Responses to Solar UV-B Exclusion and Drought Stress in Two Cultivars of Chestnut Rose with Different Leaf Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of Plant Biomass, Leaf Thickness, and Palisade/Spongy Layer Ratio
2.3. Determinations of Membrane Permeability (MP), Free Proline, and Ascorbic Acid
2.4. Measurement of Total Nitrogen, Total Phosphorus and Mineral Concentration in Plants
2.5. Antioxidant Enzymes Activity
2.6. Quantitative Flavonoid Analysis
2.7. Statistical Analysis
3. Results
3.1. Leaf Properties and Total Biomass
3.2. Leaf Membrane Permeability, Proline and Ascorbic Acid Concentration
3.3. Flavonoid Compounds
3.4. Antioxidant Enzymes Activity
3.5. Plant Nutrition
4. Discussion
4.1. Complementary Effects of Antioxidant Property and Leaf Traits against UV-B and Drought Stress
4.2. Solar UV-B Radiation Primes Chestnut Rose Plants with Increased Antioxidative Capacity against Drought Stress
4.3. Complementary Effects of Solar UV-B Radiation and Drought on Nutrition Balance in Chestnut Rose Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benca, J.P.; Duijnstee, I.A.P.; Looy, C.V. UV-B-induced forest sterility, Implications of ozone shield failure in Earth’s largest extinction. Sci. Adv. 2018, 4, e1700618. [Google Scholar] [CrossRef] [PubMed]
- Wargent, J.J.; Jordan, B.R. From ozone depletion to agriculture: Understanding the role of UV radiation in sustainable crop production. New Phytol. 2013, 197, 1058–1076. [Google Scholar] [CrossRef] [PubMed]
- Derebe, A.D.; Roro, A.G.; Asfaw, B.T.; Ayele, W.W.; Hvoslef-Eide, A.K. Effects of solar UV-B radiation exclusion on physiology; growth and yields of taro (Colocasia esculenta (L.)) at different altitudes in tropical environments of Southern Ethiopia. Sci. Hortic. 2019, 256, 108563. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.; He, H. Influence of ambient and enhanced ultraviolet-B radiation on the plant growth and physiological properties in two contrasting populations of Hippophae rhamnoides. J. Plant Res. 2008, 121, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.; Rousseaux, M.C.; Searles, P.S.; Zaller, J.G.; Giordano, C.V.; Robson, T.M.; Caldwell, M.M.; Sala, O.E.; Scopel, A.L. Impacts of solar ultraviolet-B radiation on terrestrial ecosystems of Tierra del Fuego (southern Argentina). An overview of recent progress. J. Photochem. Photobiol. 2001, 62, 67–77. [Google Scholar]
- Searles, P.S.; Caldwell, M.M.; Winter, K. The response of five tropical plant species to solar ultraviolet-B radiation. Am. J. Bot. 1995, 82, 445–453. [Google Scholar] [CrossRef]
- Hunt, J.E.; McNeil, D.L. The influence of present-day levels of ultraviolet-B radiation on seedlings of two Southern Hemisphere temperate tree species. Plant Ecol. 1999, 143, 39–50. [Google Scholar] [CrossRef]
- Rozema, J.; Van de Staaij, J.; Björn, L.O.; Caldwell, M. UV-B as an environmental factor in plant life, stress and regulation. Trends Ecol. Evol. 1997, 12, 22–28. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D.; Fountain, D. Sensitivity of white clover to UV-B radiation depends on water availability, plant productivity and duration of stress. Glob. Chang. Biol. 2003, 9, 473–477. [Google Scholar] [CrossRef]
- Petropoulou, Y.; Kyparissis, A.; Nikolopoulos, D.; Manetas, Y. Enhanced UV-B radiation alleviates the adverse effects of summer drought in two Mediterranean pines under field conditions. Physiol. Plant. 1995, 94, 37–44. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Teramura, A.H. Field study of the interaction between solar ultraviolet-B radiation and drought on photosynthesis and growth in soybean. Plant Physiol. 1990, 92, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Láposi, R.; Veres, S.; Lakatos, G.; Oláh, V.; Fieldsend, A.; Mészáros, I. Responses of leaf traits of European beech (Fagus sylvatica L.) saplings to supplemental UV-B radiation and UV-B exclusion. Agric. For. Meteor. 2009, 149, 745–755. [Google Scholar] [CrossRef]
- Randriamanana, T.R.; Lavola, A.; Julkunen-Tiitto, R. Interactive effects of supplemental UV-B and temperature in European aspen seedlings, Implications for growth; leaf traits; phenolic defense and associated organisms. Plant Physiol. Biochem. 2015, 93, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, Y.; Tu, M.; Zhang, P.; Wang, X.; Wang, T.; Li, J. Response of Zebrina pendula leaves to enhanced UV-B radiation. Funct. Plant Biol. 2021, 48, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, Y.; Li, Y.; Stanley, L. Intraspecific responses of Fagopyrum esculentum to enhanced ultraviolet B radiation. Plant. Growth Regul. 2008, 56, 297–306. [Google Scholar] [CrossRef]
- Bernado, W.P.; Rakocevic, M.; Santos, A.R.; Ruas, K.F.; Baroni, D.F.; Abraham, A.C.; Pireda, S.; Oliveira, D.D.S.; Cunha, M.D.; Ramalho, J.C.; et al. Biomass and Leaf Acclimations to Ultraviolet Solar Radiation in Juvenile Plants of Coffea arabica and C. canephora. Plants 2021, 10, 640. [Google Scholar] [CrossRef]
- Weston, E.; Thorogood, K.; Vinti, G.; López-Juez, E. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 2000, 211, 807–815. [Google Scholar] [CrossRef]
- Bidel, L.P.; Chomicki, G.; Bonini, F.; Mondolot, L.; Soulé, J.; Coumans, M.; La Fisca, P.; Baissac, Y.; Petit, V.; Loiseau, A.; et al. Dynamics of flavonol accumulation in leaf tissues under different UV-B regimes in Centella asiatica (Apiaceae). Planta 2015, 242, 545–559. [Google Scholar] [CrossRef]
- Xu, C.; Natarajan, S.; Sullivan, J.H. Impact of solar ultraviolet-B radiation on the antioxidant defensesystem in soybean lines differing in flavonoid contents. Environ. Exp. Bot. 2008, 63, 39–48. [Google Scholar] [CrossRef]
- Xu, Q.; Wen, X.P.; Deng, X.X. Genomic organization; rapid evolution and meiotic instability of NBS-encoding genes in a new fruit crop “Chestnut rose”. Genetics 2008, 178, 2081–2091. [Google Scholar] [CrossRef]
- Yao, Y.; Xuan, Z.; Li, Y.; He, Y.; Korpelainen, H.; Li, C. Effects of ultraviolet-B radiation on crop growth, development, yield and leaf pigment concentration of tartary buckwheat (Fagopyrum tataricum) under field conditions. Europ. J. Agron. 2006, 25, 215–222. [Google Scholar] [CrossRef]
- ASTM D1003–D1013; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM International: West Conshohocken, PA, USA, 2013. Available online: www.astm.org (accessed on 7 June 2021).
- Alvarez-Arenas, T.E.G.; Sancho-Knapik, D.; Peguero-Pina, J.J.; Gil-Pelegrín, E. Surface Density of the Spongy and Palisade Parenchyma Layers of Leaves Extracted From Wideband Ultrasonic Resonance Spectra. Front. Plant Sci. 2020, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dai, X.; Wang, L.; Xu, W.; He, Z.; Ma, M. Arsenate reduces copper phytotoxicity in gametophytes of Pteris vittata. J. Plant Physiol. 2008, 165, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Tear, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis (Part 2), 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Gao, Y.; Sun, Y.; Ou, Y.; Zheng, X.; Feng, Q.; Zhang, H.; Fei, Y.; Luo, J.; Resco de Dios, V.; Yao, Y. Pretreating poplar cuttings with low nitrogen ameliorates salt stress responses by increasing stored carbohydrates and priming stress signaling pathways. Ecotoxicol. Environ. Saf. 2021, 225, 112801. [Google Scholar] [CrossRef]
- Beer, R.F.; Sizer, I.W. A spectrophotometric method for measuring the break down of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Suralta, R.R.; Kano-Nakata, M.; Niones, J.M.; Inukai, Y.; Kameoka, E.; Tran, T.T.; Menge, D.; Mitsuya, S.; Yamauchi, A. Root plasticity for maintenance of productivity under abiotic stressed soil environments in rice: Progress and prospects. Field Crops Res. 2018, 220, 57–66. [Google Scholar] [CrossRef]
- Tokusoglu, O.; Unal, M.K.; Yildirum, Z. HPLC–UV and GC–MS characterization of the flavonol aglycons quercetin; kaempferol and myricetin in tomato and tomato pastes and other tomato-based products. Acta Chromatogr. 2003, 13, 196–207. [Google Scholar]
- Nagel, L.M.; Bassman, J.H.; Edwards, G.E.; Robberecht, R.; Franceshi, V.R. Leaf anatomical changes in Populus trichocarpa; Quercus rubra; Pseudotsuga menziesii and Pinus ponderosa exposed to enhanced ultraviolet-B radiation. Physiol. Plant. 1998, 104, 385–396. [Google Scholar] [CrossRef]
- Olsson, L.C.; Veit, M.; Weissenböck, G.; Bornman, J.F. Differential flavonoid response to enhanced UV-B radiation in Brassica napus. Phytochemistry 1998, 49, 1021–1028. [Google Scholar] [CrossRef]
- Bornman, J.F.; Vogelmann, T.C. Effect of UV-B radiation on leaf optical properties measured with fibre optics. J. Exp. Bot. 1990, 42, 547–554. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Proline synthesis and degradation, a model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.; Xu, G.; Li, C. Growth and physiological responses to drought and elevated ultraviolet-B in two contrasting populations of Hippophae rhamnoides. Physiol. Plant. 2005, 124, 431–440. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Swinny, E.E.; Bloor, S.J.; Markham, K.R.; Ryan, K.G.; Campbell, B.D.; Jordan, B.R.; Fountain, D.W. Responses of nine Trifolium repens L. populations to ultraviolet-B radiation, differential flavonol glycoside accumulation and biomass production. Ann. Bot. 2000, 86, 527–537. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.; Battey, N.; Hadley, P. The influence of ultraviolet radiation on growth, photosynthesis and phenolic levels of green and red lettuce: Potential for exploiting effects of ultraviolet radiation in a production system. Ann. Appl. Biol. 2010, 156, 357–366. [Google Scholar] [CrossRef]
- Morales, L.O.; Tegelberg, R.; Brosché, M.; Keinänen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Munné-Bosch, S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef]
- Quintero-Arias, D.G.; Acuña-Caita, J.F.; Asensio, C.; Valenzuela, J.L. Ultraviolet Transparency of Plastic Films Determines the Quality of Lettuce (Lactuca sativa L.) Grown in a Greenhouse. Agronomy 2021, 11, 358. [Google Scholar] [CrossRef]
- Ryan, K.; Markham, K.; Bloor, S.; Bradley, M.; Mitchell, K.; Jordan, B. UV-B radiation induces increase in quercetin, kaempferol ratio in wild-type and transgenic lines of Petunia. Photochem. Photobiol. 1998, 68, 323–330. [Google Scholar]
- Montesinos, M.C.; Ubeda, A.; Terencio, M.C.; Paya, M.; Alcaraz, M.J. Antioxidant profile of mono- and dihydroxylated flavone derivatives in free radical generating systems. Z. Naturforsch. 1995, 50, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.; Kozak, R.G.; Puntarulo, S. N-Acetylcysteine dependent protection against UV-B damage in two photosynthetic organisms. Plant Sci. 1999, 141, 129–137. [Google Scholar] [CrossRef]
- Osone, Y.; Ishida, A.; Tateno, M. Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New Phytol. 2008, 179, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Hartikainen, S.M.; Aphalo, P.J. How does solar ultraviolet-B radiation improve drought tolerance of silver birch (Betula pendula Roth.) seedlings? Plant Cell Environ. 2015, 38, 953–967. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and watering-dependent oxidative stress, effect on antioxidant content in Triticum aestivum L. leaves. J. Exp. Bot. 1999, 50, 375–383. [Google Scholar] [CrossRef]
- Mátai, A.; Nagy, D.; Hideg, É. UV-B strengthens antioxidant responses to drought in Nicotiana benthamiana leaves not only as supplementary irradiation but also as pre-treatment. Plant Physiol Biochem. 2019, 134, 9–19. [Google Scholar] [CrossRef]
- Thomas, D.T.T.; Challabathula, D.; Puthur, J.T. UV-B priming of Oryza sativa var. Kanchana seedlings augments its antioxidative potential and gene expression of stress-response proteins under various abiotic stresses. Biotech 2019, 9, 375. [Google Scholar] [CrossRef]
- Vincent, D.; Lapierre, C.; Pollet, B.; Cornic, G.; Negroni, L.; Zivy, M. Water deficits affect caffeate O-methyltransferase; lignification and related enzymes in Maize leaves. A proteomic investigation. Plant Physiol. 2005, 137, 949–960. [Google Scholar] [CrossRef]
- Lu, Y.; Duan, B.; Zhang, X.; Korpelainen, H.; Berninger, F.; Li, C. Intraspecific variation in drought response of Populus cathayana grown under ambient and enhanced UV-B radiation. Ann. For. Sci. 2009, 66, 613. [Google Scholar] [CrossRef]
- Binkert, M.; Kozma-Bognár, L.; Terecskei, K.; De Veylder, L.; Nagy, F.; Ulm, R. UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes: Including its own promoter. Plant Cell 2014, 26, 4200–4213. [Google Scholar] [CrossRef]
- Yue, M.; Li, Y.; Wang, X. Effects of enhanced ultraviolet-B radiation on plant nutrients and decomposition of spring wheat under field conditions. Environ. Exp. Bot. 1998, 40, 187–196. [Google Scholar] [CrossRef]
- Silva, E.C.D.; Nogueira, R.J.M.C.; Almeida da Silva, M.; Bandeira de Albuquerque, M. Drought Stress and Plant Nutrition.edu. In Plant Stress; Global Science Books: Petaling Jaya, Malaysia, 2011; Volume 5, pp. 32–41. [Google Scholar]
- Chimphango, S.B.M.; Musil, C.F.; Dakora, F.D. Alteration in the mineral nutrition of purely symbiotic and nitrate-fed nodulated legumes exposed to elevated UV-B radiation. J. Plant Nutr. 2012, 35, 1–20. [Google Scholar] [CrossRef]
- Liaqat, S.; Chhabra, S.; Saffeullah, P.; Iqbal, N.; Siddiqi, T.O. Role of Potassium in Drought Adaptation, Insights into Physiological and Biochemical Characteristics of Plants. In Role of Potassium in Abiotic Stress; Iqbal, N., Umar, S., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Shen, X.F.; Li, J.M.; Duan, L.S.; Li, Z.H.; Eneji, A.E. Nutrient Acquisition by Soybean Treated with and without Silicon under Ultraviolet-B Radiation. J. Plant Nutr. 2009, 32, 1731–1743. [Google Scholar] [CrossRef]
- Zancan, S.; Suglia, I.; Rocca, N.L.; Ghisi, R. Effects of UV-B radiation on antioxidant parameters of iron-deficient barley plants. Environ. Exp. Bot. 2008, 63, 71–79. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; He, X.; Si, C. Grain yield; starch; protein; and nutritional element concentrations of winter wheat exposed to enhanced UV-B during different growth stages. J. Cereal Sci. 2014, 60, 31–36. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Wang, Y.; Hu, C.; Shi, K.; Zhou, J.; Xia, X.; Zhou, Y.; Foyer, C.H.; Yu, J. The phyB-dependent induction of HY5 promotes iron uptake by systemically activating FER expression. EMBO Rep. 2021, 22, e51944. [Google Scholar] [CrossRef]
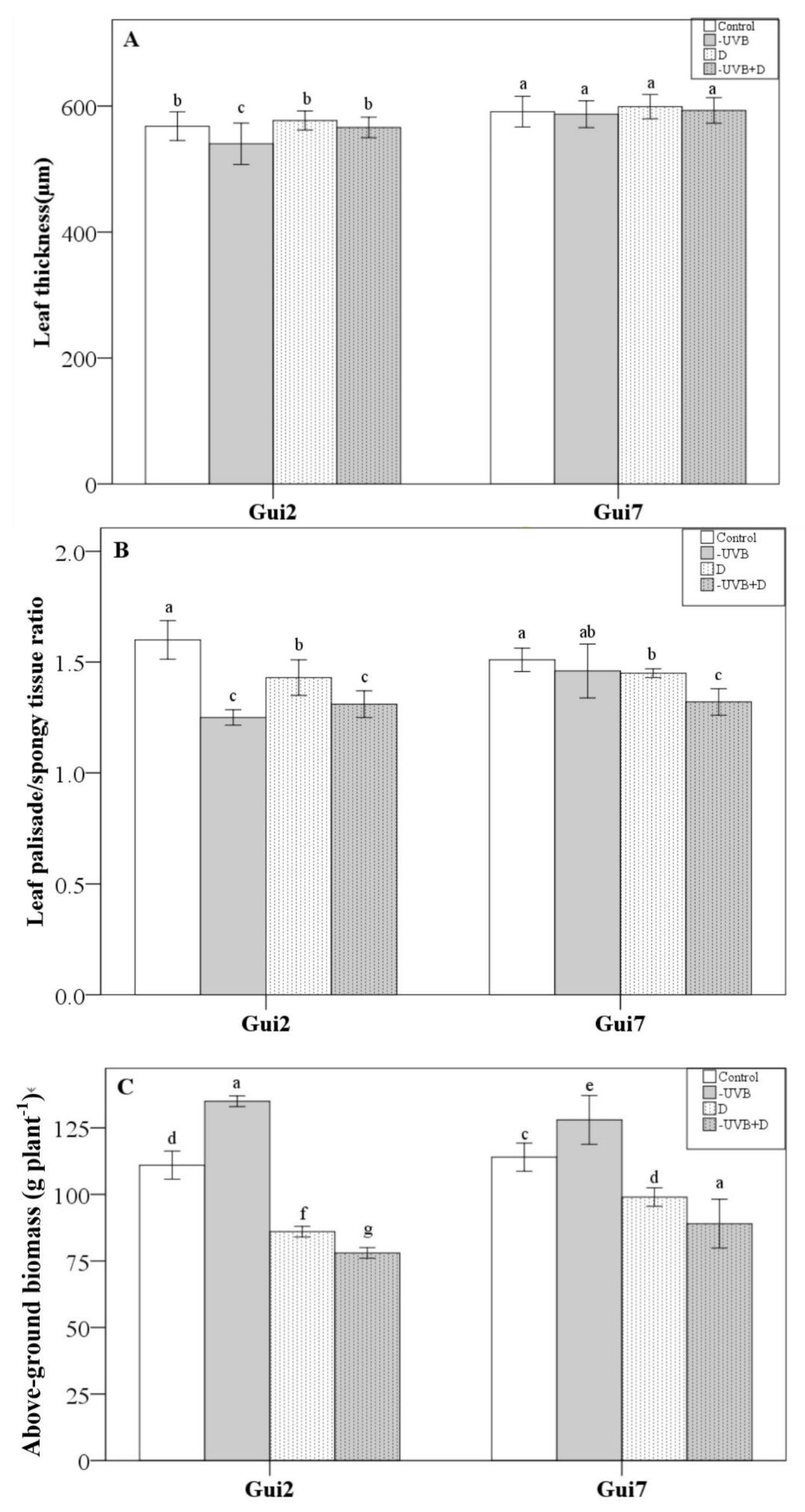
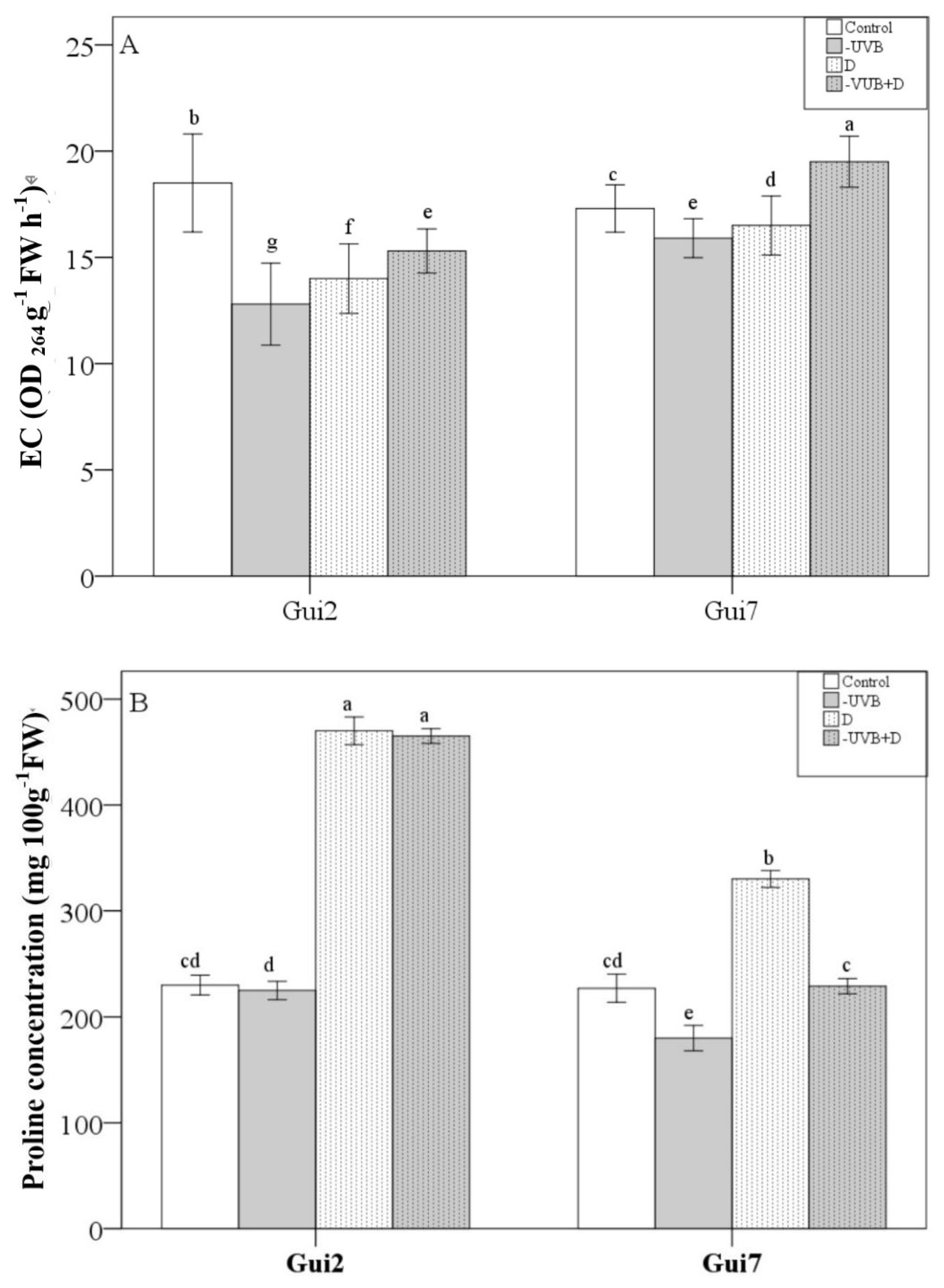
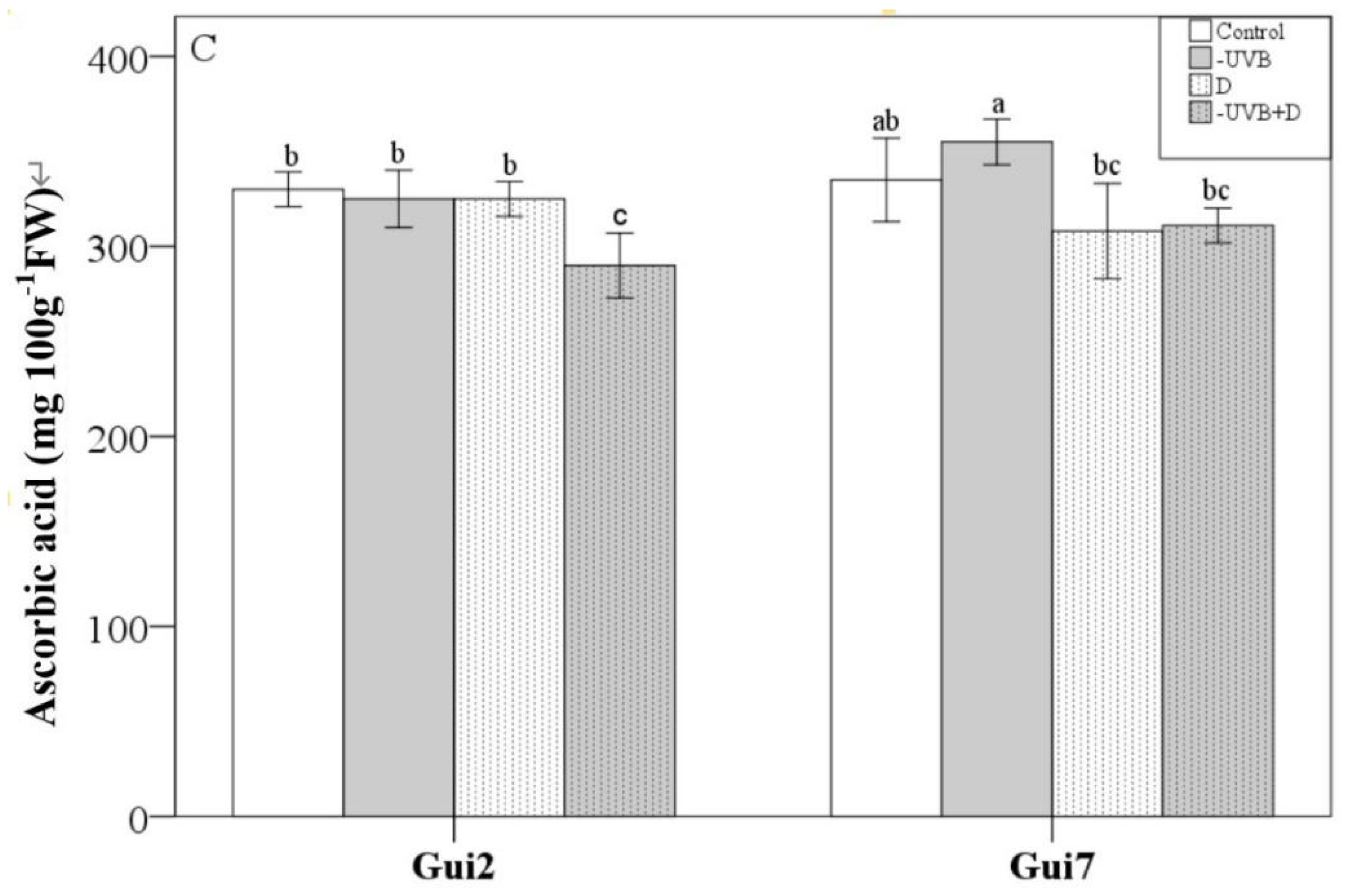
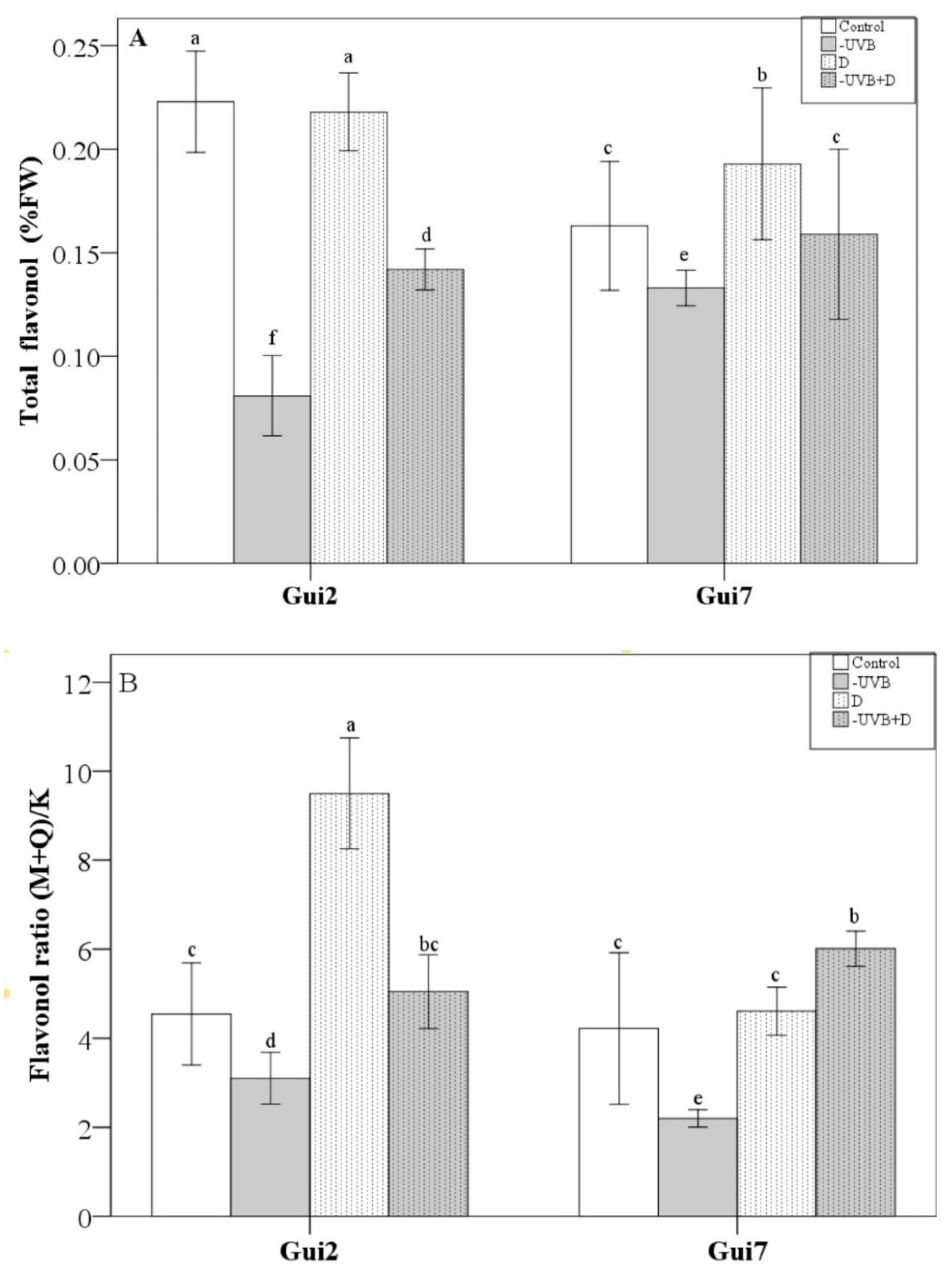

| Leaf Thickness | P/S Ratio | Biomass | EC | Proline | Asa | SOD | CAT | POD | Total Flavonol | Flavonol Ratio (M+Q)/K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 107.2 *** | 6.8 * | 163.1 *** | 691.1 *** | 5426.7 *** | 16.0 *** | 1.66 ns | 10.5 ** | 1.0 ns | 0.653 ns | 40.4 *** |
| D | 16.0 *** | 7.9 * | 8066.8 *** | 14.5 ** | 12,305.8 *** | 38.3 *** | 81.54 *** | 3.4 ns | 166.4 *** | 137.5 *** | 167.5 *** |
| U | 11.5 ** 2.127 ns | 54.0 *** | 74.8 *** | 192.4 *** | 669.0 *** | 0.25 ns | 875.13 *** | 706.0 *** | 7.0 * | 950.2 *** | 68.9 *** |
| C×D | 2.1 ns | 1.4 ns | 208.3 *** | 195.3 *** | 3966.8 *** | 2.54 ns | 1.14 ns | 26.8 *** | 0.05 ns | 1.124 ns | 14.4 ** |
| C×U | 4.2 ns | 132 ** | 95.5 *** | 278.1 * | 469.7 *** | 15.6 *** | 1.09 ns | 78.7 *** | 3.6 ns | 238.5 *** | 34.7 *** |
| D×U | 2.6 ns | 6.8 * | 998.7 *** | 1223.9 *** | 68.9 *** | 6.8 * | 171.6 *** | 16.7 *** | 0.02 ns | 52.6 *** | 0.052 ns |
| C×D×U FS×C×T | 1.8 ns | 11.6 ** | 111.5 *** | 78.0 *** | 63.0 *** | 0.26 ns | 0.99 ns | 20.8 *** | 2.1 ns | 44.7 *** | 64.3 *** |
| Caltivars | Treatments | N (mg/g) | P (mg/g) | K (%DW) | Ca (ug/g) | Mg (ug/g) | Fe (ug/g) | Mn (ug/g) | Cu (ug/g) | Zn (ug/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gui 2 | Control | 3.87 ± 0.13 b | 0.34 ± 0.017 b | 1.23 ± 0.07 a | 6329 ± 178.9 d | 1188 ± 46.57 a | 108.4 ± 10.8 c | 50.80 ± 6.1 ab | 7.20 ± 0.73 b | 8.40 ± 0.31 b |
| -UV-B | 4.05 ± 0.12 a | 0.37 ± 0.017 a | 1.02 ± 0.09 b | 7671 ± 307.5c | 991 ± 27.8 b | 289.2 ± 17.0 a | 47.65 ± 2.7 b | 9.85 ± 1.10 a | 9.30 ± 0.44 a | |
| D | 3.71 ± 0.12 b | 0.33 ± 0.024 b | 1.08 ± 0.09 ab | 9183 ± 313.2 b | 931 ± 33.78 bc | 162.3 ± 9.6 b | 45.15 ± 3.7 b | 7.80 ± 0.37 ab | 7.95 ± 0.31 c | |
| -UV-B + D | 3.37 ± 0.13 c | 0.29 ± 0.012 c | 1.02 ± 0.09 b | 10,000 ± 190.8 a | 899 ± 17.35 c | 148.3 ± 14.2 b | 56.90 ± 4.5 a | 9.05 ± 0.65 a | 7.75 ± 0.69 c | |
| Gui 7 | Control | 3.51 ± 0.10 b | 0.23 ± 0.01 b | 1.07 ± 0.17 a | 7162 ± 237.0 d | 1058 ± 33.0 a | 57.5 ± 5.6 c | 61.8 ± 7.2 b | 6.65 ± 0.79 b | 4.65 ± 0.35 c |
| -UV-B | 3.77 ± 0.12 a | 0.27 ± 0.024 a | 0.93 ± 0.09 b | 8371 ± 419.0 c | 994 ± 27.6 b | 100.7 ± 11.8 b | 67.0 ± 4.7 b | 7.90 ± 0.62 ab | 5.40 ± 0.45 b | |
| D | 3.44 ± 0.17 b | 0.22 ± 0.023 b | 1.01 ± 0.11 ab | 9014 ± 256.5 b | 1008 ± 40.4 a | 153.5 ± 8.1 a | 64.5 ± 4.4 b | 6.80 ± 0.82 b | 6.75 ± 0.28 a | |
| -UV-B + D | 3.04 ± 0.13 c | 0.20 ± 0.026 c | 0.89 ± 0.10 b | 10535 ± 493.0 a | 892 ± 47.8 c | 102.4 ± 6.5 b | 80.2 ± 3.5 a | 8.65 ± 0.37 a | 5.50 ± 0.44 b | |
| C | ** | *** | * | ns | *** | *** | *** | ns | *** | |
| D | + | ns | ns | *** | *** | ns | ns | * | *** | |
| U | ** | * | ** | *** | *** | *** | ns | ** | *** | |
| C × D | ns | ns | ns | ns | *** | *** | ns | ns | *** | |
| C × U | ns | ns | ns | ns | *** | *** | ns | ns | *** | |
| D × U | ** | ** | ns | ns | ns | *** | *** | ns | ns | |
| C × D × U | ns | ns | ns | ns | *** | *** | ns | ns | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Li, J.; Luo, J.; Ma, Y.; Wang, Y.; Liu, W.; Rodriguez, L.G.; Yao, Y. Responses to Solar UV-B Exclusion and Drought Stress in Two Cultivars of Chestnut Rose with Different Leaf Thickness. Forests 2023, 14, 50. https://doi.org/10.3390/f14010050
Luo D, Li J, Luo J, Ma Y, Wang Y, Liu W, Rodriguez LG, Yao Y. Responses to Solar UV-B Exclusion and Drought Stress in Two Cultivars of Chestnut Rose with Different Leaf Thickness. Forests. 2023; 14(1):50. https://doi.org/10.3390/f14010050
Chicago/Turabian StyleLuo, Dapeng, Jielin Li, Jianxun Luo, Yan Ma, Yongzhi Wang, Wei Liu, Lucas Gutierrez Rodriguez, and Yinan Yao. 2023. "Responses to Solar UV-B Exclusion and Drought Stress in Two Cultivars of Chestnut Rose with Different Leaf Thickness" Forests 14, no. 1: 50. https://doi.org/10.3390/f14010050
APA StyleLuo, D., Li, J., Luo, J., Ma, Y., Wang, Y., Liu, W., Rodriguez, L. G., & Yao, Y. (2023). Responses to Solar UV-B Exclusion and Drought Stress in Two Cultivars of Chestnut Rose with Different Leaf Thickness. Forests, 14(1), 50. https://doi.org/10.3390/f14010050




