A Comparative Analysis of Polyphenol Content and Biological Potential of Quercus petraea Matt. and Q. pubescens Willd. Bark Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Bacterial Strains
2.2. Plant Sample
2.3. Extraction
2.4. Quantification of Total Phenolics and Tannins
2.5. In Vitro Antioxidant Assays
2.6. Antimicrobial Activity Assay
2.7. Evaluation of Cytotoxicity
2.8. Antidiabetic (Glucosidase Inhibitory) Assay
2.9. Tyrosinase Inhibitory Activity
2.10. Acetylcholinesterase-Inhibitory Activity
2.11. Statistical Analysis
3. Results and Discussions
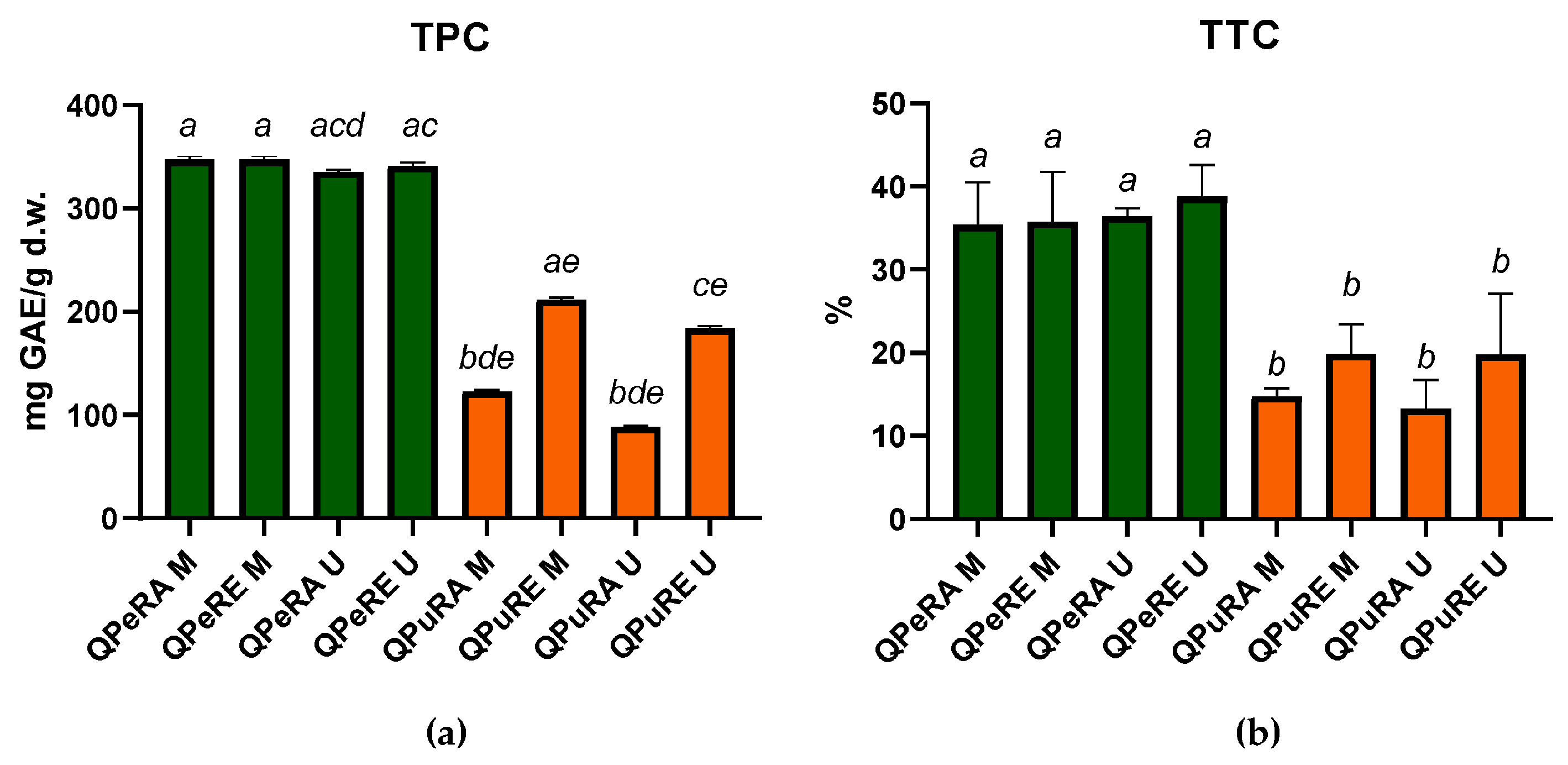
3.1. Total Phenolic Content (TPC)
3.2. Total Tannin Content (TTC)
3.3. Antioxidant Capacity
3.4. Antibacterial and Antifungal Activity
3.5. Evaluation of Cytotoxicity
3.6. Enzymatic Inhibition
3.6.1. α-Glucosidase Inhibition
3.6.2. Acetylcholinesterase Inhibition
3.6.3. Tyrosinase Inhibition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild Crop Relatives: Genomic and Breeding Resources; Kole, C. (Ed.) Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-21249-9. [Google Scholar]
- Alejano, R.; Vázquez-Piqué, J.; Domingo-Santos, J.; Fernández, M.; Andivia, E.; Martin, D.; Peérez-Carral, C.; González-Pérez, M.A. Dehesas: Open Woodland Forests of Quercus in Southwestern Spain. Ed. Nova Science Publishers: New York, NY, USA, 2011; ISBN 978-1-61942-493-7. [Google Scholar]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Morales, D. Oak Trees (Quercus spp.) as a Source of Extracts with Biological Activities: A Narrative Review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Panchal, S.K.; Brown, L. Cardioprotective and Hepatoprotective Effects of Ellagitannins from European Oak Bark (Quercus petraea L.) Extract in Rats. Eur. J. Nutr. 2013, 52, 397–408. [Google Scholar] [CrossRef]
- Lorenz, P.; Heinrich, M.; Garcia-Käufer, M.; Grunewald, F.; Messerschmidt, S.; Herrick, A.; Gruber, K.; Beckmann, C.; Knoedler, M.; Huber, R.; et al. Constituents from Oak Bark (Quercus robur L.) Inhibit Degranulation and Allergic Mediator Release from Basophils and Mast Cells in Vitro. J. Ethnopharmacol. 2016, 194, 642–650. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sabuncuoğlu, S.; Harput, Ü.Ş. Evaluation of Antioxidative, Protective Effect against H2O2 Induced Cytotoxicity, and Cytotoxic Activities of Three Different Quercus Species. Food Chem. Toxicol. 2012, 50, 141–146. [Google Scholar] [CrossRef]
- Sari, S.; Barut, B.; Özel, A.; Kuruüzüm-Uz, A.; Şöhretoğlu, D. Tyrosinase and α-Glucosidase Inhibitory Potential of Compounds Isolated from Quercus coccifera Bark: In Vitro and in Silico Perspectives. Bioorganic Chem. 2019, 86, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Khouzami, L.; Mroueh, M.; Daher, C. The Role of Methanolic Extract of Quercus infectoria Bark in Lipemia, Glycemia, Gastric Ulcer and Bacterial Growth. J. Med. Plants Res. 2009, 2, 224–230. [Google Scholar]
- Sharifi, N.; Souri, E.; Ziai, S.A.; Amin, G.; Amanlou, M. Discovery of New Angiotensin Converting Enzyme (ACE) Inhibitors from Medicinal Plants to Treat Hypertension Using an in Vitro Assay. DARU J. Pharm. Sci. 2013, 21, 74. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Avilés, E.; Martínez-Flores, H.; García-Pérez, M.; Meléndez-Herrera, E.; García-Pérez, M. Investigation of the Antibacterial Activity and Subacute Toxicity of a Quercus crassifolia Polyphenolic Bark Extract for Its Potential Use in Functional Foods. J. Food Sci. 2019, 84, 1692–1702. [Google Scholar] [CrossRef]
- Elansary, H.; Szopa, A.; Kubica, P.; Ekiert, H.A.; Mattar, M.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, M.; Orszaghova, Z.; Laubertova, L.; Vavakova, M.; Sabaka, P.; Rohdewald, P.; Durackova, Z.; Muchova, J. Effect of the French Oak Wood Extract Robuvit on Markers of Oxidative Stress and Activity of Antioxidant Enzymes in Healthy Volunteers: A Pilot Study. Oxid. Med. Cell. Longev. 2014, 2014, e639868. [Google Scholar] [CrossRef] [Green Version]
- Natella, F.; Leoni, G.; Maldini, M.; Natarelli, L.; Comitato, R.; Schonlau, F.; Virgili, F.; Canali, R. Absorption, Metabolism, and Effects at Transcriptome Level of a Standardized French Oak Wood Extract, Robuvit, in Healthy Volunteers: Pilot Study. J. Agric. Food Chem. 2014, 62, 443–453. [Google Scholar] [CrossRef]
- Rosales-Castro, M.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Rivas-Arreola, M.J.; Karchesy, J.J. Antioxidant Activity of Fractions from Quercus sideroxyla Bark and Identification of Proanthocyanidins by HPLC-DAD and HPLC-MS. Holzforschung 2012, 66, 577–584. [Google Scholar] [CrossRef]
- Dróżdż, P.; Pyrzynska, K. Extracts from Pine and Oak Barks: Phenolics, Minerals and Antioxidant Potential. Int. J. Environ. Anal. Chem. 2021, 101, 464–472. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A. Phytochemical Screening, Antimicrobial and Antioxidant Activities of Aerial Parts of Quercus robur L. Middle-East J. Med. Plants Res. 2012, 1, 1–4. [Google Scholar]
- Dania, V.O.; Fadina, O.O.; Ayodele, M.; Kumar, P.L. Efficacy of Oryza sativa Husk and Quercus phillyraeoides Extracts for the in Vitro and in Vivo Control of Fungal Rot Disease of White Yam (Dioscorea rotundata Poir). SpringerPlus 2014, 3, 711. [Google Scholar] [CrossRef] [Green Version]
- Soto-García, M.; Rosales-Castro, M.; García-Sánchez, J.R.; Rivas-Arreola, M.J.; Soto-García, M.; Rosales-Castro, M.; García-Sánchez, J.R.; Rivas-Arreola, M.J. In Vitro Cytotoxic Activity of Bark Extracts from Pinus durangensis Martinez and Quercus sideroxyla Bonpl. Biotecnia 2022, 24, 55–61. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Extracts from Quercus sp. Acorns Exhibit in Vitro Neuroprotective Features through Inhibition of Cholinesterase and Protection of the Human Dopaminergic Cell Line SH-SY5Y from Hydrogen Peroxide-Induced Cytotoxicity. Ind. Crops Prod. 2013, 45, 114–120. [Google Scholar] [CrossRef]
- Gezici, S.; Sekeroglu, N. Neuroprotective Potential and Phytochemical Composition of Acorn Fruits. Ind. Crops Prod. 2019, 128, 13–17. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Moldovan, C.; Mocan, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Muntean, D.-L.; Tanase, C. Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Mocan, A.; Coșarcă, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.-M.; Vodnar, D.C.; Muntean, D.-L.; Crișan, O. Biological and Chemical Insights of Beech (Fagus sylvatica L.) Bark: A Source of Bioactive Compounds with Functional Properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Council of Europe; European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia 8.0: Published in Accordance with the Convention on the Elaboration of a European Pharmacopoeia; European Directorate for the Quality of Medicines & Healthcare, Council of Europe: Strasbourg, France, 2013; ISBN 978-92-871-7525-0. [Google Scholar]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Phytochemical Profile and Biological Effects of Spruce (Picea abies) Bark Subjected to Ultrasound Assisted and Microwave-Assisted Extractions. Plants 2021, 10, 870. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. ANTIBACTERIAL ACTIVITIES OF BEECH BARK (Fagus sylvatica L.) POLYPHENOLIC EXTRACT. Environ. Eng. Manag. J. 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia Officinalis L., S. Glutinosa L. and S. Transsylvanica (Schur Ex Griseb. & Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) Fruit European Cultivars at Different Maturation Steps: Extraction, HPLC-DAD Analyses, and Biological Evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Nicolescu, A.; Babotă, M.; Zhang, L.; Bunea, C.I.; Gavrilaș, L.; Vodnar, D.C.; Mocan, A.; Crișan, G.; Rocchetti, G. Optimized Ultrasound-Assisted Enzymatic Extraction of Phenolic Compounds from Rosa canina L. Pseudo-Fruits (Rosehip) and Their Biological Activity. Antioxidants 2022, 11, 1123. [Google Scholar] [CrossRef]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Ferreira, J.P.A.; Miranda, I.; Quilhó, T.; Pereira, H. Quercus rotundifolia Bark as a Source of Polar Extracts: Structural and Chemical Characterization. Forests 2021, 12, 1160. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Sousa, V.B.; Pereira, H. Chemical Composition of Barks from Quercus faginea Trees and Characterization of Their Lipophilic and Polar Extracts. PLOS ONE 2018, 13, e0197135. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Avilés, E.; García-Pérez, M.E.; Garnica-Romo, M.G.; Figueroa-Cárdenas, J.D.D.; Meléndez-Herrera, E.; Salgado-Garciglia, R.; Martínez-Flores, H.E. Antioxidant Properties of Polyphenolic Extracts from Quercus laurina, Quercus crassifolia, and Quercus scytophylla Bark. Antioxidants 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Dróżdż, P.; Pyrzynska, K. Assessment of Polyphenol Content and Antioxidant Activity of Oak Bark Extracts. Eur. J. Wood Wood Prod. 2018, 76, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Tuyen, P.T.; Khang, D.T.; Thu Ha, P.T.; Hai, T.N.; Elzaawely, A.A.; Xuan, T.D. Antioxidant Capacity and Phenolic Contents of Three Quercus Species. Int. Lett. Nat. Sci. 2016, 54, 85–99. [Google Scholar] [CrossRef]
- Berahou, A.; Auhmani, A.; Fdil, N.; Benharref, A.; Jana, M.; Gadhi, C.A. Antibacterial Activity of Quercus ilex Bark’s Extracts. J. Ethnopharmacol. 2007, 112, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. UHPLC-QToF-MS Characterization of Bioactive Metabolites from Quercus robur L. Grown in South Africa for Antioxidant and Antidiabetic Properties. Arab. J. Chem. 2021, 14, 102970. [Google Scholar] [CrossRef]
- Ferreira, J.; Santos, S.; Pereira, H. In Vitro Screening for Acetylcholinesterase Inhibition and Antioxidant Activity of Quercus suber Cork and Corkback Extracts. Evid. Based Complement. Alternat. Med. 2020, 2020, e3825629. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Lee, B.M. Inhibitory Effects of Plant Extracts on Tyrosinase, l-DOPA Oxidation, and Melanin Synthesis. J. Toxicol. Environ. Health A 2007, 70, 393–407. [Google Scholar] [CrossRef]

| Tested Bacteria | MIC/MBC (mg Freeze-Dried Extract/mL Solution) | ||||||
|---|---|---|---|---|---|---|---|
| qPeRA M | qPeRE M | qPeRA U | qPeRE U | qPuRA M | qPuRE M | qPuRA U | |
| Staphylococcus aureus ATCC 25923 | 0.3/2.5 | 0.3/0.3 | 0.3/1.25 | 0.156/0.3 | 2.5/5 | 0.6/2.5 | 5/>5 |
| Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300 | 0.6/0.6 | 0.3/0.3 | 0.3/0.3 | 0.3/0.3 | 2.5/2.5 | 0.6/1.25 | >5/>5 |
| Escherichia coli ATCC 25922 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 |
| Klebsiella pneumoniae ATCC 700603 | 0.3/0.3 | 0.3/1.25 | 0.3/0.6 | 0.3/0.3 | 1.25/1.25 | 0.3/0.3 | 5/5 |
| Pseudomonas aeruginosa ATCC 2753 | 2.5/5 | 1.25/1.25 | 0.6/2.5 | 1.25/>5 | >5/>5 | 2.5/2.5 | >5/>5 |
| Tested fungi | MIC/MFC (mg Freeze-Dried Extract/mL Solution) | ||||||
| Candida albicans ATCC 90028 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 |
| Candida parapsilosis ATCC 22019 | >5/>5 | 5/>5 | 5/>5 | >5/>5 | >5/>5 | 5/5 | >5/>5 |
| Candida krusei ATCC 6258 | 1.25/>5 | 1.25/>5 | 2.5/>5 | 5/>5 | >5/>5 | 2.5/>5 | 5/>5 |
| Tested Solution | Concentration of Tested Solution | Mean |
|---|---|---|
| qPuRE M | 6% | 3.67 ± 1.16 * |
| 3% | 57.67 ± 5.03 * | |
| 1.5% | 46.00 ± 11.79 * | |
| 0.25% | 41.33 ± 15.31 * | |
| qPeRE U | 6% | 0.00 ± 0.00 * |
| 3% | 0.33 ± 0.58 * | |
| 1.5% | 1.00 ± 1.00 * | |
| 0.25% | 56.00 ± 14.42 * | |
| Control | 150.00 ± 15.62 |
| Tested Solution | Concentration of Tested Solution | Mean |
|---|---|---|
| qPuRE M | 6% | 0.00 ± 0.00 * |
| 3% | 0.00 ± 0.00 * | |
| 1.5% | 1.67 ± 1.16 * | |
| 0.25% | 1.00 ± 1.00 * | |
| qPeRE U | 6% | 0.00 ± 0.00 * |
| 3% | 0.00 ± 0.00 * | |
| 1.5% | 0.67 ± 0.58 * | |
| 0.25% | 0.00 ± 0.00 * | |
| Control | 7.67 ± 3.12 |
| Tested Solution | IC50 (µg/mL) |
|---|---|
| qPeRA M | 6.1675 |
| qPeRE M | 3.9125 |
| qPeRA U | 12.0025 |
| qPeRE U | 3.385 |
| qPuRA M | 9.64 |
| qPuRE M | 6.7 |
| qPuRA U | 11.045 |
| qPuRE U | 9.5775 |
| Control (Acarbose) | 122.275 |
| Tested Solution | IC50 (µg/mL) |
|---|---|
| qPeRA M | 176.7 |
| qPeRE M | 55.51 |
| qPeRA U | 232.9 |
| qPeRE U | 110.9 |
| qPuRA M | 272.5 |
| qPuRE M | 165.4 |
| qPuRA U | 222.7 |
| qPuRE U | 109.0 |
| Control (Galantamine) | 0.000185 |
| Tested Solution | IC50 (µg/mL) |
|---|---|
| qPeRA M | 91.8 |
| qPeRE M | 94.54 |
| qPeRA U | - |
| qPeRE U | 85.48 |
| qPuRA M | 286.8 |
| qPuRE M | 130.16 |
| qPuRA U | - |
| qPuRE U | 97.06 |
| Control (Kojic acid) | 4.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisca, A.; Ștefănescu, R.; Mocan, A.; Babotă, M.; Nicolescu, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Tanase, C. A Comparative Analysis of Polyphenol Content and Biological Potential of Quercus petraea Matt. and Q. pubescens Willd. Bark Extracts. Forests 2023, 14, 116. https://doi.org/10.3390/f14010116
Nisca A, Ștefănescu R, Mocan A, Babotă M, Nicolescu A, Mare AD, Ciurea CN, Man A, Tanase C. A Comparative Analysis of Polyphenol Content and Biological Potential of Quercus petraea Matt. and Q. pubescens Willd. Bark Extracts. Forests. 2023; 14(1):116. https://doi.org/10.3390/f14010116
Chicago/Turabian StyleNisca, Adrian, Ruxandra Ștefănescu, Andrei Mocan, Mihai Babotă, Alexandru Nicolescu, Anca Delia Mare, Cristina Nicoleta Ciurea, Adrian Man, and Corneliu Tanase. 2023. "A Comparative Analysis of Polyphenol Content and Biological Potential of Quercus petraea Matt. and Q. pubescens Willd. Bark Extracts" Forests 14, no. 1: 116. https://doi.org/10.3390/f14010116
APA StyleNisca, A., Ștefănescu, R., Mocan, A., Babotă, M., Nicolescu, A., Mare, A. D., Ciurea, C. N., Man, A., & Tanase, C. (2023). A Comparative Analysis of Polyphenol Content and Biological Potential of Quercus petraea Matt. and Q. pubescens Willd. Bark Extracts. Forests, 14(1), 116. https://doi.org/10.3390/f14010116













