Review of Managing Soil Organic C Sequestration from Vegetation Restoration on the Loess Plateau
Abstract
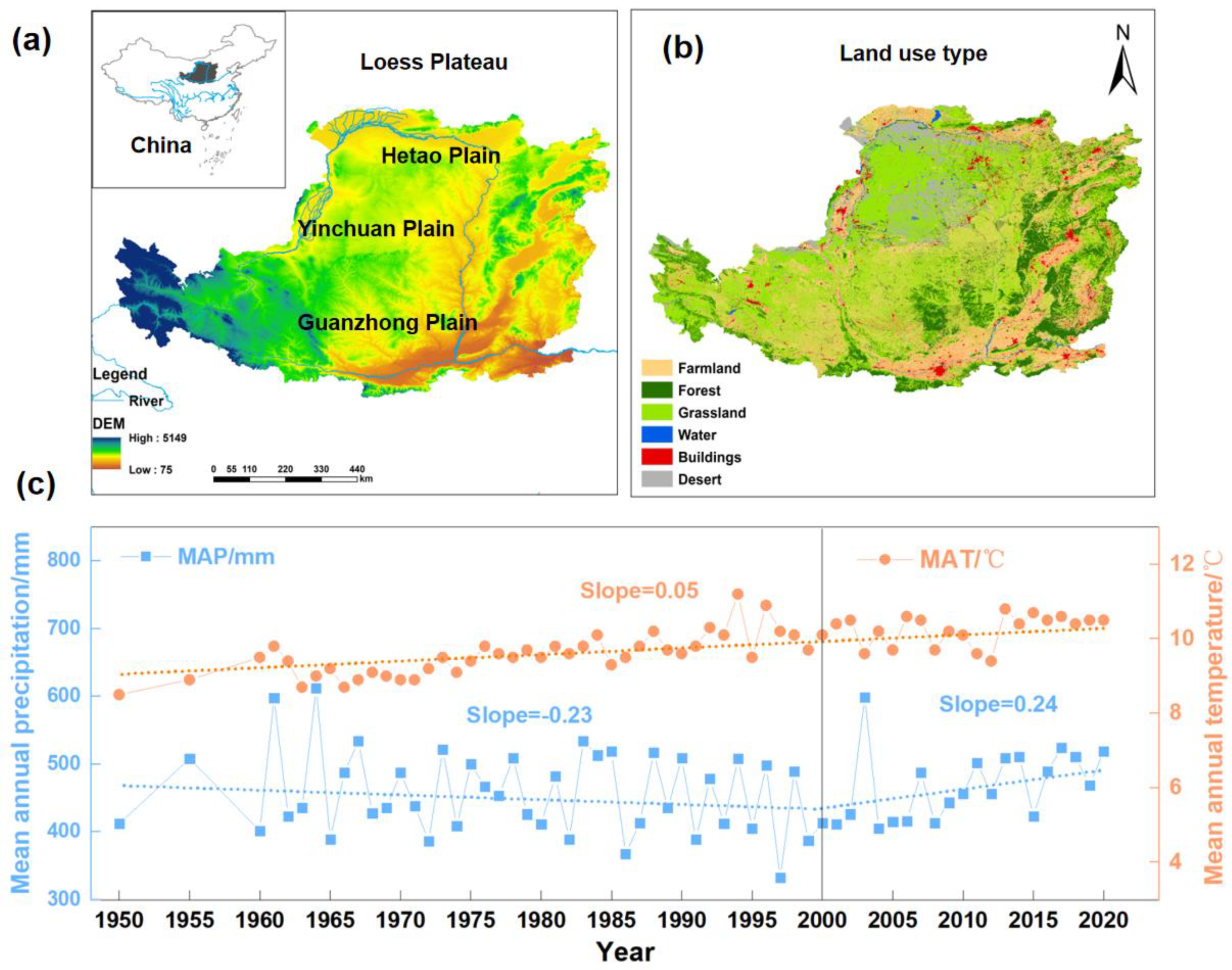
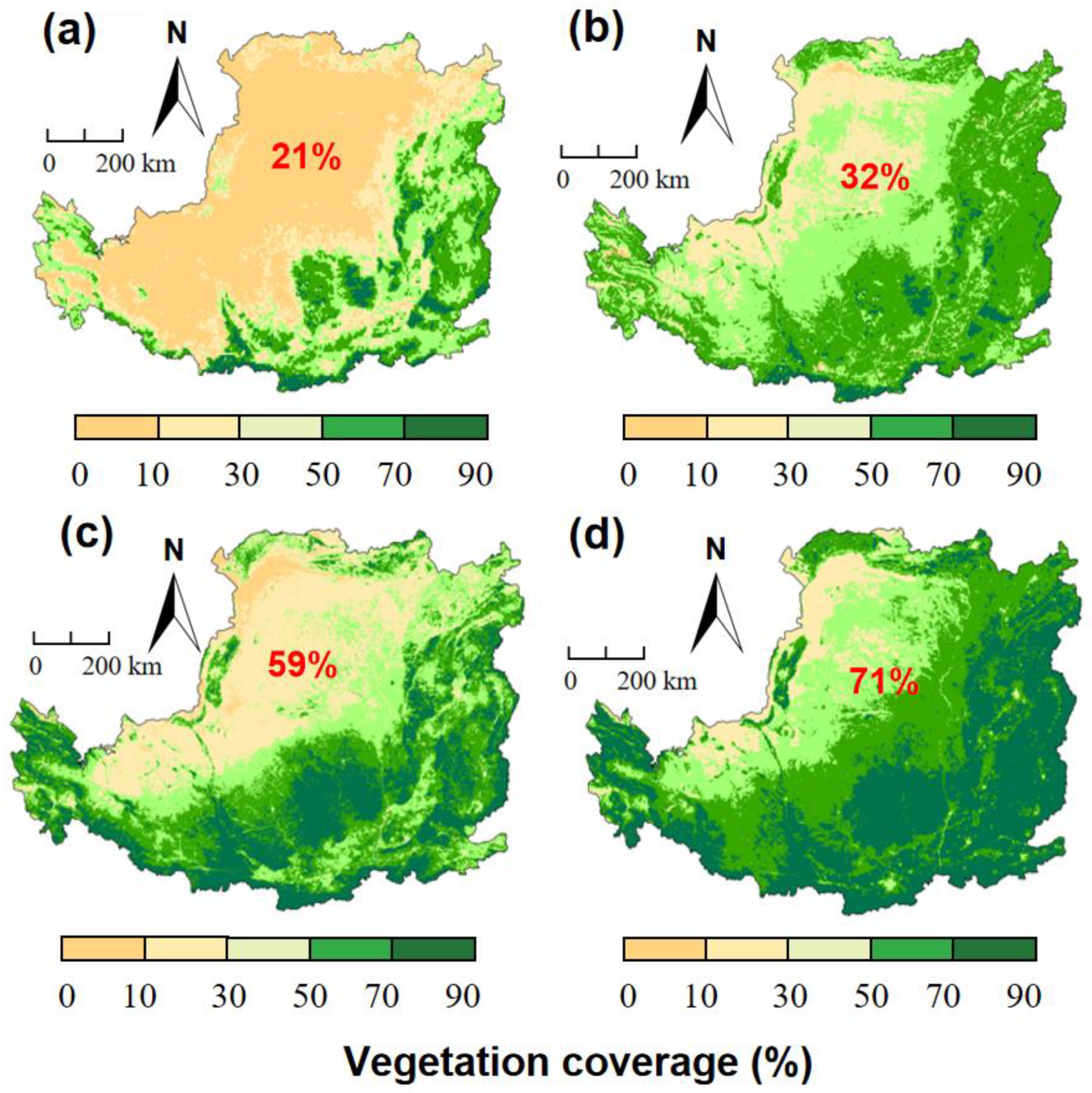
:1. Introduction
2. SOC Storage Following Vegetation Restoration on the Loess Plateau
3. SOC Sequestration Mechanism Following Vegetation Restoration
3.1. Soil Chemical C Sequestration
3.2. Soil Physical C Sequestration
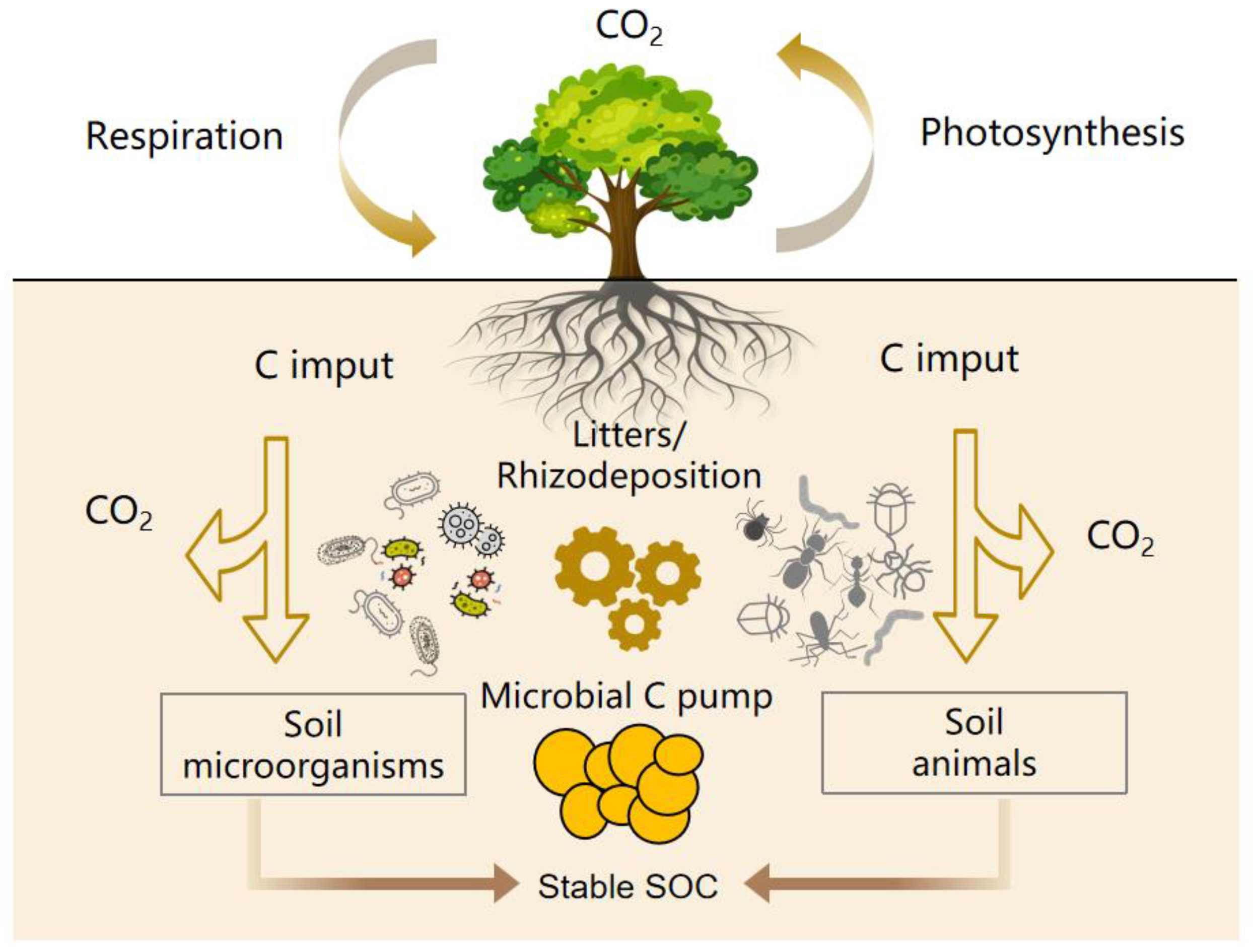
3.3. Soil Microbial C Sequestration
4. Controlling Factors of SOC on the Loess Plateau
5. Managing for SOC Sequestration on the Loess Plateau
6. Prospectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, B.; Wang, S.; Liu, Y.; Liu, J.; Liang, W.; Miao, C. Hydrogeomorphic ecosystem responses to natural and anthropogenic changes on the Loess Plateau of China. Annu. Rev. Earth Planet. Sci. 2017, 45, 223–243. [Google Scholar] [CrossRef]
- An, Z.S.; Kukla, G.J.; Porter, S.C.; Xiao, J. Magnetic susceptibility evidence of monsoon variation on the Loess Plateau of central China during the last 130,000 years. Quat. Res. 1991, 36, 29–36. [Google Scholar] [CrossRef]
- Liu, T.S. The Deposition of Loess in China; Science Press: Beijing, China, 1965. (In Chinese) [Google Scholar]
- An, Z.S.; Wu, G.X.; Li, J.P.; Sun, Y.B.; Liu, Y.M.; Zhou, W.J.; Cai, Y.J.; Duan, A.M.; Li, L.; Mao, J.Y.; et al. Global monsoon dynamics and climate change. Annu. Rev. Earth Planet. Sci. 2015, 43, 29–77. [Google Scholar] [CrossRef]
- Sun, Y.; McManus, J.F.; Clemens, S.C.; Zhang, X.; Vogel, H.; Hodell, D.A.; An, Z. Persistent orbital influence on millennial climate variability through the Pleistocene. Nat. Geosci. 2021, 14, 812–818. [Google Scholar] [CrossRef]
- Feng, X.; Fu, B.; Lu, N.; Zeng, Y.; Wu, B. How ecological restoration alters ecosystem services: An analysis of carbon sequestration in China’s Loess Plateau. Sci. Rep. 2013, 3, 2846. [Google Scholar] [CrossRef]
- Lv, Y.; Hu, J.; Fu, B.; Harris, P.; Wu, L.; Tong, X.; Comber, A.J. A framework for the regional critical zone classification: The case of the Chinese Loess Plateau. Natl. Sci. Rev. 2019, 6, 14–18. [Google Scholar] [CrossRef]
- Kou, M.; Jiao, J.; Yin, Q.; Wang, N.; Wang, Z.; Li, Y.; Cao, B. Successional trajectory over 10 years of vegetation restoration of abandoned slope croplands in the hill-gully region of the Loess Plateau. Land Degrad. Dev. 2016, 27, 919–932. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lin, Y.; Shi, W.; Song, Y.; He, X. Balancing green and grain trade. Nat. Geosci. 2015, 10, 739–741. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Cheng, W.; Fu, B.; Wang, Y.; Lü, Y. Evaluation of amsr-e retrieval by detecting soil moisture decrease following massive dryland re-vegetation on the Loess Plateau, China. Remote Sens. Environ. 2017, 196, 253–264. [Google Scholar] [CrossRef]
- Fu, B.; Wu, X.; Wang, Z.; Wu, X.; Wang, S. Coupling human and natural systems for sustainability: Experience from China’s Loess Plateau. Earth Syst. Dynam. 2022, 13, 795–808. [Google Scholar] [CrossRef]
- Greve, P.; Roderick, M.L.; Ukkola, A.M.; Wada, Y. The aridity index under global warming. Environ. Res. Lett. 2019, 14, 124006. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yu, G.; Liu, L.; Hu, S.; Chapin, F.S., III. Climate change, human impacts, and carbon sequestration in China. Proc. Natl. Acad. Sci. USA 2018, 115, 4015–4020. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Digging Deeper: A Holistic Perspective of Factors Affecting Soil Organic Carbon Sequestration in Agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Piao, S.; He, Y.; Wang, X.; Chen, F. Estimation of China’s terrestrial ecosystem carbon sink: Methods, progress and prospects. Sci. China Earth Sci. 2022, 65, 641–651. [Google Scholar] [CrossRef]
- Ouyang, Z.Y.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.G.; Xu, W.H.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E.M.; et al. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef]
- Feng, X.; Fu, B.; Piao, S.; Wang, S.; Ciais, P.; Zeng, Z. Revegetation in China’s loess plateau is approaching sustainable water resource limits. Nat. Clim. Chang. 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Li, Y.Y.; Shao, M.A. Change of soil physical properties under long-term natural vegetation restoration on the Loess Plateau of China. J. Arid Environ. 2006, 64, 77–96. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, Z.; Bai, W.; Jia, Y.; Wang, N. Assessing the ecological success of restoration by afforestation on the Chinese Loess Plateau. Restor. Ecol. 2012, 20, 240–249. [Google Scholar] [CrossRef]
- An, S.S.; Darboux, F.; Cheng, M. Revegetation as an efficient means of increasing soil aggregate stability on the Loess Plateau (China). Geoderma 2013, 209, 75–85. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.; Shangguan, Z. Land-use conversion and changing soil carbon stocks in China’s ‘grain-for-green’ program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shao, M.; Wei, X.; Horton, R. Soil organic carbon and total nitrogen as affected by vegetation types in Northern Loess Plateau of China. Geoderma 2010, 155, 31–35. [Google Scholar] [CrossRef]
- Lv, Y.; Fu, B.; Feng, X.; Zeng, Y.; Liu, Y.; Chang, R.; Wu, B. A policy-driven large scale ecological restoration: Quantifying ecosystem services changes on the Loess Plateau of China. PLoS ONE 2012, 7, e31782. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, B.; Lü, Y.; Chen, L. Effects of vegetation restoration on soil organic carbon sequestration at multiple scales in semi-arid Loess Plateau, China. Catena 2011, 85, 58–66. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Guo, Q.; Henderson, M.; Ji, W.; Li, Y.; Tao, S. Interannual variations of monthly and seasonal normalized difference vegetation index (NDVI) in China from 1982 to 1999. J. Geophys. Res. Atmos. 2003, 108, 4401. [Google Scholar] [CrossRef]
- Chen, P.; Shang, J.; Qian, B.; Jing, Q.; Liu, J. A new regionalization scheme for effective ecological restoration on the Loess Plateau in China. Remote Sens. 2017, 9, 1323. [Google Scholar] [CrossRef]
- Jiang, W.; Cheng, Y.; Yang, X.; Yang, S. Chinese Loess Plateau vegetation since the Last Glacial Maximum and its implications for vegetation restoration. J. Appl. Ecol. 2013, 50, 440–448. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, Y.; Wang, Y.; Wei, X.; Wang, Y.; Cui, B.; Zhou, W. Natural vegetation restoration is more beneficial to soil surface organic and inorganic carbon sequestration than tree plantation on the Loess Plateau of China. Sci. Total Environ. 2014, 485, 615–623. [Google Scholar] [CrossRef]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.; Torn, M.S. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, P.; Wu, F.; Wang, Y.; Xu, C.; Kuzyakov, Y. Large-scale ecosystem carbon stocks and their driving factors across Loess Plateau. Carbon Neutrality 2023, 2, 5. [Google Scholar] [CrossRef]
- van Straaten, O.; Corre, M.D.; Wolf, K.; Tchienkoua, M.; Cuellar, E.; Matthews, R.B.; Veldkamp, E. Conversion of lowland tropical forests to tree cash crop plantations loses up to one-half of stored soil organic carbon. Proc. Natl. Acad. Sci. USA 2015, 112, 9956–9960. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, J.L.; Davies, Z.G.; McCormack, S.A.; Gaston, K.J.; Leake, J.R. Land-cover effects on soil organic carbon stocks in a European city. Sci. Total Environ. 2014, 472, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dou, Y.; Wang, B.; Wang, Y.; Liang, C.; An, S.; Kuzyakov, Y. Increasing contribution of microbial residues to soil organic carbon in grassland restoration chronosequence. Soil Biol. Biochem. 2022, 170, 108688. [Google Scholar] [CrossRef]
- Cheng, M.; Xue, Z.; Xiang, Y.; Darboux, F.; An, S. Soil organic carbon sequestration in relation to revegetation on the Loess Plateau, China. Plant Soil 2015, 397, 31–42. [Google Scholar] [CrossRef]
- Tong, X.W.; Brandt, M.; Yue, Y.M.; Horion, S.; Wang, K.L.; Keersmaecker, W.; Tian, F.; Schurgers, G.; Xiao, X.M.; Luo, Y.Q.; et al. Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat. Sustain. 2018, 1, 44–50. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.P.; Wu, G.L.; Chang, X.F. Effects of grazing exclusion on carbon sequestration in China’s grassland. Earth-Sci. Rev. 2017, 173, 84–95. [Google Scholar] [CrossRef]
- Fu, B.; Yu, L.; Lü, Y.; He, C.; Yuan, Z.; Wu, B. Assessing the soil erosion control service of ecosystems change on the Loess Plateau of China. Ecol. Complex. 2011, 8, 284–293. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Hu, P.M.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; Wang, K.L.; Su, Y.R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.; Bell, S.M.; Soromotin, A.V.; Peng, C.; An, S.; Kuzyakov, Y. Carbon in Chinese grasslands: Meta-analysis and theory of grazing effects. Carbon Res. 2023, 2, 19. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, A.J.; Wilson, K.P.; Dudley Williams, D.; Simpson, M.J. Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat. Geosci. 2008, 1, 836–839. [Google Scholar] [CrossRef]
- An, S.S.; Mentler, A.; Mayer, H.; Blum, W.E. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Mitchell, E.; Scheer, C.; Rowlings, D.; Cotrufo, F.; Conant, R.T.; Grace, P. Important constraints on soil organic carbon formation efficiency in subtropical and tropical grasslands. Glob. Chang. Biol. 2021, 27, 5383–5391. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Plaza, C.; Courtier-Murias, D.; Fernández, J.M.; Polo, A.; Simpson, A.J. Physical, chemical, and biochemical mechanisms of soil organic matter stabilization under conservation tillage systems: A central role for microbes and microbial by-products in C sequestration. Soil Biol. Biochem. 2013, 57, 124–134. [Google Scholar] [CrossRef]
- Miralles, I.; Ortega, R.; Sánchez-Marañón, M.; Soriano, M.; Almendros, G. Assessment of biogeochemical trends in soil organic matter sequestration in Mediterranean calcimorphic mountain soils (Almería, Southern Spain). Soil Biol. Biochem. 2007, 39, 2459–2470. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Schroth, A.W.; Bostick, B.C.; Kaste, J.M.; Friedland, A.J. Lead sequestration and species redistribution during soil organic matter decomposition. Environ. Sci. Technol. 2008, 42, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Krause, H.M.; Fliessbach, A.; Mäder, P.; Steffens, M. Fertilizer quality and labile soil organic matter fractions are vital for organic carbon sequestration in temperate arable soils within a long-term trial in Switzerland. Geoderma 2022, 426, 116080. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, W.; Ma, M.; Zhang, X.; Bao, Z.; Xie, S.; Yan, S. The role of laccase in stabilization of soil organic matter by iron in various plant-DOCinated peatlands: Degradation or sequestration? Plant Soil 2019, 443, 575–590. [Google Scholar] [CrossRef]
- Del Galdo, I.; Six, J.; Peressotti, A.; Francesca Cotrufo, M. Assessing the impact of land-use change on soil C sequestration in agricultural soils by means of organic matter fractionation and stable C isotopes. Glob. Chang. Biol. 2003, 9, 1204–1213. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Amundson, R. Managing for soil carbon sequestration: Let’s get realistic. Glob. Chang. Biol. 2019, 25, 386–389. [Google Scholar] [CrossRef]
- Yu, H.; Zha, T.; Zhang, X.; Ma, L. Vertical distribution and influencing factors of soil organic carbon in the Loess Plateau, China. Sci. Total Environ. 2019, 693, 133632. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, M.A.; Wang, Y. Effect of environmental factors on regional soil organic carbon stocks across the Loess Plateau region, China. Agric. Ecosyst. Environ. 2011, 142, 184–194. [Google Scholar] [CrossRef]
- Han, F.; Hu, W.; Zheng, J.; Du, F.; Zhang, X. Estimating soil organic carbon storage and distribution in a catchment of Loess Plateau, China. Geoderma 2010, 154, 261–266. [Google Scholar] [CrossRef]
- Zhu, F.; Li, Y.; Xue, S.; Hartley, W.; Wu, H. Effects of iron-aluminium oxides and organic carbon on aggregate stability of bauxite residues. Environ. Sci. Pollut. R. 2016, 23, 9073–9081. [Google Scholar] [CrossRef]
- Fan, X.; Gao, D.; Zhao, C.; Wang, C.; Qu, Y.; Zhang, J.; Bai, E. Improved model simulation of soil carbon cycling by representing the microbially derived organic carbon pool. ISME J. 2021, 15, 2248–2263. [Google Scholar] [CrossRef]
- Just, C.; Armbruster, M.; Barkusky, D.; Baumecker, M.; Diepolder, M.; Döring, T.F.; Wiesmeier, M. Soil organic carbon sequestration in agricultural long-term field experiments as derived from particulate and mineral-associated organic matter. Geoderma 2023, 434, 116472. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Jilkova, V.; Jandová, K.; Sim, A.; Thornton, B.; Paterson, E. Soil organic matter decomposition and carbon sequestration in temperate coniferous forest soils affected by soluble and insoluble spruce needle fractions. Soil Biol. Biochem. 2019, 138, 107595. [Google Scholar] [CrossRef]
- Hedges, J.I.; Baldock, J.A.; Gélinas, Y.; Lee, C.; Peterson, M.; Wakeham, S.G. Evidence for non-selective preservation of organic matter in sinking marine particles. Nature 2001, 409, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Benbi, D.K.; Boparai, A.K.; Brar, K. Decomposition of particulate organic matter is more sensitive to temperature than the mineral associated organic matter. Soil Biol. Biochem. 2014, 70, 183–192. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Weiwen, Q.I.U.; Sharp, J. Does particulate organic matter fraction meet the criteria for a model soil organic matter pool? Pedosphere 2019, 29, 195–203. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Helfrich, M.; Ludwig, B.; Buurman, P.; Flessa, H. Effect of land use on the composition of soil organic matter in density and aggregate fractions as revealed by solid-state 13C NMR spectroscopy. Geoderma 2006, 136, 331–341. [Google Scholar] [CrossRef]
- Liu, F.; Qin, S.; Fang, K.; Chen, L.; Peng, Y.; Smith, P.; Yang, Y. Divergent changes in particulate and mineral-associated organic carbon upon permafrost thaw. Nat. Commun. 2022, 13, 5073. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Liu, X.; Xiao, L.; Shi, P.; Zhao, B. Effects of farmland conversion on the stoichiometry of carbon, nitrogen, and phosphorus in soil aggregates on the Loess Plateau of China. Geoderma 2019, 351, 188–196. [Google Scholar] [CrossRef]
- Henson, S.A.; Sanders, R.; Madsen, E. Global patterns in efficiency of particulate organic carbon export and transfer to the deep ocean. Glob. Biogeochem. Cycles 2012, 26, GB1028. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Wander, M.M.; Bidart, M.G. Tillage practice influences on the physical protection, bioavailability and composition of particulate organic matter. Biol. Fert. Soils 2000, 32, 360–367. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Kan, Z.R.; Liu, W.X.; Liu, W.S.; Lal, R.; Dang, Y.P.; Zhao, X.; Zhang, H.L. Mechanisms of soil organic carbon stability and its response to no-till: A global synthesis and perspective. Glob. Chang. Biol. 2022, 28, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wu, W.; Lin, D.; Yang, K. Adsorption of soil organic matter by gel-like ferrihydrite and dense ferrihydrite. Sci. Total Environ. 2022, 835, 155507. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Chen, Y.; An, S.; Zhang, L.; Chen, F.; Cai, W. Influence of Dissolved Organic Carbon on the Adsorption and Desorption of Cadmium in Reclaimed Soil. Nat. Environ. Pollut. Technol. 2022, 21, 405–412. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Yang, R.; Chen, T.; Li, G. The rare earth elements of sequentially leached phases in the loess-paleosol sequence at Weinan on the southeastern Chinese Loess Plateau. Quatern. Int. 2023, 669, 43–52. [Google Scholar] [CrossRef]
- Arenas, L.R.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef]
- Wu, H.; Wei, H.; Yang, X.; Jin, C.; Sun, W.; Deng, K.; Sun, C. Spherical activated carbons derived from resin-microspheres for the adsorption of acetic acid. J. Environ. Chem. Eng. 2023, 11, 109394. [Google Scholar] [CrossRef]
- Rong, Y.; Pan, C.; Song, K.; Nam, J.C.; Wu, F.; You, Z.; Zhang, Z. Bamboo-derived hydrophobic porous graphitized carbon for adsorption of volatile organic compounds. Chem. Eng. J. 2023, 461, 141979. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; Van Nostrand, J.; Wu, L.; et al. Warming enhances old organic carbon decomposition through altering functional microbial communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, S.R.; Kemanian, A.R.; Ernst, O.R.; Jackson, R.B.; Piñeiro, G. Priming of soil organic carbon decomposition induced by corn compared to soybean crops. Soil Biol. Biochem. 2014, 75, 273–281. [Google Scholar] [CrossRef]
- Hopkins, F.M.; Filley, T.R.; Gleixner, G.; Lange, M.; Top, S.M.; Trumbore, S.E. Increased belowground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biol. Biochem. 2014, 76, 57–69. [Google Scholar] [CrossRef]
- Moinet, G.Y.; Hunt, J.E.; Kirschbaum, M.U.; Morcom, C.P.; Midwood, A.J.; Millard, P. The temperature sensitivity of soil organic matter decomposition is constrained by microbial access to substrates. Soil Biol. Biochem. 2018, 116, 333–339. [Google Scholar] [CrossRef]
- Mueller, P.; Jensen, K.; Megonigal, J.P. Plants mediate soil organic matter decomposition in response to sea level rise. Glob. Chang. Biol. 2016, 22, 404–414. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Lavallee, J.M. Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. Adv. Agron. 2022, 172, 1–66. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, M.J. Molecular-level methods for monitoring soil organic matter responses to global climate change. J. Environm. Monitor. 2011, 13, 1246–1254. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Gunina, A.; Kuzyakov, Y. From energy to (soil organic) matter. Global Chang. Biol. 2022, 28, 2169–2182. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, J.; Bastida, F.; Delgado-Baquerizo, M.; Yang, Y.; Wang, J.; Zhao, F. Microbial traits determine soil C emission in response to fresh carbon inputs in forests across biomes. Glob. Chang. Biol. 2022, 28, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Guo, J.; Wang, X.; Du, Z.; Ren, R.; Lu, S.; He, C. Afforestation alters the molecular composition of soil organic matter in the central Loess Plateau of China. Forests 2023, 14, 1502. [Google Scholar] [CrossRef]
- Yuan, H.; Ge, T.; Chen, C.; O’Donnell, A.G.; Wu, J. Significant role for microbial autotrophy in the sequestration of soil carbon. Appl. Environm. Microb. 2012, 78, 2328–2336. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef]
- Yuan, H.; Ge, T.; Zou, S.; Wu, X.; Liu, S.; Zhou, P. Effect of land use on the abundance and diversity of autotrophic bacteria as measured by ribulose-1,5-biphosphate carboxylase/oxygenase (rubisco) large subunit gene abundance in soils. Biol. Fert. Soils 2013, 49, 609–616. [Google Scholar] [CrossRef]
- Huang, L.; Shao, M. Advances and perspectives on soil water research in China’s Loess Plateau. Earth-Sci. Rev. 2019, 199, 102962. [Google Scholar] [CrossRef]
- Tuo, D.; Gao, G.; Chang, R.; Li, Z.; Ma, Y.; Wang, S.; Fu, B. Effects of revegetation and precipitation gradient on soil carbon and nitrogen variations in deep profiles on the Loess Plateau of China. Sci. Total Environ. 2008, 626, 399–411. [Google Scholar] [CrossRef]
- Lan, Z.; Zhao, Y.; Zhang, J.; Jiao, R.; Khan, M.N.; Sial, T.A.; Si, B. Long-term vegetation restoration increases deep soil carbon storage in the Northern Loess Plateau. Sci. Rep. 2021, 11, 13758. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Comparing the effects of soil fauna on litter decomposition and organic matter turnover in sustainably and conventionally managed olive orchards. Geoderma 2020, 372, 114393. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.; Cheng, H.; An, S.; Chang, S.X. Soil extracellular enzyme stoichiometry reflects the shift from P-to N-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Bagchi, S.; Roy, S.; Maitra, A.; Sran, R.S. Herbivores suppress soil microbes to influence carbon sequestration in the grazing ecosystem of the Trans-Himalaya. Agric. Ecosyst. Environ. 2017, 239, 199–206. [Google Scholar] [CrossRef]
- Aynekulu, E.; Mekuria, W.; Tsegaye, D.; Feyissa, K.; Angassa, A.; de Leeuw, J.; Shepherd, K. Long-term livestock exclosure did not affect soil carbon in southern Ethiopian rangelands. Geoderma 2017, 307, 1–7. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.A.; Wang, P.; Zhang, J.; Li, Y.; Munir, M.Z.; Ji, B. Formation of common mycorrhizal networks significantly affects plant biomass and soil properties of the neighboring plants under various nitrogen levels. Microorganisms 2020, 8, 230. [Google Scholar] [CrossRef]
- Kong, W.; Wei, X.; Wu, Y.; Shao, M.; Zhang, Q.; Sadowsky, M.J.; Liu, L. Afforestation can lower microbial diversity and functionality in deep soil layers in a semiarid region. Glob. Chang. Biol. 2022, 28, 6086–6101. [Google Scholar] [CrossRef]
- Wen, S.; Chen, J.; Yang, Z.; Deng, L.; Feng, J.; Zhang, W.; Liu, Y.R. Climatic seasonality challenges the stability of microbial-driven deep soil carbon accumulation across China. Glob. Chang. Biol. 2023, 29, 4430–4439. [Google Scholar] [CrossRef]
- Wood, S.A.; Bowman, M. Large-scale farmer-led experiment demonstrates positive impact of cover crops on multiple soil health indicators. Nat. Food 2021, 2, 97–103. [Google Scholar] [CrossRef]
- Wani, O.A.; Kumar, S.S.; Hussain, N. Multi-scale processes influencing global carbon storage and land-carbon-climate nexus: A critical review. Pedosphere 2023, 33, 250–267. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A.; Schomberg, H.H.; Wilkinson, S.R. Soil organic C and N pools under long-term pasture management in the Southern Piedmont USA. Soil Biol. Biochem. 2000, 32, 469–478. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Li, N.; Yao, H.; Yang, E.; Soromotin, A.V.; An, S. Initial soil formation by biocrusts: Nitrogen demand and clay protection control microbial necromass accrual and recycling. Soil Biol. Biochem. 2022, 167, 108607. [Google Scholar] [CrossRef]
- Oelmann, Y.; Lange, M.; Leimer, S.; Roscher, C.; Aburto, F.; Alt, F.; Wilcke, W. Above-and belowground biodiversity jointly tighten the P cycle in agricultural grasslands. Nat. Commun. 2021, 12, 4431. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.S.; Kaštovská, E.; Körner, C.; Lambers, H.; Ostonen, I. Surplus carbon drives allocation and plant–soil interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Wei, Y.X.; Li, R.C.; Chen, Z.; Wang, H.D.; Virk, A.L.; Zhang, H.L. Improving soil aggregates stability and soil organic carbon sequestration by no-till and legume-based crop rotations in the North China Plain. Sci. Total Environ. 2022, 847, 157518. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, Y.; Wu, J.; Liao, Y.; Li, J.; Yu, J.; Deng, L. The distribution of soil C and N along the slope is regulated by vegetation type on the Loess Plateau. Catena 2023, 226, 107094. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Carvalho, J.L.N.; Cerri, C.E.P.; Lal, R.; Bernoux, M.; Galdos, M.V.; Cerri, C.C. Contrasting approaches for estimating soil carbon changes in Amazon and Cerrado biomes. Soil Tillage Res. 2013, 133, 75–84. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, D.; Yao, P.; Zhao, N.; Liu, N.; Zhai, B.; Gao, Y. Coupling life-cycle assessment and the RothC model to estimate the carbon footprint of green manure-based wheat production in China. Sci. Total Environ. 2017, 607, 433–442. [Google Scholar] [CrossRef]
- Hirte, J.; Leifeld, J.; Abiven, S.; Oberholzer, H.R.; Mayer, J. Below ground carbon inputs to soil via root biomass and rhizodeposition of field-grown maize and wheat at harvest are independent of net primary productivity. Agric. Ecosyst. Environ. 2018, 265, 556–566. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Sun, H.; Zhang, P.; Wu, F.; Qiao, J.; Li, T.; Wang, Y.; An, S. Review of Managing Soil Organic C Sequestration from Vegetation Restoration on the Loess Plateau. Forests 2023, 14, 1964. https://doi.org/10.3390/f14101964
Yang Y, Sun H, Zhang P, Wu F, Qiao J, Li T, Wang Y, An S. Review of Managing Soil Organic C Sequestration from Vegetation Restoration on the Loess Plateau. Forests. 2023; 14(10):1964. https://doi.org/10.3390/f14101964
Chicago/Turabian StyleYang, Yang, Hui Sun, Pingping Zhang, Fan Wu, Jiangbo Qiao, Tongchuan Li, Yunqiang Wang, and Shaoshan An. 2023. "Review of Managing Soil Organic C Sequestration from Vegetation Restoration on the Loess Plateau" Forests 14, no. 10: 1964. https://doi.org/10.3390/f14101964
APA StyleYang, Y., Sun, H., Zhang, P., Wu, F., Qiao, J., Li, T., Wang, Y., & An, S. (2023). Review of Managing Soil Organic C Sequestration from Vegetation Restoration on the Loess Plateau. Forests, 14(10), 1964. https://doi.org/10.3390/f14101964






