Linking Forest Management Practices to the Functional Composition of Plant Communities
Abstract
1. Introduction
- (1)
- As previously shown, different forest management types can determine various ecosystem functions and services driven by plant functional traits [5,12]. For example, wood production is closely associated with stem conduit density and wood vessel element length in tree species [18,19]. Here, we propose the first hypothesis (H1): different forest management types can contribute to the variation in the FCPC.
- (2)
- An ecozone is a biogeographical unit consisting of a biological community formed in response to environmental change [20,21,22]. Ecozone-level relationships may exist between forest management types and ecosystem functions. Here, we propose the second hypothesis (H2): the effects of historical woodland changes on contemporary plant functional diversity vary among different forest ecozones. Different forest ecozones are meant to regulate the relationship between forest management and the FCPC.
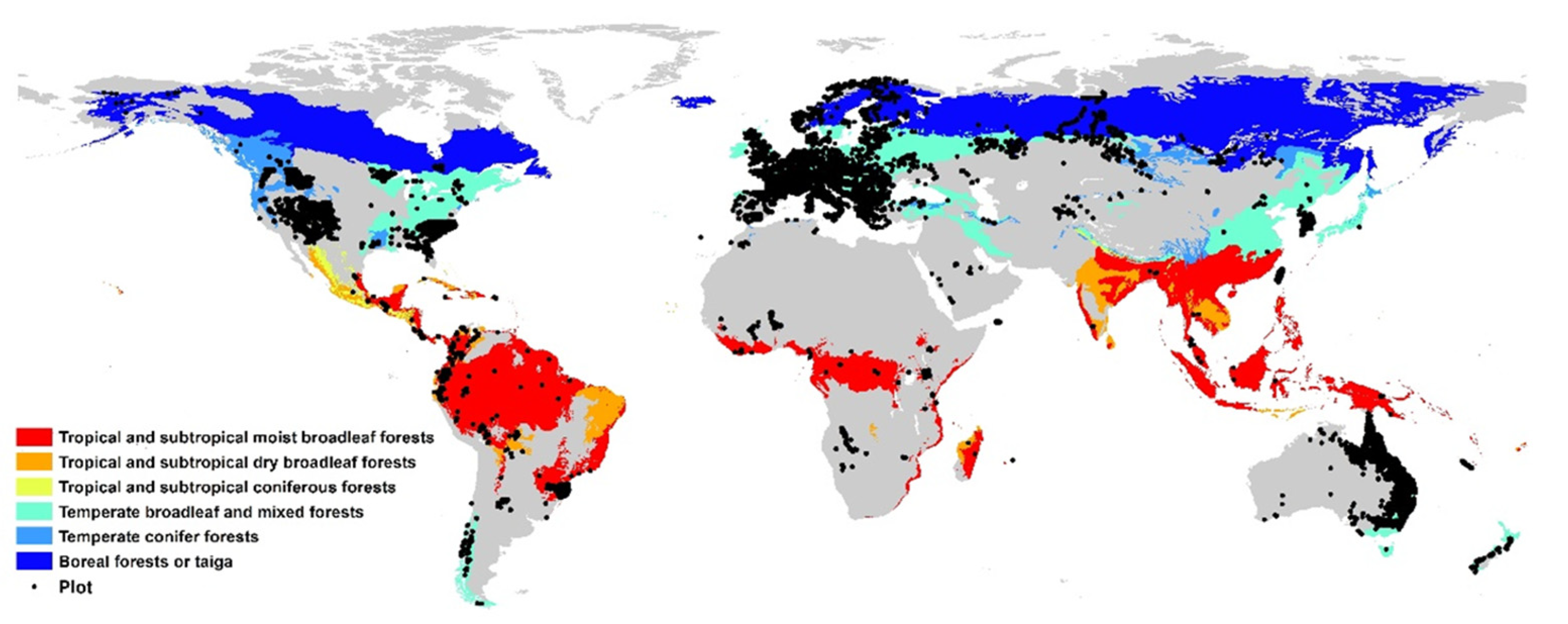
2. Materials and Methods
2.1. Data on Plant Functional Composition
2.2. Data on Forest Management Types
2.3. Analyses
3. Results
4. Discussion
4.1. Linking Forest Management to CWM and CWV
4.2. Ecozone Effects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; Lamarque, P.; Colace, M.P.; Garden, D.; Girel, J.; Pellet, G.; Douzet, R. Using plant functional traits to understand the landscape distribution of multiple ecosystem services. J. Ecol. 2011, 99, 135–147. [Google Scholar] [CrossRef]
- Ostertag, R.; Warman, L.; Cordell, S.; Vitousek, P.M. Using plant functional traits to restore Hawaiian rainforest. J. Appl. Ecol. 2015, 52, 805–809. [Google Scholar] [CrossRef]
- Carlucci, M.B.; Brancalion, P.H.; Rodrigues, R.R.; Loyola, R.; Cianciaruso, M.V. Functional traits and ecosystem services in ecological restoration. Restor. Ecol. 2020, 28, 1372–1383. [Google Scholar] [CrossRef]
- Miedema Brown, L.; Anand, M. Plant functional traits as measures of ecosystem service provision. Ecosphere 2022, 13, e3930. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jandt, U. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- DeMalach, N.; Ke, P.J.; Fukami, T. The effects of ecological selection on species diversity and trait distribution: Predictions and an empirical test. Ecology 2022, 103, e03567. [Google Scholar] [CrossRef] [PubMed]
- Boinot, S.; Mony, C.; Fried, G.; Ernoult, A.; Aviron, S.; Ricono, C.; Couthouis, E.; Alignier, A. Weed communities are more diverse, but not more abundant, in dense and complex bocage landscapes. J. Appl. Ecol. 2023, 60, 4–16. [Google Scholar] [CrossRef]
- Allan, E.; Manning, P.; Alt, F.; Binkenstein, J.; Blaser, S.; Blüthgen, N.; Böhm, S.; Grassein, F.; Hölzel, N.; Fischer, M. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D.B. Indicators of biodiversity for ecologically sustainable forest management. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- Oettel, J.; Lapin, K. Linking forest management and biodiversity indicators to strengthen sustainable forest management in Europe. Ecol. Indic. 2021, 122, 107275. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Balmford, A. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Lesiv, M.; Schepaschenko, D.; Buchhorn, M.; See, L.; Dürauer, M.; Georgieva, I.; Jung, M.; Hofhansl, F.; Schulze, K.; Fritz, S. Global forest management data for 2015 at a 100 m resolution. Sci. Data 2022, 9, 199. [Google Scholar] [CrossRef]
- Hérault, B.; Bachelot, B.; Poorter, L.; Rossi, V.; Bongers, F.; Chave, J.; Paine, C.E.T.; Wagner, F.; Baraloto, C. Functional traits shape ontogenetic growth trajectories of rain forest tree species. J. Ecol. 2011, 99, 1431–1440. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lachat, T.; Brunet, J.; Isacsson, G.; Bouget, C.; Brustel, H.; Brandl, R.; Weisser, W.W.; Mueller, J. Current near-to-nature forest management effects on functional trait composition of saproxylic beetles in beech forests. Conserv. Biol. 2013, 27, 605–614. [Google Scholar] [CrossRef]
- Hao, M.; Messier, C.; Geng, Y.; Zhang, C.; Zhao, X.; von Gadow, K. Functional traits influence biomass and productivity through multiple mechanisms in a temperate secondary forest. Eur. J. For. Res. 2020, 139, 959–968. [Google Scholar] [CrossRef]
- Le Provost, G.; Badenhausser, I.; Le Bagousse-Pinguet, Y.; Clough, Y.; Henckel, L.; Violle, C.; Bretagnolle, V.; Roncoroni, M.; Manning, P.; Gross, N. Land-use history impacts functional diversity across multiple trophic groups. Proc. Natl. Acad. Sci. USA 2020, 117, 1573–1579. [Google Scholar] [CrossRef]
- Searson, M.J.; Thomas, D.S.; Montagu, K.D.; Conroy, J.P. Wood density and anatomy of water-limited eucalypts. Tree Physiol. 2004, 24, 1295–1302. [Google Scholar] [CrossRef]
- Rodriguez-Zaccaro, F.D.; Groover, A. Wood and water: How trees modify wood development to cope with drought. Plants People Planet 2019, 1, 346–355. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority ecoregions for global conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Saleem, M. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Kier, G.; Mutke, J.; Dinerstein, E.; Ricketts, T.H.; Küper, W.; Kreft, H.; Barthlott, W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005, 32, 1107–1116. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Lenoir, J.; Hattab, T.; Arnst, E.A.; Chytrý, M.; Dengler, J.; De Ruffray, P.; Hennekens, S.M.; Jandt, U.; Wagner, V. sPlotOpen–An environmentally balanced, open-access, global dataset of vegetation plots. Global Ecol. Biogeogr. 2021, 30, 1740–1764. [Google Scholar] [CrossRef]
- Muscarella, R.; Uriarte, M. Do community-weighted mean functional traits reflect optimal strategies? Proc. R. Soc. B Biol. Sci. 2016, 283, 20152434. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Violle, C.; Mouillot, D.; Mouquet, N.; Enquist, B.J.; Munoz, F.; Münkemüller, T.; Ostling, A.; Zimmermann, N.E.; Thuiller, W. Plant community impact on productivity: Trait diversity or key (stone) species effects? Ecol. Lett. 2022, 25, 913–925. [Google Scholar] [CrossRef]
- Dengler, J.; Jansen, F.; Glöckler, F.; Peet, R.K.; De Cáceres, M.; Chytrý, M.; Ewald, J.; Oldeland, J.; Lopez-Gonzalez, G.; Spencer, N. The Global Index of Vegetation-Plot Databases (GIVD): A new resource for vegetation science. J. Veg. Sci. 2011, 22, 582–597. [Google Scholar] [CrossRef]
- Gaüzère, P.; Doulcier, G.; Devictor, V.; Kéfi, S. A framework for estimating species-specific contributions to community indicators. Ecol. Indic. 2019, 99, 74–82. [Google Scholar] [CrossRef]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Mahecha, M.D. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F. Changing drivers of species dominance during tropical forest succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Bongers, F. Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 2015, 96, 1242–1252. [Google Scholar] [CrossRef]
- Reid, J.L.; Holl, K.D.; Zahawi, R.A. Seed dispersal limitations shift over time in tropical forest restoration. Ecol. Appl. 2015, 25, 1072–1082. [Google Scholar] [CrossRef]
- Jesus, F.M.; Pivello, V.R.; Meirelles, S.T.; Franco, G.A.; Metzger, J.P. The importance of landscape structure for seed dispersal in rain forest fragments. J. Veg. Sci. 2012, 23, 1126–1136. [Google Scholar] [CrossRef]
- Palma, A.C.; Goosem, M.; Fensham, R.J.; Goosem, S.; Preece, N.D.; Stevenson, P.R.; Laurance, S.G. Dispersal and recruitment limitations in secondary forests. J. Veg. Sci. 2021, 32, e12975. [Google Scholar] [CrossRef]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Coomes, D.A.; Gibson, L.; Hu, G.; Liu, J.; Luo, Y.; Wu, C.; Yu, M. Forest fragmentation in China and its effect on biodiversity. Biol. Rev. 2019, 94, 1636–1657. [Google Scholar] [CrossRef]
- Damschen, E.I.; Baker, D.V.; Bohrer, G.; Nathan, R.; Orrock, J.L.; Turner, J.R.; Brudvig, L.A.; Haddad, N.M.; Levey, D.J.; Tewksbury, J.J. How fragmentation and corridors affect wind dynamics and seed dispersal in open habitats. Proc. Natl. Acad. Sci. USA 2014, 111, 3484–3489. [Google Scholar] [CrossRef]
- Chen, S.C.; Poschlod, P.; Antonelli, A.; Liu, U.; Dickie, J.B. Trade-off between seed dispersal in space and time. Ecol. Lett. 2020, 23, 1635–1642. [Google Scholar] [CrossRef]
- Poorter, L.; Van de Plassche, M.; Willems, S.; Boot, R.G.A. Leaf traits and herbivory rates of tropical tree species differing in successional status. Plant Ecol. 2004, 6, 746–754. [Google Scholar] [CrossRef]
- Sonnier, G.; Shipley, B.; Navas, M.L. Quantifying relationships between traits and explicitly measured gradients of stress and disturbance in early successional plant communities. J. Veg. Sci. 2010, 21, 1014–1024. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.E.N.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Dahlgren, J.P.; Eriksson, O.; Bolmgren, K.; Strindell, M.; Ehrlén, J. Specific leaf area as a superior predictor of changes in field layer abundance during forest succession. J. Veg. Sci. 2006, 17, 577–582. [Google Scholar] [CrossRef]
- Larocque, G.R.; Luckai, N.; Adhikary, S.N.; Groot, A.; Bell, F.W.; Sharma, M. Competition theory—Science and application in mixed forest stands: Review of experimental and modelling methods and suggestions for future research. Environ. Rev. 2013, 21, 71–84. [Google Scholar] [CrossRef]
- Gan, H.; Jiao, Y.; Jia, J.; Wang, X.; Li, H.; Shi, W.; Peng, C.; Polle, A.; Luo, Z.B. Phosphorus and nitrogen physiology of two contrasting poplar genotypes when exposed to phosphorus and/or nitrogen starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, B.; Munoz, F.; Fried, G.; Mahaut, L.; Armengot, L.; Denelle, P.; Storkey, J.; Gaba, S.; Violle, C. What makes a weed a weed? A large-scale evaluation of arable weeds through a functional lens. Am. J. Bot. 2019, 106, 90–100. [Google Scholar] [CrossRef]
- Ros-Barceló, A.; Gómez-Ros, L.V.; Ferrer, M.A.; Hernández, J.A. The apoplastic antioxidant enzymatic system in the wood-forming tissues of trees. Trees 2006, 20, 145–156. [Google Scholar] [CrossRef]
- Abreu, I.N.; Johansson, A.I.; Sokołowska, K.; Niittylä, T.; Sundberg, B.; Hvidsten, T.R.; Street, N.R.; Moritz, T. A metabolite roadmap of the wood-forming tissue in Populus tremula. New Phytol. 2020, 228, 1559–1572. [Google Scholar] [CrossRef]
- Gardner, C.J.; Bicknell, J.E.; Baldwin-Cantello, W.; Struebig, M.J.; Davies, Z.G. Quantifying the impacts of defaunation on natural forest regeneration in a global meta-analysis. Nat. Commun. 2019, 10, 4590. [Google Scholar] [CrossRef] [PubMed]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring forests: Regeneration and ecosystem function for the future. New For. 2019, 50, 139–151. [Google Scholar] [CrossRef]
- Temperton, V.M.; Buchmann, N.; Buisson, E.; Durigan, G.; Kazmierczak, Ł.; Perring, M.P.; de Sá Dechoum, M.; Veldman, J.W.; Overbeck, G.E. Step back from the forest and step up to the Bonn Challenge: How a broad ecological perspective can promote successful landscape restoration. Restor. Ecol. 2019, 27, 705–719. [Google Scholar] [CrossRef]
- Cubino, J.P.; Biurrun, I.; Bonari, G.; Braslavskaya, T.; Font, X.; Jandt, U.; Jansen, F.; Rašomavičius, V.; Škvorc, Ž.; Chytrý, M. The leaf economic and plant size spectra of European forest understory vegetation. Ecography 2021, 44, 1311–1324. [Google Scholar] [CrossRef]
- Ramovs, B.V.; Roberts, M.R. Understory vegetation and environment responses to tillage, forest harvesting, and conifer plantation development. Ecol. Appl. 2003, 13, 1682–1700. [Google Scholar] [CrossRef]
- Duan, W.; Ren, H.; Fu, S.; Wang, J.; Zhang, J.; Yang, L.; Huang, C. Community comparison and determinant analysis of understory vegetation in six plantations in South China. Restor. Ecol. 2010, 18, 206–214. [Google Scholar] [CrossRef]
- Ali, A.; Dai, D.; Akhtar, K.; Teng, M.; Yan, Z.; Urbina-Cardona, N.; Mullerova, J.; Zhou, Z. Response of understory vegetation, tree regeneration, and soil quality to manipulated stand density in a Pinus massoniana plantation. Glob. Ecol. Conserv. 2019, 20, e00775. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Deng, J.; Wang, B. Afforestation affects soil seed banks by altering soil properties and understory plants on the eastern Loess Plateau, China. Ecol. Indic. 2021, 126, 107670. [Google Scholar] [CrossRef]
- Michelsen, A.; Lisanework, N.; Friis, I.; Holst, N. Comparisons of understorey vegetation and soil fertility in plantations and adjacent natural forests in the Ethiopian highlands. J. Appl. Ecol. 1996, 33, 627–642. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Sundarapandian, S.M.; Swamy, P.S. Litter production and leaf-litter decomposition of selected tree species in tropical forests at Kodayar in the Western Ghats, India. Forest Ecol. Manag. 1999, 123, 231–244. [Google Scholar] [CrossRef]
- Xuluc-Tolosa, F.J.; Vester, H.F.M.; Ramırez-Marcial, N.; Castellanos-Albores, J.; Lawrence, D. Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, Mexico. For. Ecol. Manag. 2003, 174, 401–412. [Google Scholar] [CrossRef]
- Bakker, M.A.; Carreño-Rocabado, G.; Poorter, L. Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct. Ecol. 2011, 25, 473–483. [Google Scholar] [CrossRef]
- Ulrich, W.; Piwczyński, M.; Zaplata, M.K.; Winter, S.; Schaaf, W.; Fischer, A. Soil conditions and phylogenetic relatedness influence total community trait space during early plant succession. J. Plant Ecol. 2014, 7, 321–329. [Google Scholar] [CrossRef]


| Trait | CWM-PC1 | CWM-PC2 | CWM-PC3 | CWV-PC1 | CWV-PC2 | CWV-PC3 | CWV-PC4 | CWV-PC5 |
|---|---|---|---|---|---|---|---|---|
| Leaf area | 0.241 | 0.334 | 0.028 | 0.312 | −0.091 | −0.006 | −0.605 | 0.049 |
| Stem-specific density | 0.306 | −0.089 | −0.081 | 0.149 | −0.012 | 0.138 | −0.009 | −0.154 |
| Specific leaf area | −0.162 | 0.448 | 0.033 | 0.346 | −0.164 | −0.322 | 0.162 | 0.105 |
| Leaf carbon concentration | 0.046 | 0.087 | −0.056 | 0.130 | −0.006 | 0.080 | 0.122 | 0.448 |
| Leaf nitrogen concentration | −0.055 | 0.433 | 0.105 | 0.186 | −0.187 | 0.173 | 0.174 | 0.275 |
| Leaf phosphorus concentration | −0.230 | 0.257 | 0.384 | 0.156 | −0.168 | 0.463 | 0.136 | 0.272 |
| Mean plant height | 0.340 | −0.074 | 0.192 | 0.301 | 0.070 | −0.315 | 0.102 | −0.075 |
| Seed mass | 0.356 | 0.083 | 0.218 | 0.194 | 0.518 | 0.034 | 0.068 | 0.015 |
| Seed length | 0.322 | 0.064 | 0.348 | 0.189 | 0.518 | 0.141 | 0.028 | 0.005 |
| Leaf dry matter content | 0.207 | −0.351 | −0.005 | 0.189 | −0.176 | 0.119 | 0.117 | −0.532 |
| Leaf N per area unit | 0.199 | −0.301 | 0.018 | 0.313 | −0.156 | −0.282 | 0.203 | 0.087 |
| Leaf N:P ratio | 0.279 | 0.097 | −0.361 | 0.181 | −0.133 | 0.447 | 0.087 | 0.092 |
| leaf δ15N | 0.101 | 0.043 | −0.304 | 0.149 | 0.009 | 0.293 | 0.067 | 0.003 |
| Seed number per reproductive unit | −0.165 | −0.153 | 0.209 | 0.273 | −0.035 | −0.059 | 0.136 | −0.107 |
| Fresh leaf mass | 0.280 | 0.242 | −0.004 | 0.287 | −0.097 | 0.060 | −0.645 | 0.015 |
| Stem conduit density | −0.195 | −0.242 | 0.253 | 0.270 | −0.071 | −0.284 | 0.081 | 0.145 |
| Dispersal unit length | 0.296 | 0.035 | 0.416 | 0.250 | 0.493 | 0.079 | 0.011 | −0.066 |
| Wood vessel element length | 0.128 | 0.189 | −0.347 | 0.199 | −0.168 | 0.181 | 0.140 | −0.521 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.-Q.; Wang, C.-J.; Chen, Z.; Yu, F.-H.; Wan, J.-Z. Linking Forest Management Practices to the Functional Composition of Plant Communities. Forests 2023, 14, 1939. https://doi.org/10.3390/f14101939
Ma Y-Q, Wang C-J, Chen Z, Yu F-H, Wan J-Z. Linking Forest Management Practices to the Functional Composition of Plant Communities. Forests. 2023; 14(10):1939. https://doi.org/10.3390/f14101939
Chicago/Turabian StyleMa, Yu-Qi, Chun-Jing Wang, Zhi Chen, Fei-Hai Yu, and Ji-Zhong Wan. 2023. "Linking Forest Management Practices to the Functional Composition of Plant Communities" Forests 14, no. 10: 1939. https://doi.org/10.3390/f14101939
APA StyleMa, Y.-Q., Wang, C.-J., Chen, Z., Yu, F.-H., & Wan, J.-Z. (2023). Linking Forest Management Practices to the Functional Composition of Plant Communities. Forests, 14(10), 1939. https://doi.org/10.3390/f14101939







