Low-Cost and High-Strength Soybean Meal Adhesives Modified by Tannin–Phenol–Formaldehyde Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method
2.2.1. Synthesis of Tannin–Phenol–Formaldehyde Resin
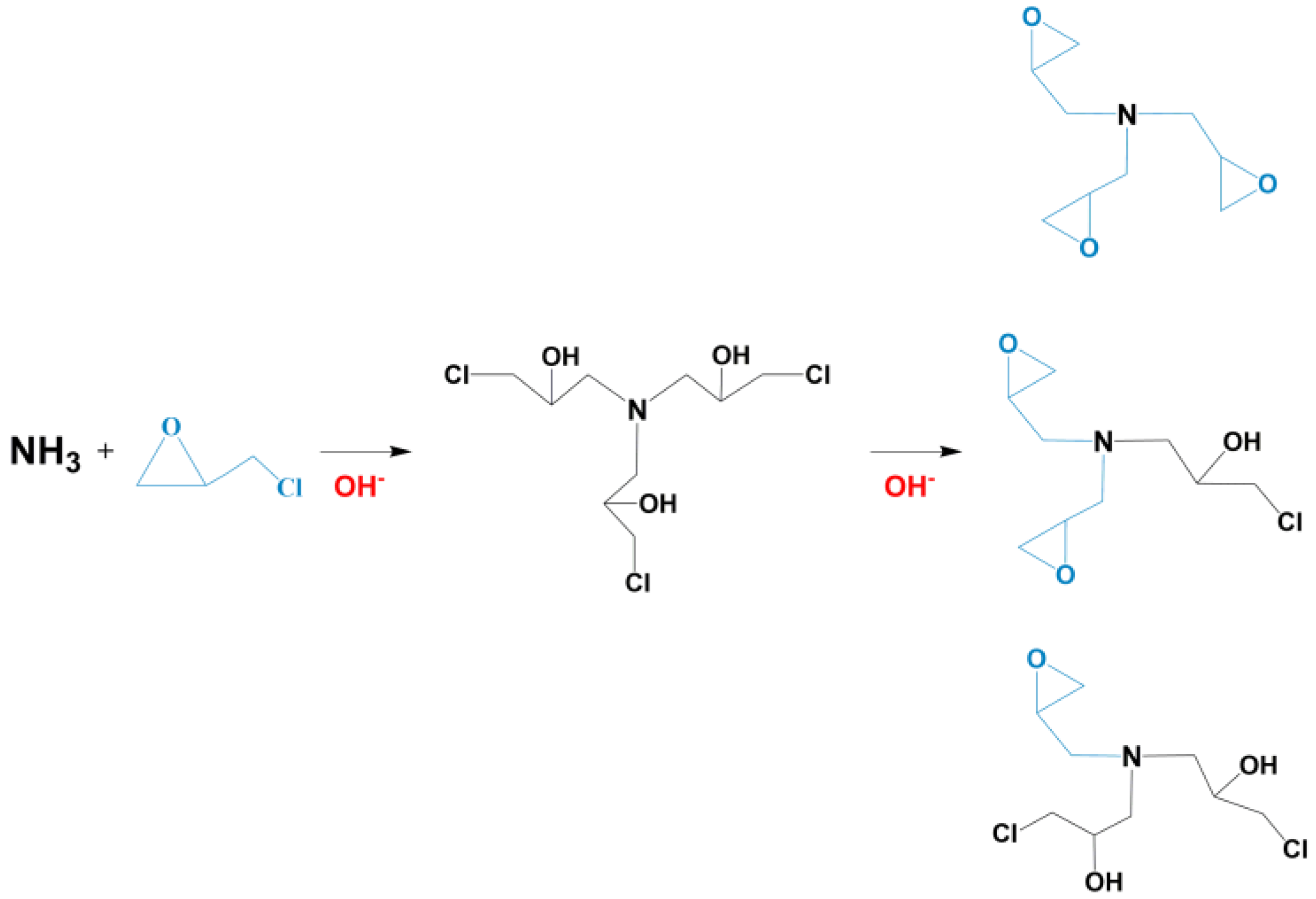
2.2.2. Preparation of Epoxy Cross-Linking Agent (CA)
2.2.3. Preparation of Soybean Meal Adhesive
2.2.4. Solid Content
2.2.5. Residual Rate
2.2.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.7. X-ray Diffraction (XRD)
2.2.8. Thermogravimetric (TG) Analysis
2.2.9. Scanning Electron Microscope (SEM)
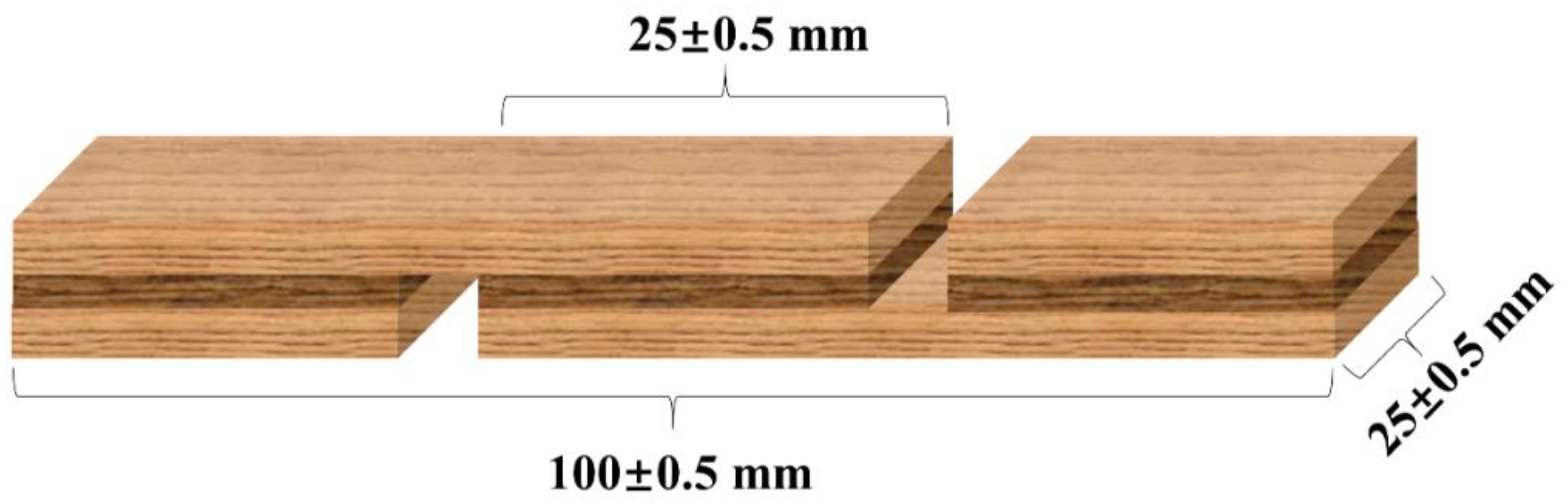
2.2.10. Preparation and Performance Testing of Plywood
3. Results and Discussion
3.1. Effect of Solid Content
3.2. FTIR Spectroscopic Analysis of Adhesives
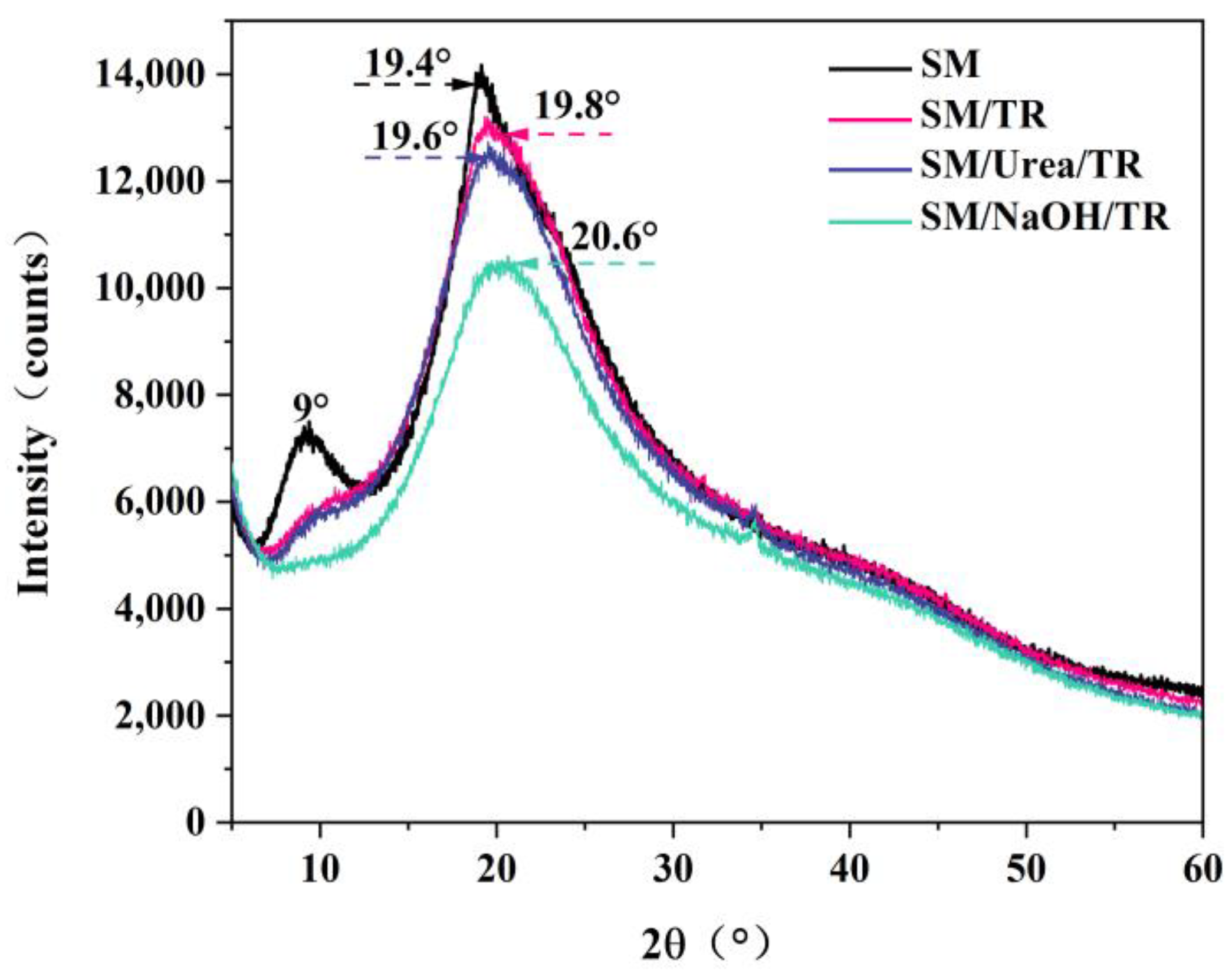
3.3. XRD Spectroscopic Analysis of Adhesives
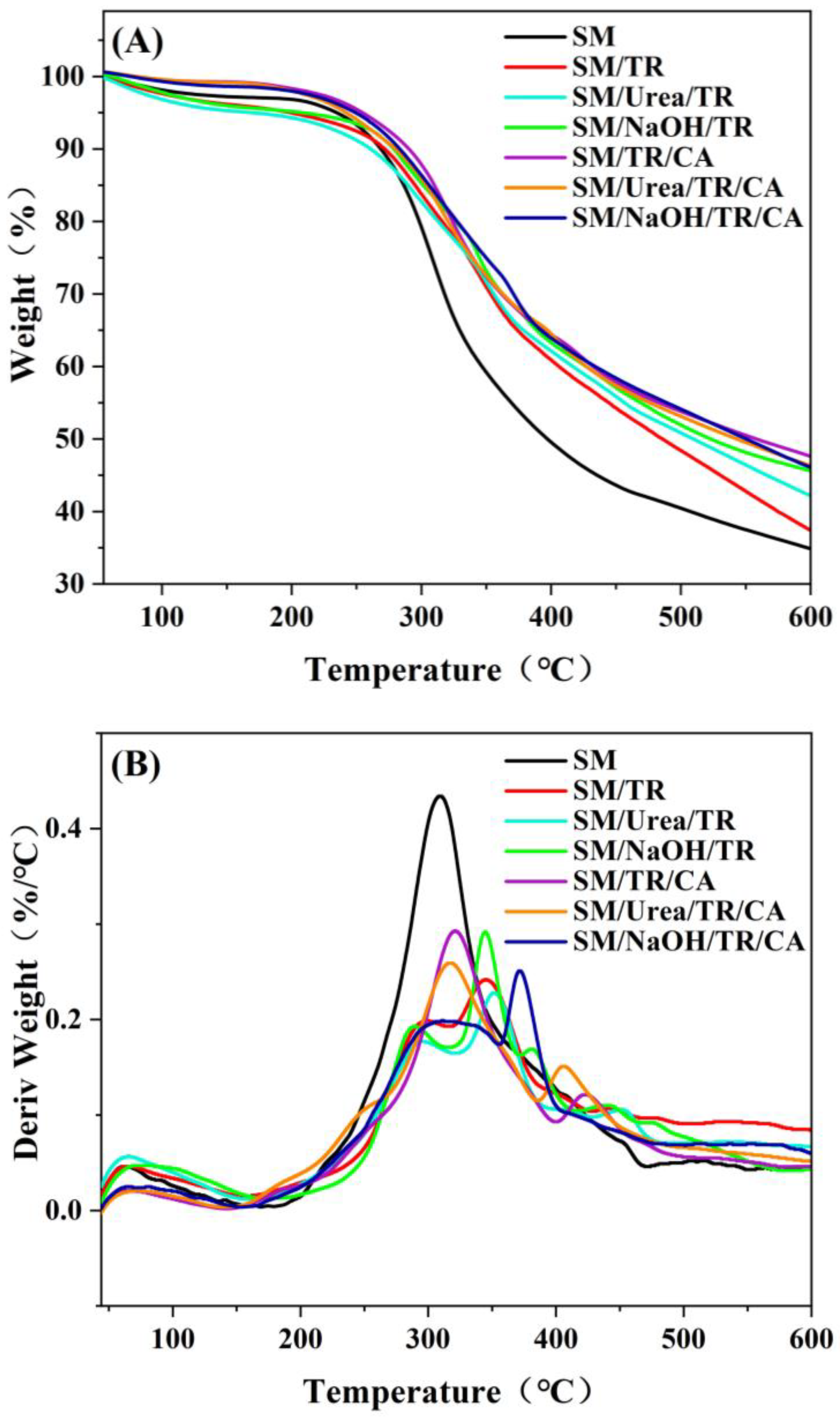
3.4. Thermal Property Analysis of Different SM Adhesives
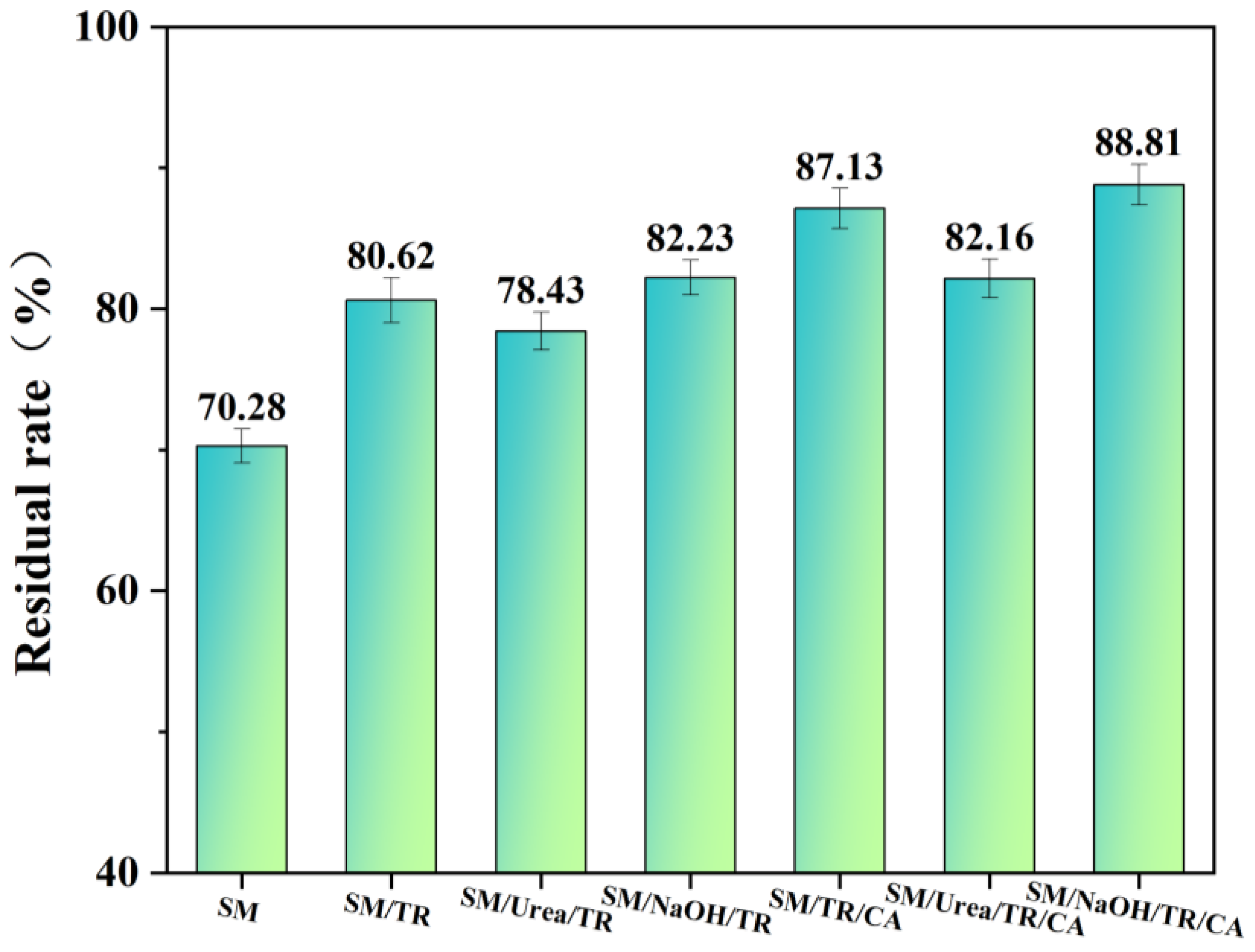
3.5. Residual Analysis of Adhesives
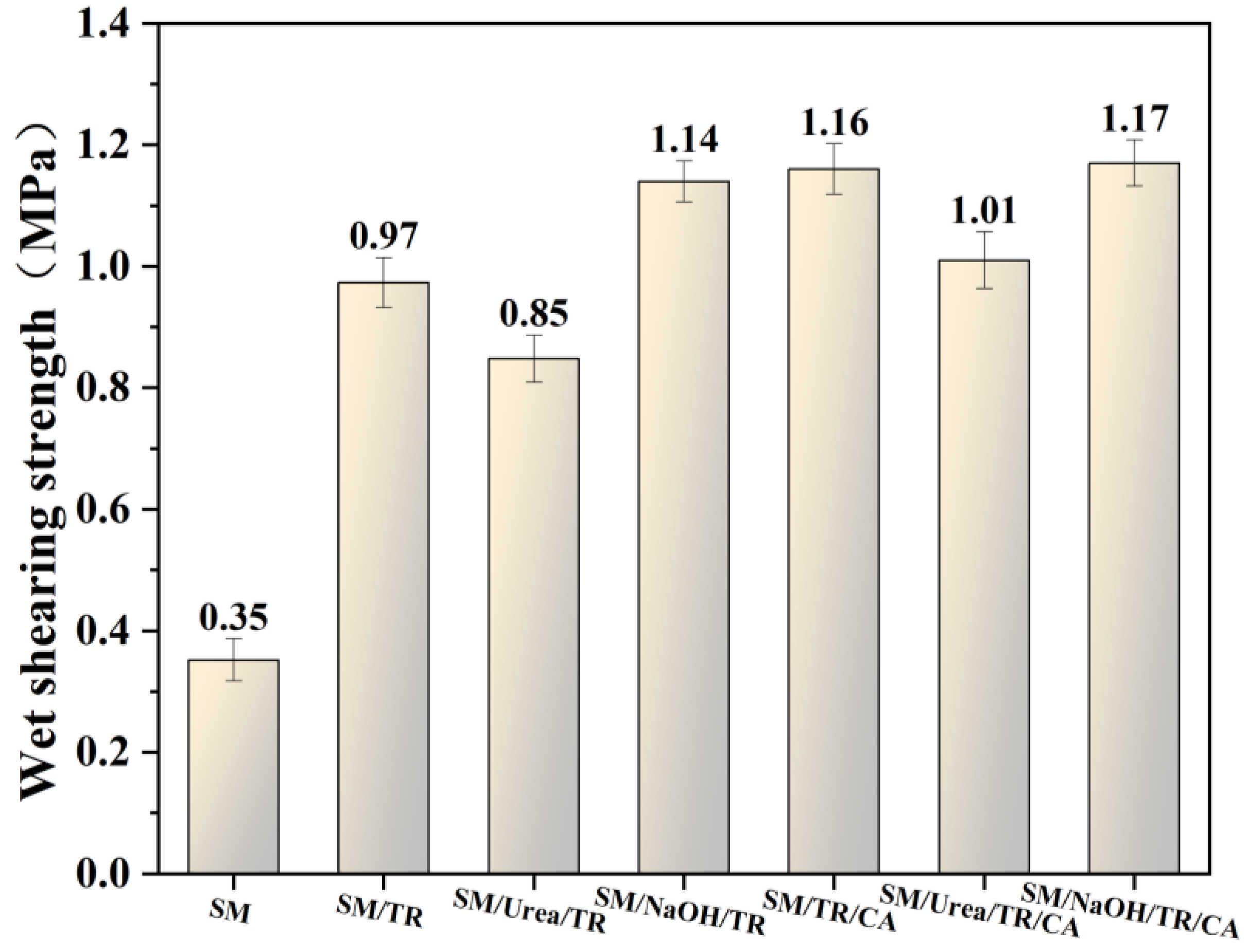
3.6. Analysis of the Performance of Plywood Prepared with Adhesives
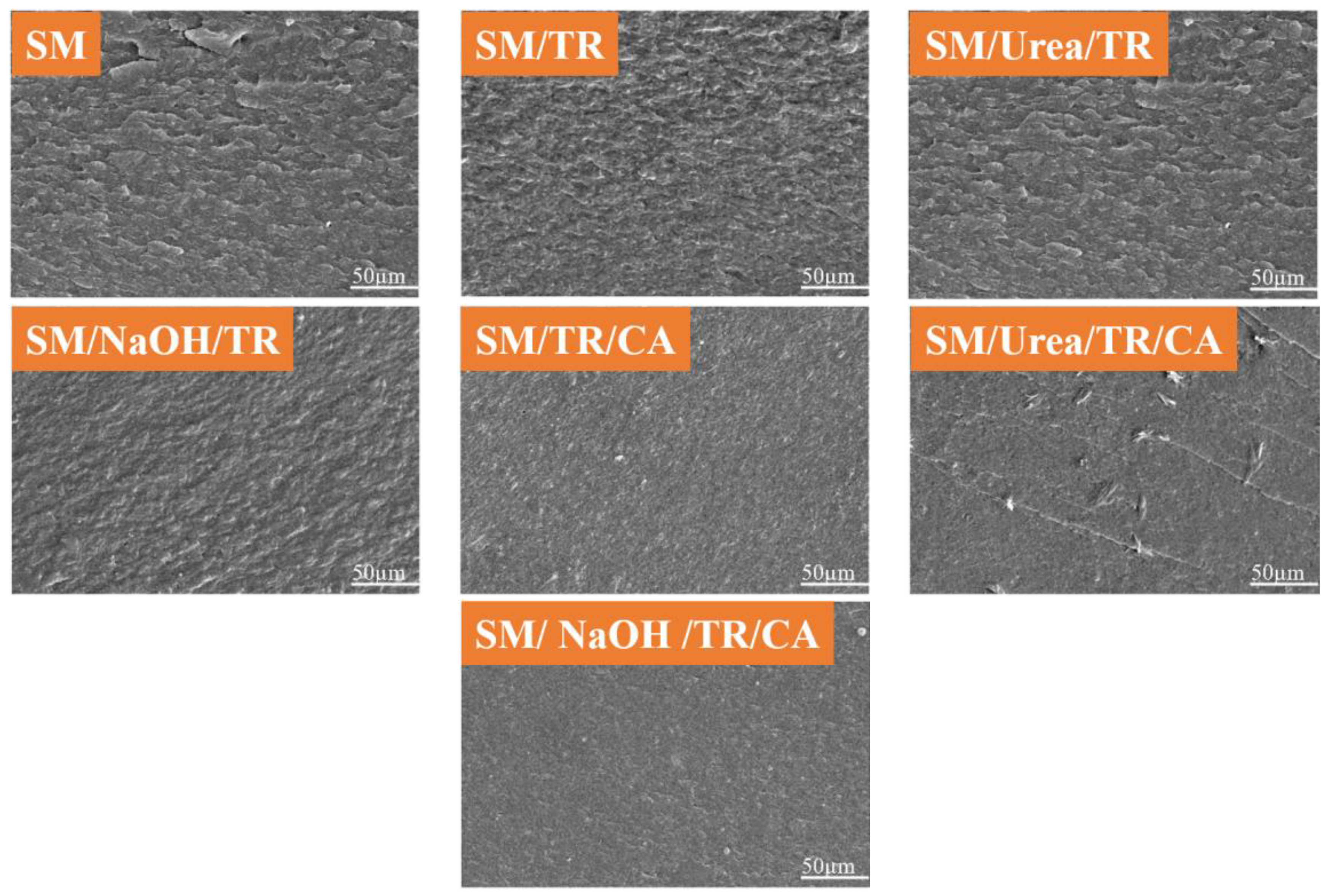
3.7. Electron Microscopy Observation of Adhesives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lamaming, S.Z.; Lamaming, J.; Rawi, N.F.M.; Hashim, R.; Kassim, M.H.M.; Hussin, M.H.; Bustami, Y.; Sulaiman, O.; Amini, M.H.M.; Hiziroglu, S. Improvements and limitation of soy protein-based adhesive: A review. Polym. Eng. Sci. 2021, 61, 2393–2405. [Google Scholar] [CrossRef]
- Huang, C.; Peng, Z.; Li, J.; Li, X.; Jiang, X.; Dong, Y. Unlocking the role of lignin for preparing the lignin-based wood adhesive: A review. Ind. Crops Prod. 2022, 187, 115388. [Google Scholar] [CrossRef]
- Tran, M.H.; Lee, E.Y. Production of polyols and polyurethane from biomass: A review. Environ. Chem. Lett. 2023, 21, 2199–2223. [Google Scholar] [CrossRef]
- Li, W.; Sun, H.; Wang, G.; Sui, W.; Dai, L.; Si, C. Lignin as a green and multifunctional alternative to phenol for resin synthesis. Green Chem. 2023, 25, 2241–2261. [Google Scholar] [CrossRef]
- Liu, S.; Du, G.; Yang, H.; Su, H.; Ran, X.; Li, J.; Zhang, L.; Gao, W.; Yang, L. Developing High-Performance Cellulose-Based Wood Adhesive with a Cross-Linked Network. ACS Sustain. Chem. Eng. 2021, 9, 16849–16861. [Google Scholar] [CrossRef]
- Chen, L.; Din, Z.-U.; Yang, D.; Hu, C.; Cai, J.; Xiong, H. Functional nanoparticle reinforced starch-based adhesive emulsion: Toward robust stability and high bonding performance. Carbohydr. Polym. 2021, 269, 118270. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Fredon, E.; Gerardin, C.; Li, J.; Zhou, X.; Du, G. Preparation and properties of a novel type of tannin-based wood adhesive. J. Adhes. 2022, 98, 871–888. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, G.; Zhang, F.; Li, K.; Li, X.; Luo, J.; Li, J.; Li, J. Design of tough, strong and recyclable plant protein-based adhesive via dynamic covalent crosslinking chemistry. Chem. Eng. J. 2023, 460, 141774. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An overview of different types and potential of bio-based adhesives used for wood products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar] [CrossRef]
- Brubaker, C.E.; Messersmith, P.B. The Present and Future of Biologically Inspired Adhesive Interfaces and Materials. Langmuir 2012, 28, 2200–2205. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Wood adhesive properties of cottonseed protein with denaturant additives. J. Adhes. Sci. Technol. 2017, 31, 2657–2666. [Google Scholar] [CrossRef]
- Xiaobo, W.; Wang, X.; Li, Y.; Ma, Y. Properties of a new renewable sesame protein adhesive modified by urea in the absence and presence of zinc oxide. RSC Adv. 2017, 7, 46388–46394. [Google Scholar]
- Liu, X.; Yu, Z.; Li, H.; Zhang, T.; Dong, Y.; Wang, K.; Zhan, X.; Li, Y.; Li, J. Constructing phenylboronic acid-tethered hierarchical kenaf fibers to develop strong soy meal adhesives with excellent water resistant, mildew proof, and flame-retardant properties. Cellulose 2023, 30, 5669–5686. [Google Scholar] [CrossRef]
- Kan, Y.; Kan, H.; Bai, Y.; Zhang, S.; Gao, Z. Effective and environmentally safe self-antimildew strategy to simultaneously improve the mildew and water resistances of soybean flour-based adhesives. J. Clean. Prod. 2023, 392, 136319. [Google Scholar] [CrossRef]
- Jin, S.; Song, X.; Li, K.; Xia, C.; Li, J. A mussel-inspired strategy toward antimicrobial and bacterially anti-adhesive soy protein surface. Polym. Compos. 2020, 41, 633–644. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, W.; Xu, P.; Cai, X.; Dong, W.; Chen, M.; Du, M.; Liu, T.; Lemstra, P.J.; Ma, P. The bonding strength, water resistance and flame retardancy of soy protein-based adhesive by incorporating tailor-made core–shell nanohybrid compounds. Chem. Eng. J. 2022, 428, 132390. [Google Scholar]
- Yue, L.; Meng, Z.; Yi, Z.; Gao, Q.; Mao, A.; Li, J. Effects of Different Denaturants on Properties and Performance of Soy Protein-Based Adhesive. Polymers 2019, 11, 1262. [Google Scholar]
- Doustdar, F.; Olad, A.; Ghorbani, M. Effect of glutaraldehyde and calcium chloride as different crosslinking agents on the characteristics of chitosan/cellulose nanocrystals scaffold. Int. J. Biol. Macromol. 2022, 208, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Yao, S.; Wang, B.-J.; Weng, Y.-M. Preparation and characterization of mung bean starch edible films using citric acid as cross-linking agent. Food Packag. Shelf Life 2022, 32, 100845. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Fan, D.; Wang, Y. Preparation and Characterization of a Robust, High Strength, and Mildew Resistant Fully Biobased Adhesive from Agro-Industrial Wastes. ACS Appl. Polym. Mater. 2021, 3, 5197–5206. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Liu, Z.; Zhang, X.; Luo, J.; Li, J.; Shi, S.Q.; Li, J.; Gao, Q. Constructing SiO2 nanohybrid to develop a strong soy protein adhesive with excellent flame-retardant and coating ability. Chem. Eng. J. 2022, 446, 137065. [Google Scholar] [CrossRef]
- Halstenberg, S.; Panitch, A.; Rizzi, S.; Hall, H.; Hubbell, J.A. Biologically Engineered Protein-graft-Poly(ethylene glycol) Hydrogels: A Cell Adhesive and Plasmin-Degradable Biosynthetic Material for Tissue Repair. Biomacromolecules 2002, 3, 710–723. [Google Scholar] [CrossRef]
- Nivala, O.; Nordlund, E.; Kruus, K.; Ercili-Cura, D. The effect of heat and transglutaminase treatment on emulsifying and gelling properties of faba bean protein isolate. LWT 2021, 139, 110517. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, Y.; Zheng, J.; Cao, Q.; Li, S.; He, L.; Wei, B.; Zhang, J.; Xu, C.; Wang, H. Refolding Behavior of Urea-Induced Denaturation Collagen. Macromol. Res. 2021, 29, 402–410. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, E.; Tu, Y.; Ye, M.; Chen, N. An Eco-Friendly Wood Adhesive Consisting of Soybean Protein and Cardanol-Based Epoxy for Wood Based Composites. Polymers 2022, 14, 2831. [Google Scholar]
- Wang, X.; Zhu, J.; Liu, X.; Zhang, H.J.; Zhu, X. Novel Gelatin-based Eco-friendly Adhesive with a Hyperbranched Cross-linked Structure. Ind. Eng. Chem. Res. 2020, 59, 5500–5511. [Google Scholar] [CrossRef]
- Faggio, N.; Marotta, A.; Ambrogi, V.; Cerruti, P.; Gentile, G. Fully bio-based furan/maleic anhydride epoxy resin with enhanced adhesive properties. J. Mater. Sci. 2023, 58, 7195–7208. [Google Scholar] [CrossRef]
- Sandomierski, M.; Buchwald, T.; Strzemiecka, B.; Voelkel, A. Carbon black modified with 4-hydroxymethylbenzenediazonium salt as filler for phenol-formaldehyde resins and abrasive tools. J. Appl. Polym. Sci. 2020, 137, 48160. [Google Scholar] [CrossRef]
- Li, C. Synthesis, Characterization and Curing Mechanism of Biomass Modified Phenol Formaldehyde Resin; Beijing Forestry University: Beijing, China, 2018. [Google Scholar]
- Li, H.; Wang, Y.; Xie, W.; Tang, Y.; Yang, F.; Gong, C.; Wang, C.; Li, X.; Li, C. Preparation and Characterization of Soybean Protein Adhesives Modified with an Environmental-Friendly Tannin-Based Resin. Polymers 2023, 15, 2289. [Google Scholar]
- Zhang, Y.; Shi, R.; Xu, Y.; Chen, M.; Zhang, J.; Gao, Q.; Li, J. Developing a stable high-performance soybean meal-based adhesive using a simple high-pressure homogenization technology. J. Clean. Prod. 2020, 256, 120336. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Ye, Q.; Gao, Q.; Gong, S.; Li, J.; Shi, S.Q. Bio-inspired co-deposition strategy of aramid fibers to improve performance of soy protein isolate-based adhesive. Ind. Crops Prod. 2020, 150, 112424. [Google Scholar] [CrossRef]
- Lei, H.; Wu, Z.; Cao, M.; Du, G. Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol. Polymers 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, H.; Li, C. Development of Eco-Friendly Soy Meal Adhesives Enhanced by Ethylene Glycol Diglycidyl Ether. Adv. Polym. Technol. 2019, 2019, 8697047. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Gao, Q.; Zhang, S.; Li, J. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crops Prod. 2014, 59, 35–40. [Google Scholar] [CrossRef]
- Mo, X.; Sun, X.; Wang, D. Thermal properties and adhesion strength of modified soybean storage proteins. J. Am. Oil Chem. Soc. 2004, 81, 395–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. Preparation and characterization of a soy protein-based high-performance adhesive with a hyperbranched cross-linked structure. Chem. Eng. J. 2018, 354, 1032–1041. [Google Scholar] [CrossRef]
- Zhiyong, Q.; Qiang, G.; Shifeng, Z.; Jianzhang, L. Glycidyl Methacrylate Grafted onto Enzyme-Treated Soybean Meal Adhesive with Improved Wet Shear Strength. BioResources 2013, 8, 5369–5379. [Google Scholar] [CrossRef]
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Yang, S.; Fan, D. Developing an antifungal and high-strength soy protein-based adhesive modified by lignin-based polymer. Ind. Crops Prod. 2021, 170, 113795. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, G.; Zhang, F.; Tang, Z.; Luo, J.; Li, K.; Li, X.; Li, J.; Shi, S.Q. Preparation of functional fiber hybrid enhanced high strength and multifunctional protein based adhesive. Mater. Des. 2022, 224, 111289. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Y.; Gao, Q.; Li, J.; Yan, N. From Wastes to Functions: A New Soybean Meal and Bark-Based Adhesive. ACS Sustain. Chem. Eng. 2020, 8, 10767–10773. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Zhang, Y.; Liu, Z.; Luo, J.; Li, J.; Li, J.; Gao, Q. A high bonding performance and antibacterial soybean meal adhesive with Maillard reaction based cross-linked structure. Compos. Part B Eng. 2021, 227, 109403. [Google Scholar] [CrossRef]
- Ma, C.; Pang, H.; Shen, Y.; Liang, Z.; Li, J.; Zhang, S.; Shi, J. Plant Polyphenol-Inspired Crosslinking Strategy toward High Bonding Strength and Mildew Resistance for Soy Protein Adhesives. Macromol. Mater. Eng. 2021, 306, 2100543. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Y.; Bian, Y.; Gao, Q.; Shi, S.Q.; Cao, J.; Zhang, Q.; Li, J. A novel universal strategy for fabricating soybean protein adhesive with excellent adhesion and anti-mildew performances. Chem. Eng. J. 2023, 452, 139359. [Google Scholar] [CrossRef]

| Adhesives | SM (g) | Deionized Water (g) | Urea (g) | NaOH (g) | TR (g) | CA (g) | Solid Content (%) |
|---|---|---|---|---|---|---|---|
| SM | 20 | 100 | 0 | 0 | 0 | 0 | 14.23 ± 0.32 |
| SM/TR | 20 | 100 | 0 | 0 | 9 | 0 | 20.16 ± 0.14 |
| SM/Urea/TR | 20 | 100 | 1 | 0 | 9 | 0 | 19.92 ± 0.43 |
| SM/NaOH/TR | 20 | 100 | 0 | 1 | 9 | 0 | 21.98 ± 0.33 |
| SM/TR/CA | 20 | 100 | 0 | 0 | 9 | 4.5 | 24.32 ± 0.31 |
| SM/Urea/TR/CA | 20 | 100 | 1 | 0 | 9 | 4.5 | 22.96 ± 0.42 |
| SM/NaOH/TR/CA | 20 | 100 | 0 | 1 | 9 | 4.5 | 25.06 ± 0.58 |
| Adhesive | SM | SM/TR | SM/Urea/TR | SM/NaOH/TR |
|---|---|---|---|---|
| Crystallinity (%) | 16.76 | 13.34 | 12.91 | 10.73 |
| Adhesive | First-Stage Tmax (°C) | Second-Stage Tmax (°C) | Third-Stage Tmax (°C) | Total Weight Loss Rate (%) |
|---|---|---|---|---|
| SM | – | 302.53 | – | 66.21 |
| SM/TR | – | 298.6 | 340.26 | 64.47 |
| SM/Urea/TR | – | 293.29 | 350.24 | 57.85 |
| SM/NaOH/TR | – | 288.47 | 344.34 | 54.41 |
| SM/TR/CA | – | 320.33 | 420.06 | 52.39 |
| SM/Urea/TR/CA | – | 315.04 | 390.3 | 53.68 |
| SM/NaOH/TR/CA | – | 310.16 | 370.74 | 53.92 |
| Adhesive | Wet Shear Strength (MPa) (63 ± 3 °C) | References |
|---|---|---|
| SM/EPL | 1.02 | [41] |
| SM/CFF-LDH-TA2 | 1.60 | [42] |
| SM/BEP-10 | 0.89 | [43] |
| SM/BD/5%HPFC | 1.05 | [44] |
| SMPT-2 | 1.03 | [45] |
| SM@BDAB-HDE-6 | 1.25 | [46] |
| SM/NaOH/TR/CA | 1.17 | Our study |
| SM/NaOH/TR | 1.16 | Our study |
| SM/TR/CA | 1.14 | Our study |
| SM/PMDI * | 1.21 | Commercial |
| SM/ER * | 1.19 | Commercial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Gao, Y.; Zhao, Z.; Yang, F.; Zou, Y.; Wang, Y.; Tang, Y.; Zhou, Q.; Li, C. Low-Cost and High-Strength Soybean Meal Adhesives Modified by Tannin–Phenol–Formaldehyde Resin. Forests 2023, 14, 1947. https://doi.org/10.3390/f14101947
Li H, Gao Y, Zhao Z, Yang F, Zou Y, Wang Y, Tang Y, Zhou Q, Li C. Low-Cost and High-Strength Soybean Meal Adhesives Modified by Tannin–Phenol–Formaldehyde Resin. Forests. 2023; 14(10):1947. https://doi.org/10.3390/f14101947
Chicago/Turabian StyleLi, Hanyin, Yan Gao, Zijie Zhao, Fan Yang, Yunming Zou, Yujie Wang, Yang Tang, Qiongqiong Zhou, and Cheng Li. 2023. "Low-Cost and High-Strength Soybean Meal Adhesives Modified by Tannin–Phenol–Formaldehyde Resin" Forests 14, no. 10: 1947. https://doi.org/10.3390/f14101947
APA StyleLi, H., Gao, Y., Zhao, Z., Yang, F., Zou, Y., Wang, Y., Tang, Y., Zhou, Q., & Li, C. (2023). Low-Cost and High-Strength Soybean Meal Adhesives Modified by Tannin–Phenol–Formaldehyde Resin. Forests, 14(10), 1947. https://doi.org/10.3390/f14101947






