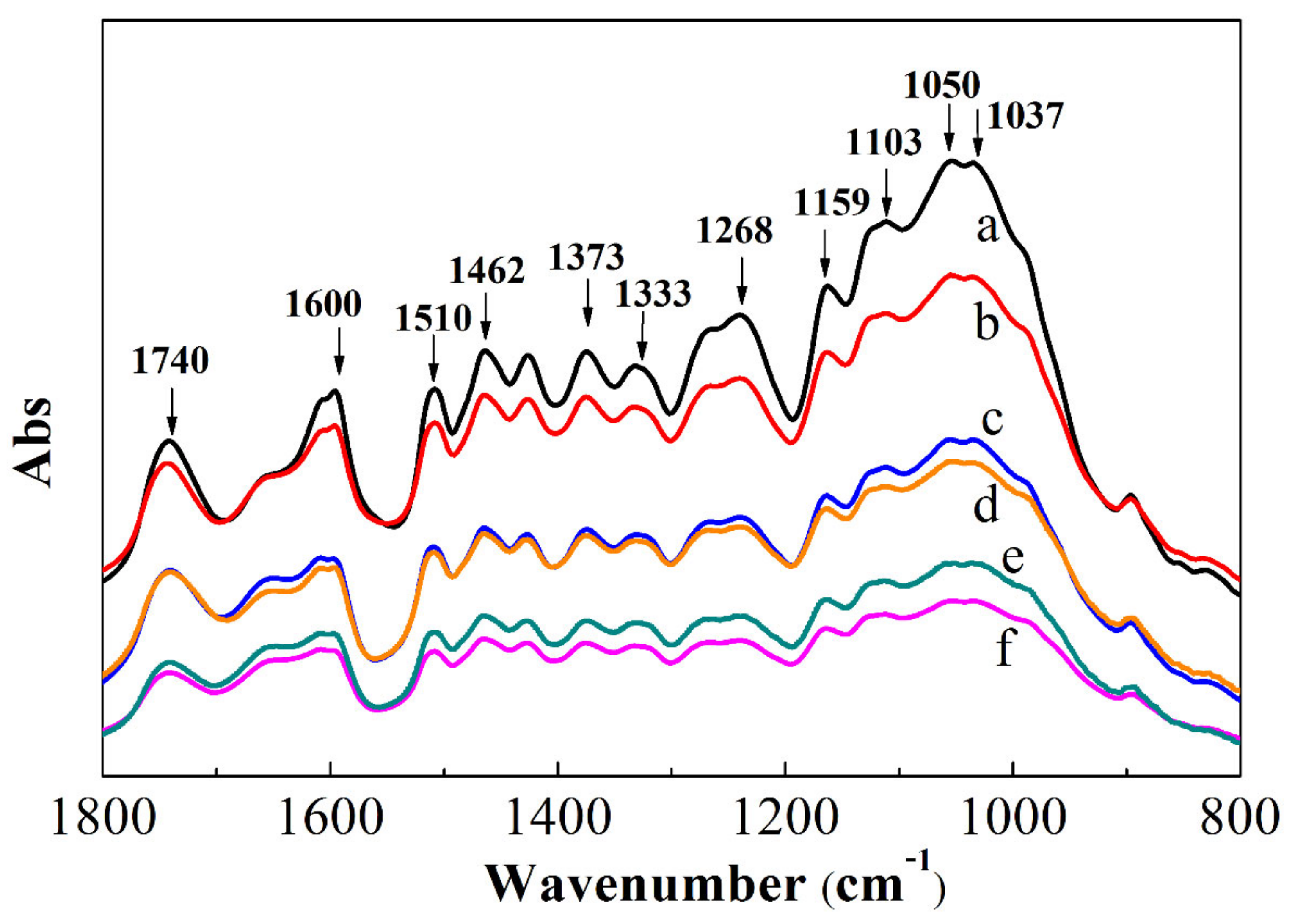
3.3. FTIR Test
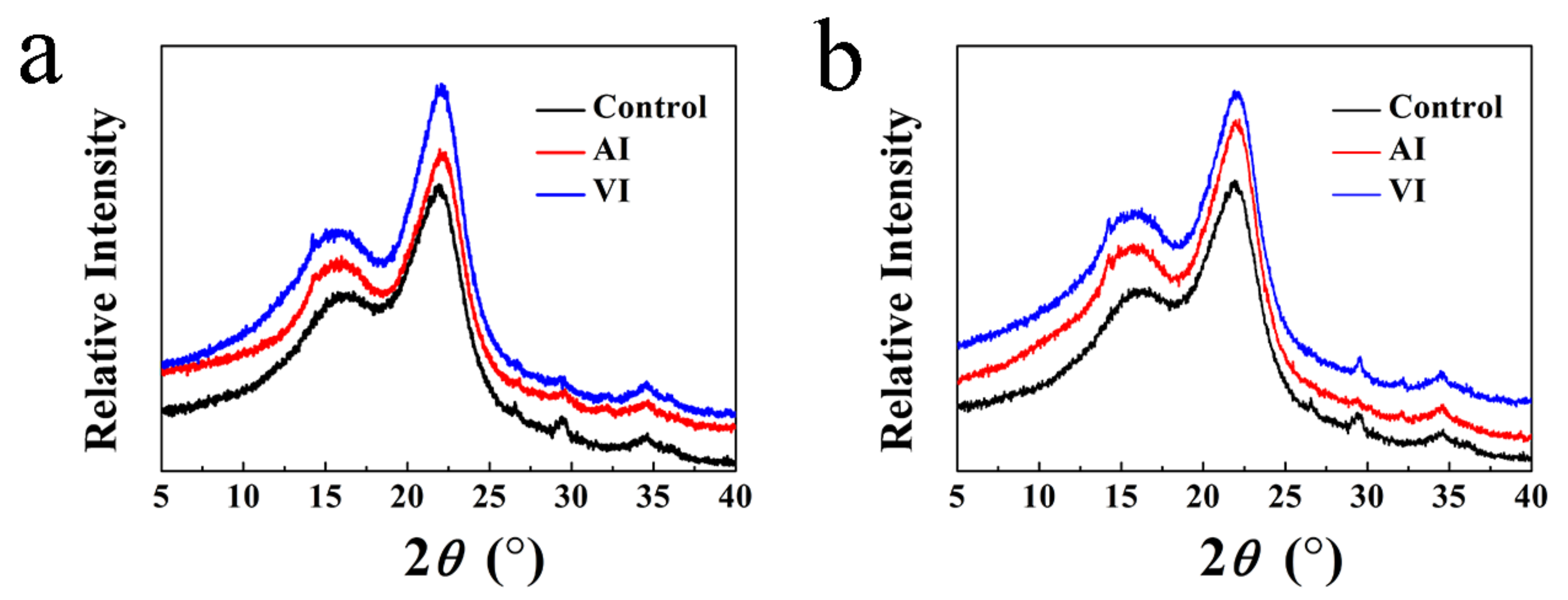
3.4. XRD Test
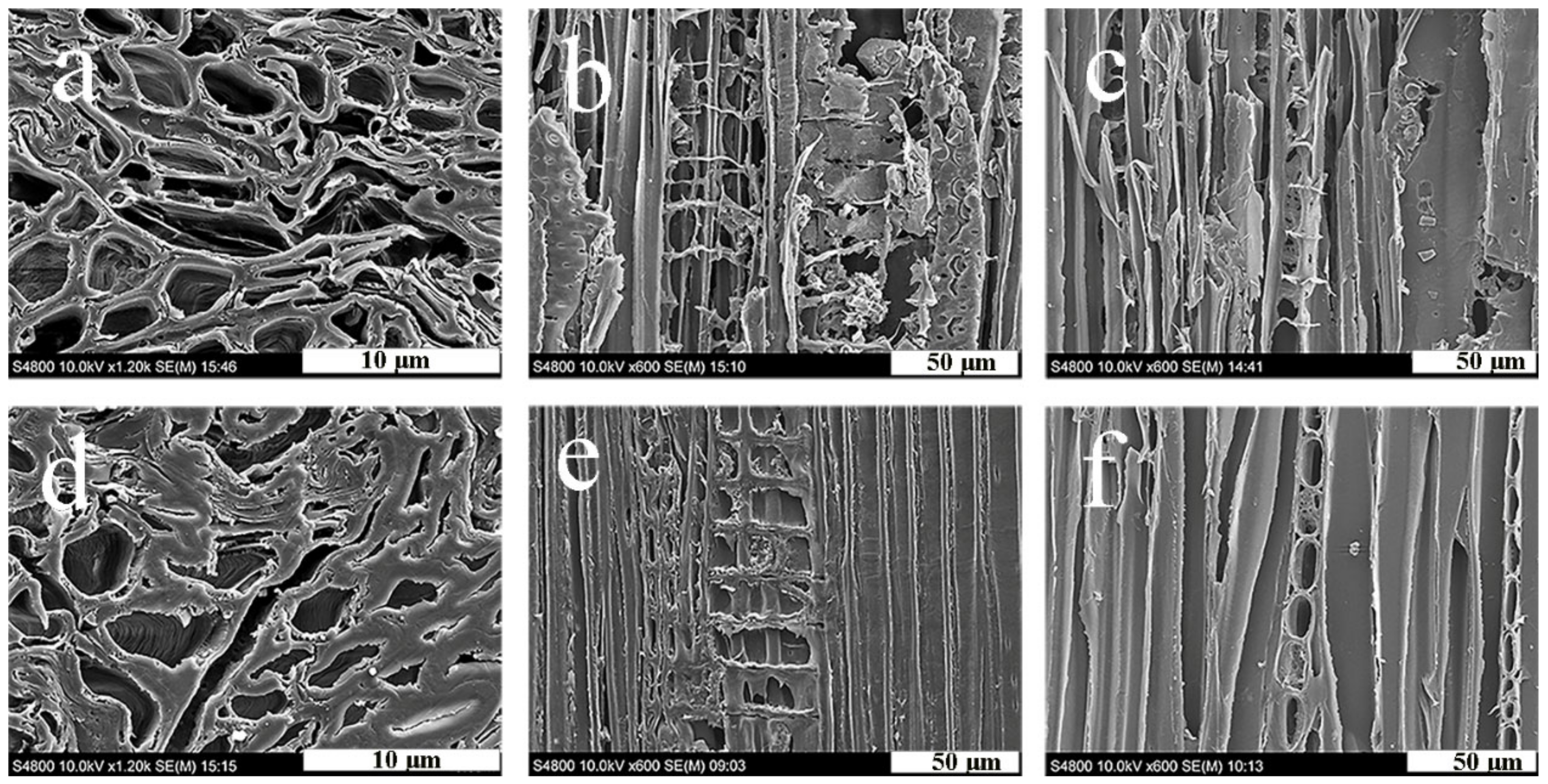
3.5. Microscope Observation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.H.; Qi, Y.; Huang, Y.X.; Yu, Y.L.; Liang, Y.J.; Yu, W.J. Influence of veneer thickness, mat formation and resin content on some properties of novel poplar scrimbers. Holzforschung 2018, 72, 673–680. [Google Scholar]
- Bao, M.Z.; Li, N.; Huang, C.J.; Chen, Y.H.; Yu, W.J.; Yu, Y.L. Fabrication, physical–mechanical properties and morphological characterizations of novel scrimber composite. Eur. J. Wood Wood Prod. 2019, 77, 741–747. [Google Scholar]
- Meyer-Veltrup, L.; Brischke, C.; Alfredsen, G.; Humar, M.; Flæte, P.-O.; Isaksson, T.; Brelid, P.L.; Westin, M.; Jermer, J. The combined effect of wetting ability and durability on outdoor performance of wood: Development and verification of a new prediction approach. Wood Sci. Technol. 2017, 51, 615–637. [Google Scholar]
- Yang, Z.; Jiang, Z.H.; Hse, C.Y.; Liu, R. Assessing the impact of wood decay fungi on the modulus of elasticity of slash pine (Pinus elliottii) by stress wave non-destructive testing. Int. Biodeterior. Biodegrad. 2017, 117, 123–127. [Google Scholar]
- Bao, M.Z.; Rao, F.; He, S.; Bao, Y.J.; Wu, Z.X.; Li, N.; Chen, Y.H. A note on the surface deterioration of scrimber composites exposed to artificial ageing. Coatings 2019, 9, 846. [Google Scholar]
- Kumar, A.; Ryparová, P.; Škapin, A.S.; Humar, M.; Pavlič, M.; Tywoniak, J.; Hajek, P.; Žigon, J.; Petrič, M. Influence of surface modification of wood with octadecyltrichlorosilane on its dimensional stability and resistance against Coniophora puteana and molds. Cellulose 2016, 23, 3249–3263. [Google Scholar]
- Witomski, P.; Olek, W.; Bonarski, J.T. Changes in strength of Scots pine wood (Pinus silvestris L.) decayed by brown rot (Coniophora puteana) and white rot (Trametes versicolor). Constr. Build. Mater. 2016, 102, 162–166. [Google Scholar]
- Kim, J.H.; Sutley, E.J.; Martin, F. Review of modern wood fungal decay research for implementation into a building standard of practice. J. Mater. Civ. Eng. 2019, 31, 03119005. [Google Scholar]
- Sun, F.L.; Bao, B.F.; Ma, L.F.; Chen, A.L.; Duan, X.F. Mould-resistance of bamboo treated with the compound of chitosan-copper complex and organic fungicides. J. Wood Sci. 2012, 58, 51–56. [Google Scholar]
- Mohajerani, A.; Vajna, J.; Ellcock, R. Chromated copper arsenate timber: A review of products, leachate studies and recycling. J. Clean. Prod. 2018, 179, 292–307. [Google Scholar]
- Morais, S.; Fonseca, H.M.; Oliveira, S.M.; Oliveira, H.; Gupta, V.K.; Sharma, B.; de Lourdes Pereira, M. Environmental and health hazards of chromated copper arsenate-treated wood: A review. Int. J. Environ. Res. Public Health 2021, 18, 5518. [Google Scholar] [PubMed]
- Freeman, M.H.; McIntyre, C.R. A comprehensive review of copper-based wood preservatives. For. Prod. J. 2008, 58, 6–27. [Google Scholar]
- Liu, M.; Zhong, H.; Ma, E.N.; Liu, R. Resistance to fungal decay of paraffin wax emulsion/copper azole compound system treated wood. Int. Biodeterior. Biodegrad. 2018, 129, 61–66. [Google Scholar]
- Karunasekera, H.; Terziev, N.; Daniel, G. Does copper tolerance provide a competitive advantage for degrading copper treated wood by soft rot fungi? Int. Biodeterior. Biodegrad. 2017, 117, 105–114. [Google Scholar]
- Jiang, H.H.; Kamdem, D.P. Differential scanning calorimetry characterization of the cure of phenol-formaldehyde adhesive in the presence of copper-based preservative treated wood. Wood Sci. Technol. 2007, 41, 637–644. [Google Scholar]
- Lorenz, L.F.; Frihart, C. Adhesive bonding of wood treated with ACQ and copper azole preservatives. For. Prod. J. 2006, 56, 90–93. [Google Scholar]
- Miyazaki, J.; Nakano, T.; Hirabayashi, Y.; Kishino, M. Effect of wood preservatives on adhesive properties of phenol-resorcinol-formaldehyde resin. Mokuzai Gakkaishi 1999, 45, 34–41. (In Japanese) [Google Scholar]
- GB/T 27651-2011; Use Category and Specification for Preservative-Treated Wood. Standard Administration of China: Beijing, China, 2011.
- GB/T 13942.1-2009; Durability of Wood-Part 1: Method for Laboratory Test of Natural Decay Resistance. Standard Administration of China: Beijing, China, 2009.
- TAAPPI T 222 om-98; Standard Methods for Acid-Insoluble Lignin in Wood and Pulp. Technical Association of Pulp and Paper Industry: Atlanta, GA, USA, 1998.
- TAAPPI T 249-75; Carbohydrate Composition of Extractive-Free Wood and Wood Pulp by Gaseliquid Chromatography. Technical Association of Pulp and Paper Industry: Atlanta, GA, USA, 1989.
- TAAPPI T 203 om-93; Standard Methods for Alpha-, Beta- and Gamma-Cellulose in Pulp and Wood. Technical Association of Pulp and Paper Industry: Atlanta, GA, USA, 1988.
- Loh, Y.F.; Paridah, T.M.; Hoong, Y.B.; Bakar, E.S.; Anis, M.; Hamdan, H. Resistance of phenolic-treated oil palm stem plywood against subterranean termites and white rot decay. Int. Biodeterior. Biodegrad. 2011, 65, 14–17. [Google Scholar]
- Freitag, C.; Kamke, F.A.; Morrell, J.J. Resistance of resin-impregnated VTC processed hybrid-poplar to fungal attack. Int. Biodeterior. Biodegrad. 2015, 99, 174–176. [Google Scholar]
- Kartal, S.N.; Ayrilmis, N.; Imamura, Y. Decay and termite resistance of plywood treated with various fire retardants. Build. Environ. 2007, 42, 1207–1211. [Google Scholar]
- Kumar, A.; Ryparovà, P.; Kasal, B.; Adamopoulos, S.; Hajek, P. Resistance of bamboo scrimber against white-rot and brown-rot fungi. Wood Mater. Sci. Eng. 2020, 15, 57–63. [Google Scholar]
- Meng, F.D.; Liu, R.; Zhang, Y.H.; Huang, Y.X.; Yu, Y.L.; Yu, W.J. Improvement of the water repellency, dimensional stability, and biological resistance of bamboo–based fiber reinforced composites. Polym. Compos. 2019, 40, 506–513. [Google Scholar]
- Green III, F.; Highley, T.L. Mechanism of brown-rot decay: Paradigm or paradox. Int. Biodeterior. Biodegrad. 1997, 39, 113–124. [Google Scholar]
- Kirk, T.K.; Highley, T.L. Quantitative changes in structural components of conifer woods during decay by white-and brown-rot fungi. Phytopathology 1973, 63, 1338–1342. [Google Scholar]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar]
- Adaskaveg, J.; Blanchette, R.; Gilbertson, R. Decay of date palm wood by white-rot and brown-rot fungi. Can. J. Bot. 1991, 69, 615–629. [Google Scholar]
- Lekounougou, S.; Kocaefe, D. Comparative study on the durability of heat-treated White Birch (Betula papyrifera) subjected to the attack of brown and white rot fungi. Wood Mater. Sci. Eng. 2012, 7, 101–106. [Google Scholar]
- Jin, J.W.; Wang, J.T.; Qin, D.C.; Luo, N.; Niu, H.J. Effects of veneer treatment with copper-based preservatives on the decay resistance and mechanical properties of poplar LVL. J. Wood Chem. Technol. 2016, 36, 329–338. [Google Scholar]
- Green Iii, F.; Clausen, C.A. Copper tolerance of brown-rot fungi: Time course of oxalic acid production. Int. Biodeterior. Biodegrad. 2003, 51, 145–149. [Google Scholar]
- Young, G.Y. Copper Tolerance of Some Wood-Rotting Fungi; Forest Service, Forest Products Laboratory, USDA: Madison, WI, USA, 1961. [Google Scholar]
- Humar, M.; Lesar, B. Fungicidal properties of individual components of copper–ethanolamine-based wood preservatives. Int. Biodeterior. Biodegrad. 2008, 62, 46–50. [Google Scholar]
- Kartal, S.N.; Terzi, E.; Yılmaz, H.; Goodell, B. Bioremediation and decay of wood treated with ACQ, micronized ACQ, nano-CuO and CCA wood preservatives. Int. Biodeterior. Biodegrad. 2015, 99, 95–101. [Google Scholar] [CrossRef]
- Woo, C.; Daniels, B.; Stirling, R.; Morris, P. Tebuconazole and propiconazole tolerance and possible degradation by Basidiomycetes: A wood-based bioassay. Int. Biodeterior. Biodegrad. 2010, 64, 403–408. [Google Scholar] [CrossRef]
- Palanti, S.; Susco, D. A new wood preservative based on heated oil treatment combined with triazole fungicides developed for above-ground conditions. Int. Biodeterior. Biodegrad. 2004, 54, 337–342. [Google Scholar] [CrossRef]
- Zabel, R.A.; Morrell, J.J. Wood Microbiology: Decay and Its Prevention; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Kubicek, C.P. Fungi and Lignocellulosic Biomass; John Wiley & Sons: New Delhi, India, 2012. [Google Scholar]
- Schmidt, O. Wood and Tree Fungi: Biology, Damage, Protection, and Use; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cowling, E.B. Comparative Biochemistry of the Decay of Sweetgum Sapwood by White-Rot and Brown-Rot Fungi; US Department of Agriculture: Washington, DC, USA, 1961. [Google Scholar]
- Liese, W. Ultrastructural aspects of woody tissue disintegration. Ann. Rev. Phytopathol. 1970, 8, 231–258. [Google Scholar] [CrossRef]
- Anagnost, S.E. Light microscopic diagnosis of wood decay. Iawa J. 1998, 19, 141–167. [Google Scholar] [CrossRef]
- Winandy, J.E.; Morrell, J.J. Relationship between incipient decay, strength, and chemical composition of Douglas-fir heartwood. Wood Fiber Sci. 1993, 25, 278–288. [Google Scholar]
- Bouslimi, B.; Koubaa, A.; Bergeron, Y. Effects of biodegradation by brown-rot decay on selected wood properties in eastern white cedar (Thuja occidentalis L.). Int. Biodeterior. Biodegrad. 2014, 87, 87–98. [Google Scholar] [CrossRef]
- Mohebby, B. Attenuated total reflection infrared spectroscopy of white-rot decayed beech wood. Int. Biodeterior. Biodegrad. 2005, 55, 247–251. [Google Scholar] [CrossRef]
- Pandey, K.; Pitman, A. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Pandey, K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Zhang, J.; Kamdem, D.P. Interaction of copper-amine with southern pine: Retention and migration. Wood Fiber Sci. 2000, 32, 332–339. [Google Scholar]
- Ruddick, J. Basic copper wood preservatives, preservative depletion: Factors which influence loss. Proc. Can. Wood Preserv. Assoc. 2003, 24, 26–59. [Google Scholar]
- Jiang, X. Fixation Chemistry of Amine-Copper Preservatives; National Library of Canada: Ottawa, ON, Canada, 2002. [Google Scholar]
- Cooper, P.A. Diffusion of copper in wood cell walls following vacuum treatment. Wood Fiber Sci. 1998, 30, 382–395. [Google Scholar]
- Jin, L.; Archer, K. Copper based wood preservatives: Observations on fixation, distribution and performance. Proc. Annu. Meet.-Am. Wood-Preserv Assoc. 1991, 87, 169–183. [Google Scholar]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Howell, C.; Steenkjær Hastrup, A.C.; Goodell, B.; Jellison, J. Temporal changes in wood crystalline cellulose during degradation by brown rot fungi. Int. Biodeterior. Biodegrad. 2009, 63, 414–419. [Google Scholar] [CrossRef]
- Bari, E.; Nazarnezhad, N.; Kazemi, S.M.; Tajick Ghanbary, M.A.; Mohebby, B.; Schmidt, O.; Clausen, C.A. Comparison between degradation capabilities of the white rot fungi Pleurotus ostreatus and Trametes versicolor in beech wood. Int. Biodeterior. Biodegrad. 2015, 104, 231–237. [Google Scholar] [CrossRef]
- Darwish, S.S.; EL Hadidi, N.; Mansour, M. The effect of fungal decay on ficus sycomorus wood. Int. J. Conserv. Sci. 2013, 4, 271–282. [Google Scholar]
- Chi, Y.J. FTIR analysis on function groups of david poplar wood and lignin degraded by 6 species of wood white--rot fungi. Sci. Silvae Sin. 2005, 41, 136–140. [Google Scholar]
- Schubert, M.; Volkmer, T.; Lehringer, C.; Schwarze, F.W.M.R. Resistance of bioincised wood treated with wood preservatives to blue-stain and wood-decay fungi. Int. Biodeterior. Biodegrad. 2011, 65, 108–115. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Hou, B.Y.; Ma, E. Decay of populus cathay treated with paraffin wax emulsion and copper azole compound. J. Korean Wood Sci. Technol. 2019, 47, 21–32. [Google Scholar] [CrossRef]


| Samples | Fungus | Holocellulose (%) | α–Cellulose (%) | Acid–Insoluble Lignin (%) |
|---|---|---|---|---|
| control | — | 64.19 (0.13) b | 38.61 (0.14) b | 28.12 (0.11) b |
| AI | — | 64.56 (0.21) c | 38.44 (0.32) a | 27.51 (0.19) a |
| VI | — | 63.35 (0.17) a | 38.33 (0.21) a | 27.37 (0.23) a |
| control | T. versicolor | 61.51 (0.08) b | 37.85 (0.21) b | 32.63 (0.31) b |
| AI | 61.85 (0.16) c | 37.84 (0.06) b | 32.34 (0.25) a | |
| VI | 60.84 (0.13) a | 37.03 (0.24) a | 33.69 (0.22) c | |
| control | G. trabeum | 61.68 (0.11) b | 38.12 (0.15) b | 31.18 (0.31) a |
| AI | 61.36 (0.13) a | 38.01 (0.19) b | 31.60 (0.26) b | |
| VI | 60.32 (0.22) a | 36.80 (0.05) a | 32.93 (0.21) b |
| Samples | Fungus | Cr (%) |
|---|---|---|
| Control | — | 21.31 |
| AI | — | 22.07 |
| VI | — | 22.81 |
| Control | T. versicolor | 16.64 |
| AI | 18.26 | |
| VI | 18.87 | |
| Control | G. trabeum | 17.12 |
| AI | 18.98 | |
| VI | 19.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, M.; Tang, R.; Bao, Y.; He, S.; Chen, Y.; Li, N. Changes in Chemical Composition, Crystallizability, and Microstructure of Decayed Wood-Fiber-Mat-Reinforced Composite Treated with Copper Triazole Preservative. Forests 2022, 13, 1387. https://doi.org/10.3390/f13091387
Bao M, Tang R, Bao Y, He S, Chen Y, Li N. Changes in Chemical Composition, Crystallizability, and Microstructure of Decayed Wood-Fiber-Mat-Reinforced Composite Treated with Copper Triazole Preservative. Forests. 2022; 13(9):1387. https://doi.org/10.3390/f13091387
Chicago/Turabian StyleBao, Minzhen, Rongqiang Tang, Yongjie Bao, Sheng He, Yuhe Chen, and Neng Li. 2022. "Changes in Chemical Composition, Crystallizability, and Microstructure of Decayed Wood-Fiber-Mat-Reinforced Composite Treated with Copper Triazole Preservative" Forests 13, no. 9: 1387. https://doi.org/10.3390/f13091387





