Illuminating Plant Community Assembly on Karst Mountain Road Slopes through Plant Traits and Environmental Filters
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Sampling Design
2.3. Materials and Methods
2.3.1. Species Identification
2.3.2. Trait Measurement
2.3.3. Environmental Factor
2.4. Statistical Analysis
2.4.1. Assessment of Plant Trait and Structural Attributes
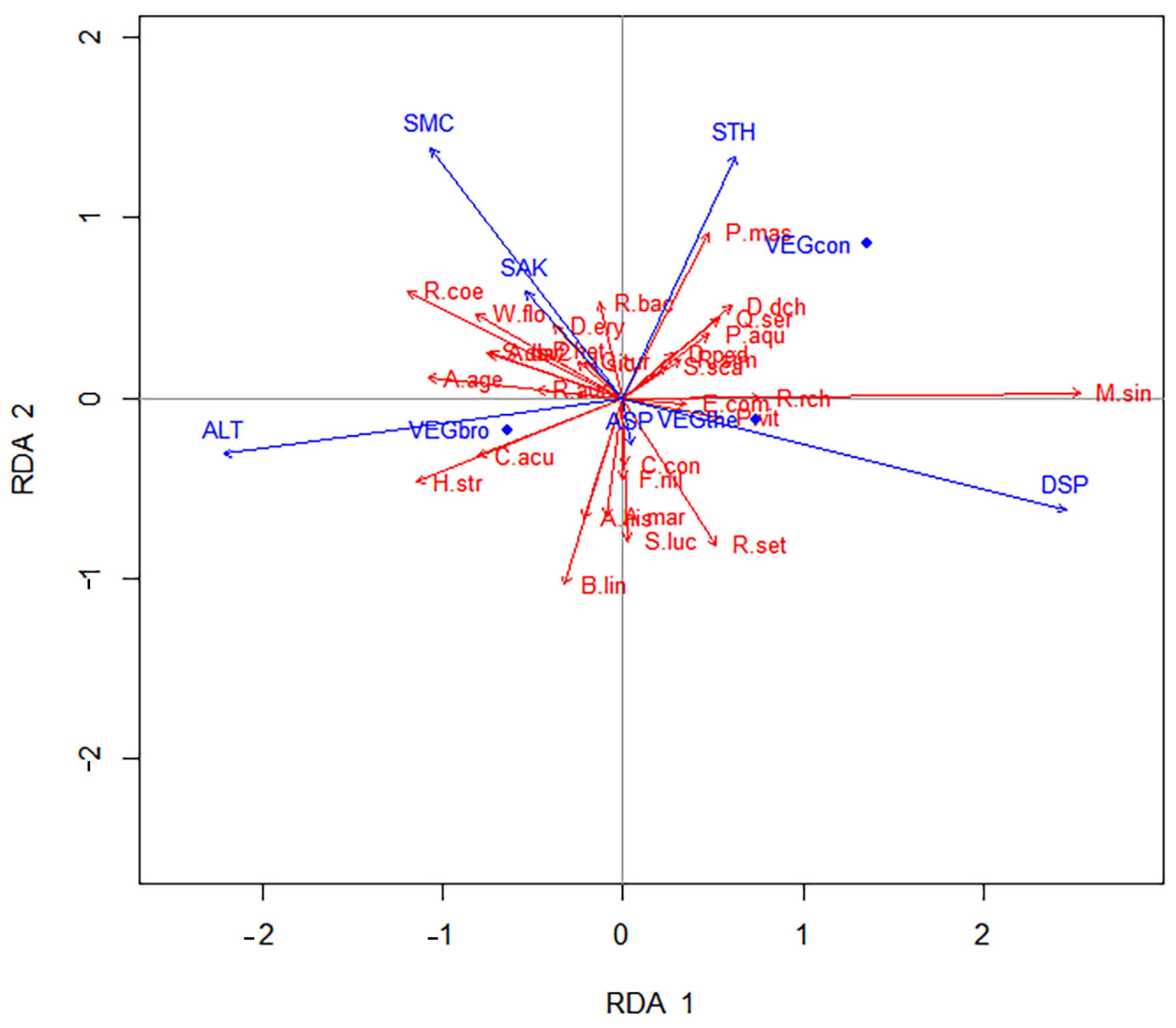
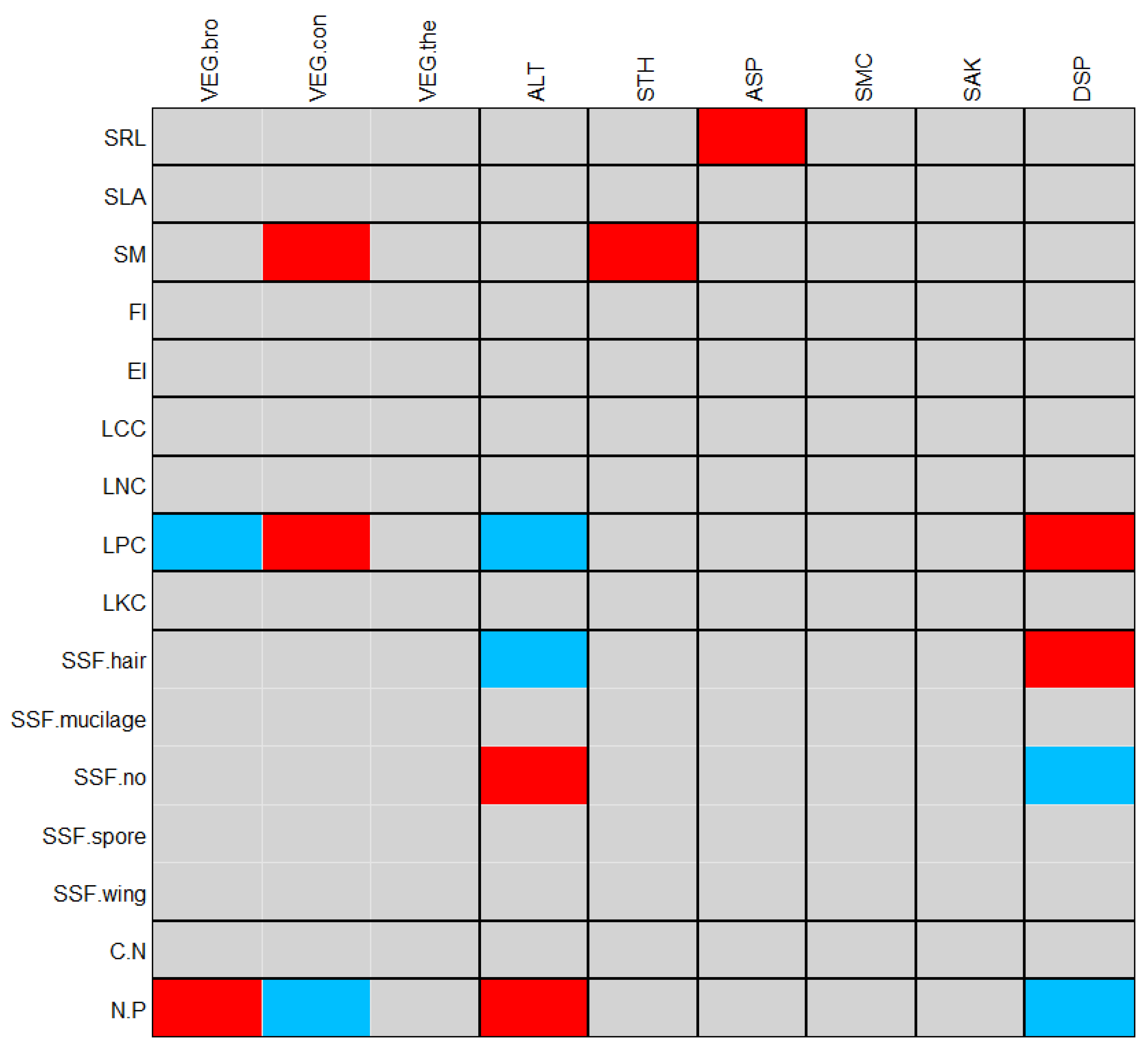
2.4.2. Linking Species Distribution and Plant Traits with Environment Variables
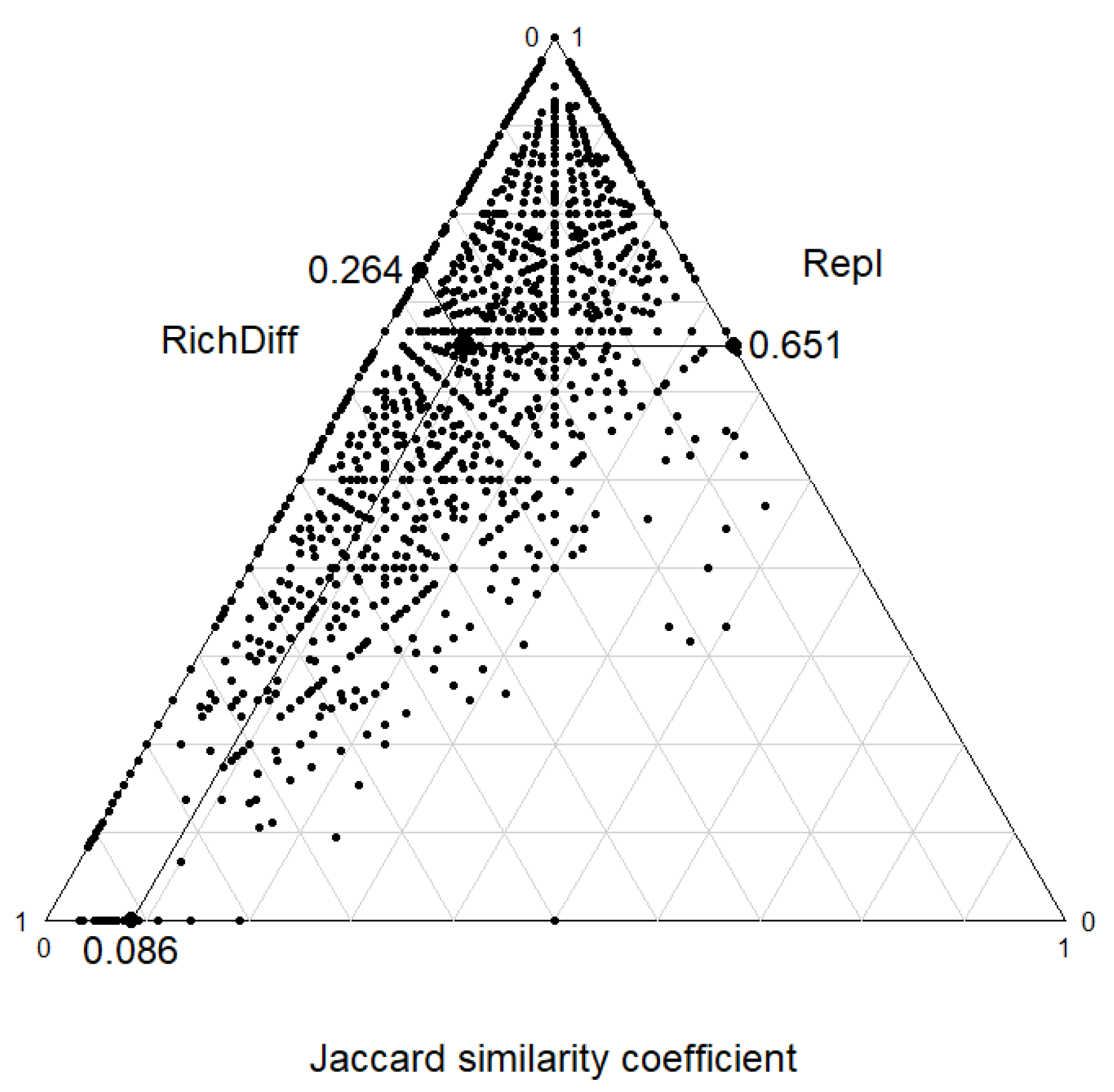
2.4.3. Mechanisms of Community Assembly
3. Results
3.1. Composition of Self-Established Plants on Road Slopes
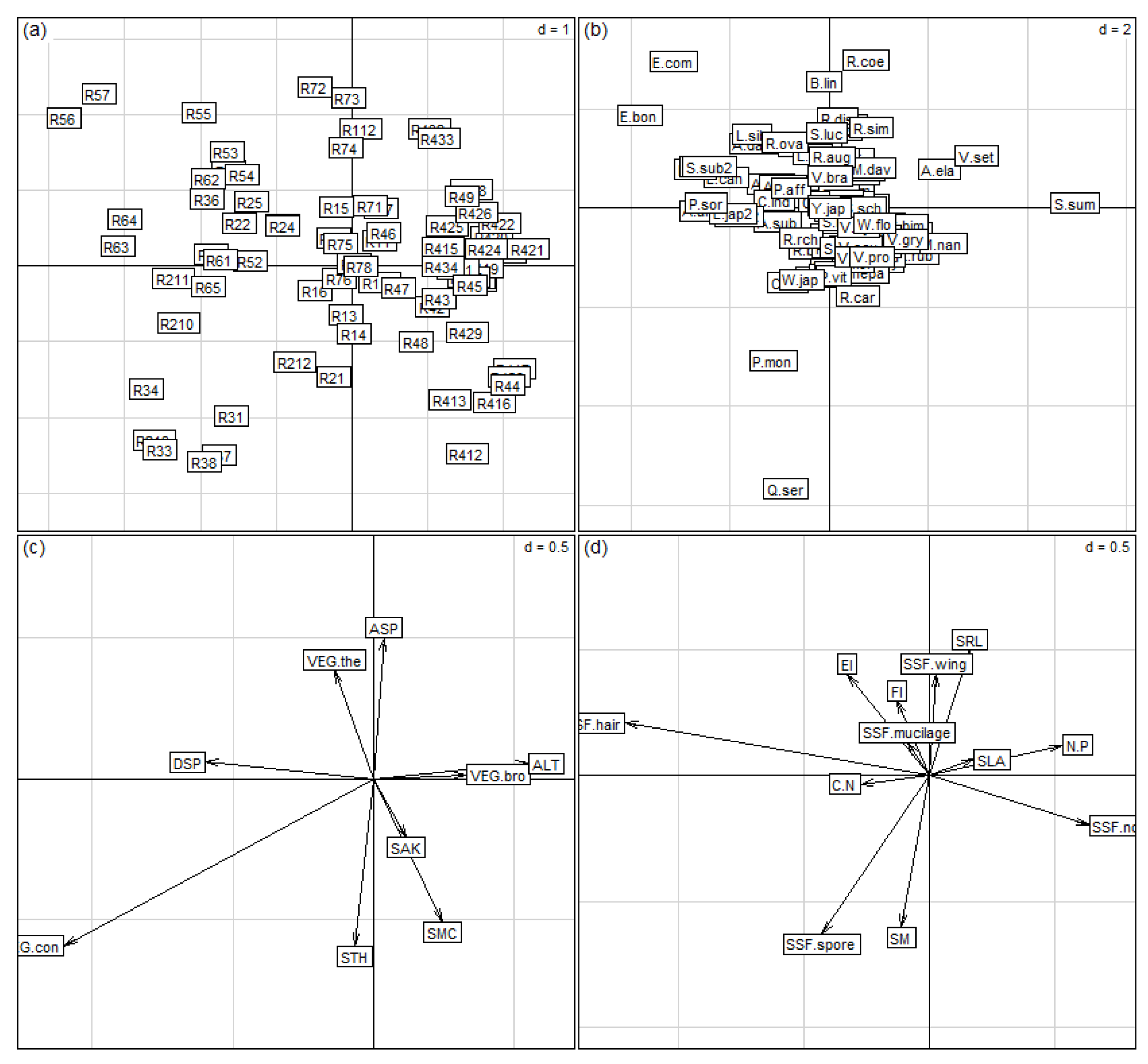
3.2. Plant Distribution and Environmental Characteristics
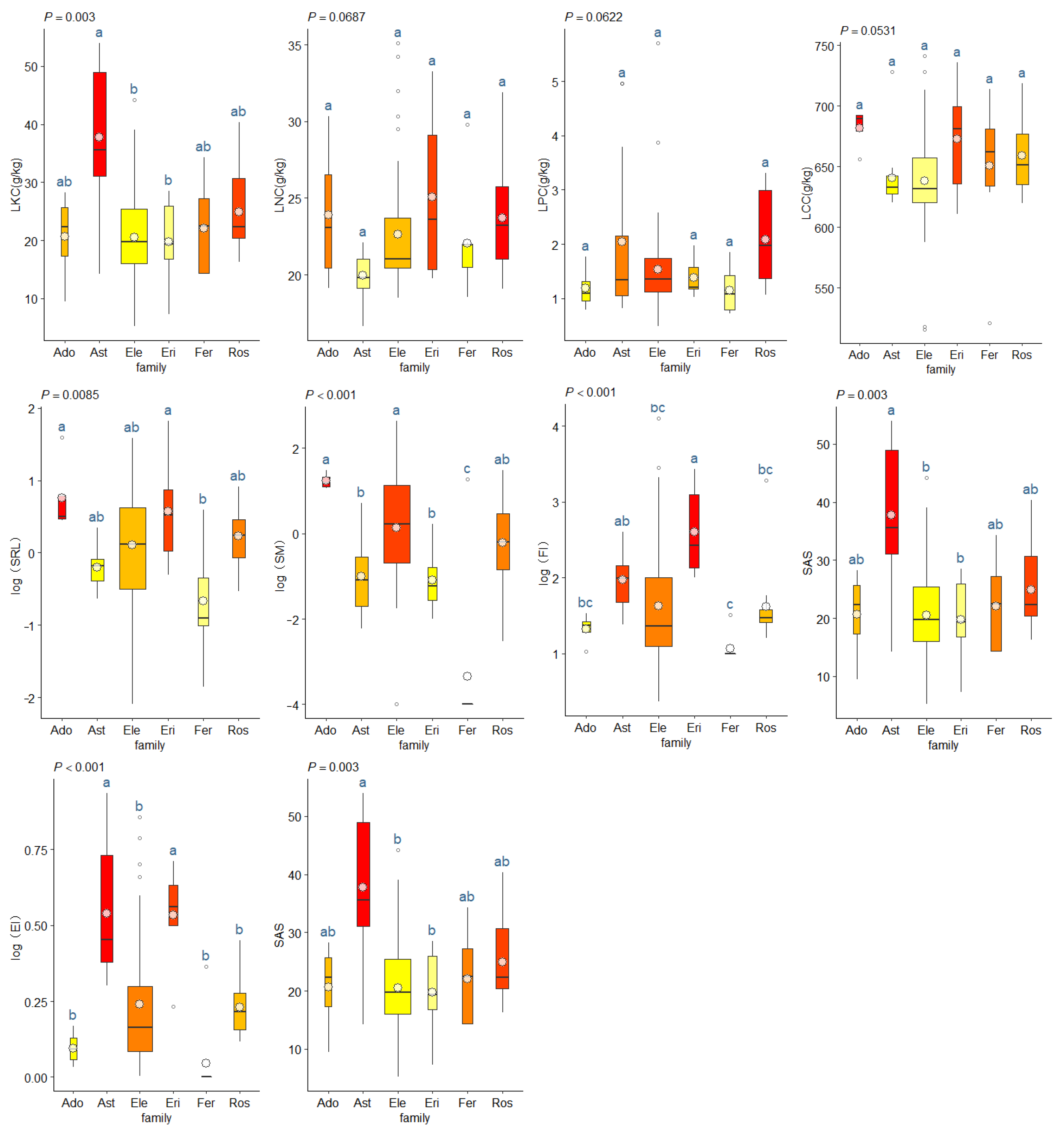
3.3. Plant Trait Characteristics and Structure
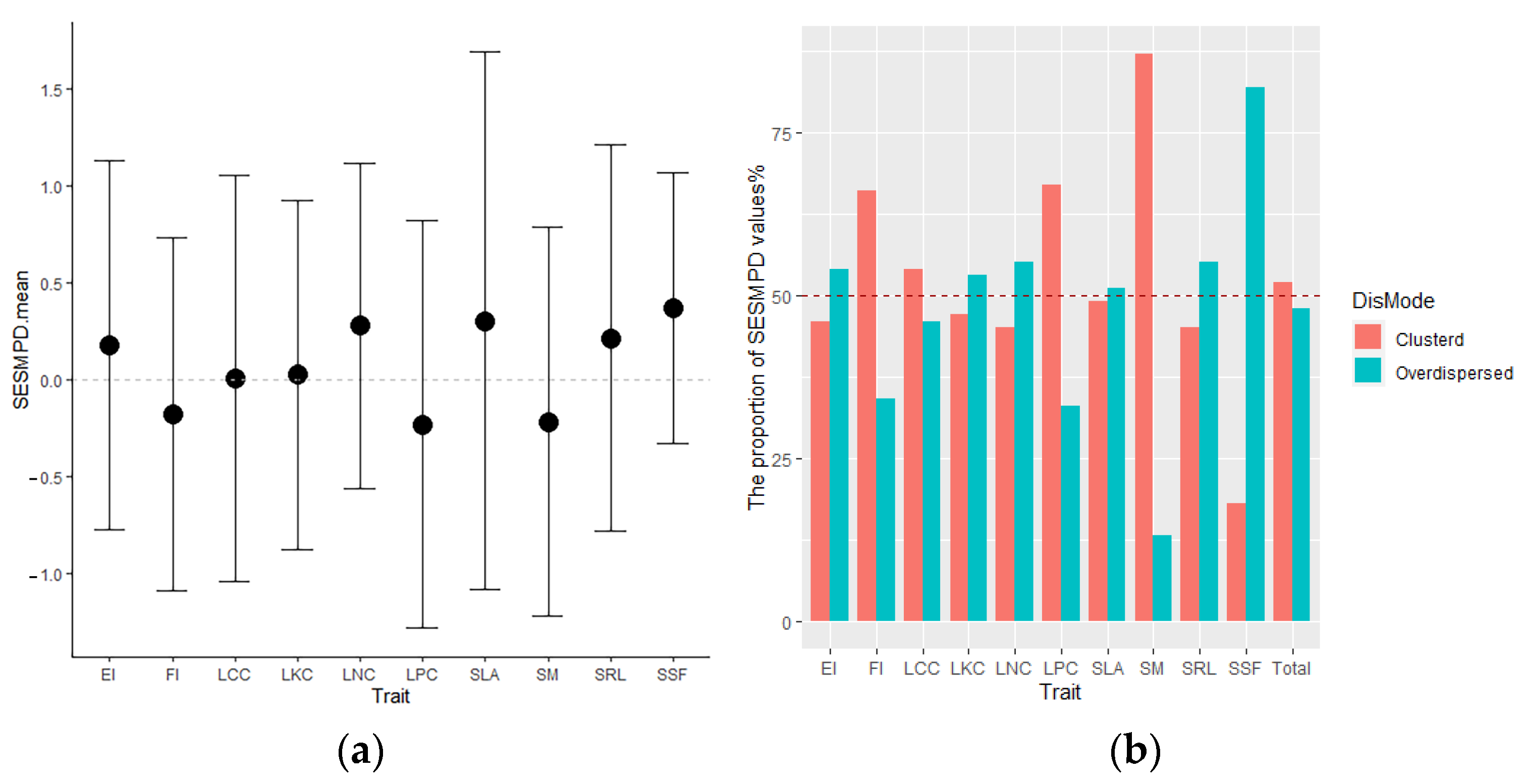
3.4. Root Trait Distributions along Environmental Gradients
4. Discussion
4.1. Community Composition and Distribution on Karst Mountain Road Slopes
4.2. Establishment of Plant Communities on Road Slopes
4.3. Distributional Structure of Slope Plant Traits
4.4. Inspiration for Road Slope Revegetation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahbek, C.; Borregaard, M.K.; Antonelli, A.; Colwell, R.K.; Holt, B.G.; Nogues-Bravo, D.; Rasmussen, C.M.; Richardson, K.; Rosing, M.T.; Whittaker, R.J.; et al. Building mountain biodiversity: Geological and evolutionary processes. Science 2019, 365, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Alday, J.G.; Chu, Y.; Lee, E.J.; Park, S.; Lee, H. Plant species colonization in newly created road habitats of South Korea: Insights for more effective restoration. Sci. Total Environ. 2020, 719, 137476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, M.; Xie, X. Optimal allocation model of plant community in slope ecological restoration. Glob. Nest. J. 2020, 22, 554–564. [Google Scholar]
- Rivera, D.; Mejias, V.; Jauregui, B.M.; Costa-Tenorio, M.; Lopez-Archilla, A.I.; Peco, B. Spreading topsoil encourages ecological restoration on embankments: Soil fertility, microbial activity and vegetation cover. PLoS ONE 2014, 9, e101413. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Yang, H.; Wang, L.; Yang, S.; Li, R.; Zhang, W.; Ai, X.; Ai, Y. Vegetation and soil nutrient restoration of cut slopes using outside soil spray seeding in the plateau region of southwestern China. J. Environ. Manag. 2018, 228, 47–54. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, J.; Ma, Q.; Wan, J.; Li, L.; Peng, Q.; Rezaeimalek, S. Experimental study on the soil mixture to promote vegetation for slope protection and landslide prevention. Landslides 2017, 14, 287–297. [Google Scholar] [CrossRef]
- Ai, X.; Sheng, M.; Su, X.; Ai, S.; Jiang, X.; Yang, S.; Huang, Z.; Ai, Y. Effects of frame beam on structural characteristics of artificial soil on railway cut-slopes in southwestern China. Land Degrad. Dev. 2021, 32, 482–493. [Google Scholar] [CrossRef]
- Novak, J.; Prach, K. Vegetation succession in basalt quarries: Pattern on a landscape scale. Appl. Veg. Sci. 2003, 6, 111–116. [Google Scholar] [CrossRef]
- Matesanz, S.; Valladares, F.; Tena, D.; Costa-Tenorio, M.; Bote, D. Early dynamics of plant communities on revegetated motorway slopes from southern Spain: Is hydroseeding always needed? Restor. Ecol. 2006, 14, 297–307. [Google Scholar] [CrossRef]
- Bochet, E.; Garcia-Fayos, P.; Tormo, J. Road slope revegetation in semiarid mediterranean environments. Part I: Seed dispersal and spontaneous colonization. Restor. Ecol. 2007, 15, 88–96. [Google Scholar] [CrossRef]
- Ulrich, W.; Zaplata, M.K. Trait driven or neutral: Understanding the change in functional trait diversity during early plant succession using Price partitions. Plant Ecol. 2023. [Google Scholar] [CrossRef]
- Holzapfel, C.; Schmidt, W. Roadside vegetation along transects in the Judean Desert. Isr. J. Bot. 1990, 39, 263–270. [Google Scholar]
- Godefroid, S.; Tanghe, M. Influence of small climatic variations on the species composition of roadside grasslands. Phytocoenologia 2000, 30, 655–664. [Google Scholar] [CrossRef]
- Guzman, P.; Benitez, A.; Carrion-Paladines, V.; Salinas, P.; Cumbicus, N. Elevation and soil properties determine community composition, but not vascular plant richness in tropical andean roadside. Forests 2022, 13, 685. [Google Scholar] [CrossRef]
- Backes, A.R.; Römermann, C.; Alexander, J.M.; Arévalo, J.R.; Keil, P.; Padrón-Mederos, M.A.; Trogisch, S.; Haider, S. Mechanisms behind elevational plant species richness patterns revealed by a trait-based approach. J. Veg. Sci. 2023, 34, e13171. [Google Scholar]
- Bochet, E.; Garcia-Fayos, P. Identifying plant traits: A key aspect for species selection in restoration of eroded roadsides in semiarid environments. Ecol. Eng. 2015, 83, 444–451. [Google Scholar] [CrossRef]
- Beck, J.J.; Richards, J.H. Functional traits influence local plant distributions and spatial patterns of diversity within a heterogeneous bedrock glade. Plant Ecol. 2023, 224, 729–740. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Ackerly, D.D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 2009, 79, 109–126. [Google Scholar] [CrossRef]
- Lopez-Angulo, J.; de la Cruz, M.; Pescador, D.S.; Sanchez, A.M.; Escudero, A. A dimmer shade of pale: Revealing the faint signature of local assembly processes on the structure of strongly filtered plant communities. Ecography 2021, 44, 87–97. [Google Scholar] [CrossRef]
- Silva, J.L.A.; Souza, A.F.; Santiago, L.S. Traits uncover quasi-neutral community assembly in a coastal heath vegetation. J. Plant Ecol. 2019, 12, 703–712. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Bittebiere, A.K.; Mony, C. Plant traits respond to the competitive neighbourhood at different spatial and temporal scales. Ann. Bot. 2015, 115, 117–126. [Google Scholar] [CrossRef]
- May, F.; Giladi, I.; Ristow, M.; Ziv, Y.; Jeltsch, F. Plant functional traits and community assembly along interacting gradients of productivity and fragmentation. Perspect. Plant Ecol. 2013, 15, 304–318. [Google Scholar] [CrossRef]
- Dubuis, A.; Rossier, L.; Pottier, J.; Pellissier, L.; Vittoz, P.; Guisan, A. Predicting current and future spatial community patterns of plant functional traits. Ecography 2013, 36, 1158–1168. [Google Scholar] [CrossRef]
- Chauvet, M.; Kunstler, G.; Roy, J.; Morin, X. Using a forest dynamics model to link community assembly processes and traits structure. Funct. Ecol. 2017, 31, 1452–1461. [Google Scholar] [CrossRef]
- Thompson, K.; Petchey, O.L.; Askew, A.P.; Dunnett, N.P.; Beckerman, A.P.; Willis, A.J. Little evidence for limiting similarity in a long-term study of a roadside plant community. J. Ecol. 2010, 98, 480–487. [Google Scholar] [CrossRef]
- Backhaus, L.; Albert, G.; Cuchietti, A.; Jaimes Nino, L.M.; Fahs, N.; Lisner, A.; Kolář, V.; Kermavnar, J.; Widmer, S.; Zimmermann, Z.; et al. Shift from trait convergence to divergence along old-field succession. J. Veg. Sci. 2021, 32, e12986. [Google Scholar] [CrossRef]
- Erfanzadeh, R.; Garbutt, A.; Petillon, J.; Maelfait, J.P.; Hoffmann, M. Factors affecting the success of early salt-marsh colonizers: Seed availability rather than site suitability and dispersal traits. Plant Ecol. 2010, 206, 335–347. [Google Scholar] [CrossRef]
- Pescador, D.S.; Sierra-Almeida, A.; Torres, P.J.; Escudero, A. Summer freezing resistance: A critical filter for plant community assemblies in mediterranean high mountains. Front. Plant Sci. 2016, 7, 194. [Google Scholar] [CrossRef]
- Janse-ten Klooster, S.H.; Thomas, E.J.P.; Sterck, F.J. Explaining interspecific differences in sapling growth and shade tolerance in temperate forests. J. Ecol. 2007, 95, 1250–1260. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martinez-Ramos, M.; Rodriguez-Velazquez, J.; van Breugel, M.; Bongers, F. Changing drivers of species dominance during tropical forest succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef]
- van der, M.E.; Franklin, J. Vegetation Ecology, 2nd ed.; Wiley: Chichester, UK, 2013; pp. 154–196. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Cerda, A.; Garcia-Fayos, P. The influence of seed size and shape on their removal by water erosion. Catena 2002, 48, 293–301. [Google Scholar] [CrossRef]
- Luo, W.; Lan, R.; Chen, D.; Zhang, B.; Xi, N.; Li, Y.; Fang, S.; Valverde-Barrantes, O.J.; Eissenstat, D.M.; Chu, C.; et al. Limiting similarity shapes the functional and phylogenetic structure of root neighborhoods in a subtropical forest. New Phytol. 2021, 229, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Legendre, P. Testing the Species Traits-Environment Relationships: The fourth-corner problem revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef]
- Gibert, C.; Escarguel, G. PER-SIMPER-A new tool for inferring community assembly processes from taxon occurrences. Glob. Ecol. Biogeogr. 2019, 28, 374–385. [Google Scholar] [CrossRef]
- Saikia, P.; Deka, J.; Bharali, S.; Kumar, A.; Tripathi, O.P.; Singha, L.B.; Dayanandan, S.; Khan, M.L. Plant diversity patterns and conservation status of eastern Himalayan forests in Arunachal Pradesh, Northeast India. For. Ecosyst. 2017, 4, 28. [Google Scholar] [CrossRef]
- Wani, Z.A.; Khan, S.; Bhat, J.A.; Malik, A.H.; Alyas, T.; Pant, S.; Siddiqui, S.; Moustafa, M.; Ahmad, A.E. Pattern of beta-diversity and plant species richness along Vertical Gradient in Northwest Himalaya, India. Biology 2022, 11, 1064. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.H.; Byun, C.; Hong, S.Y.; Lee, E.J. Identification of restoration species for early roadcut slope regeneration using functional group approach. Restor. Ecol. 2021, 29, e134241. [Google Scholar] [CrossRef]
- Wang, X.; Wiegand, T.; Anderson-Teixeira, K.J.; Bourg, N.A.; Hao, Z.; Howe, R.; Jin, G.; Orwig, D.A.; Spasojevic, M.J.; Wang, S.; et al. Ecological drivers of spatial community dissimilarity, species replacement and species nestedness across temperate forests. Glob. Ecol. Biogeogr. 2018, 27, 581–592. [Google Scholar] [CrossRef]
- Laiolo, P.; Illera, J.C.; Obeso, J.R. Stuck on top of a mountain: Consequences of dispersal limitations for alpine diversity. J. Biogeogr. 2023, 50, 282–290. [Google Scholar] [CrossRef]
- Azcarate, F.M.; Robleno, I.; Seoane, J.; Manzano, P.; Peco, B. Drove roads as local biodiversity reservoirs: Effects on landscape pattern and plant communities in a Mediterranean region. Appl. Veg. Sci. 2013, 16, 480–490. [Google Scholar] [CrossRef]
- Arenas, J.M.; Escudero, A.; Mola, I.; Casado, M.A. Roadsides: An opportunity for biodiversity conservation. Appl. Veg. Sci. 2017, 20, 527–537. [Google Scholar] [CrossRef]
- Schleicher, A.; Peppler-Lisbach, C.; Kleyer, M. Functional traits during succession: Is plant community assembly trait-driven? Preslia 2011, 83, 347–370. [Google Scholar]
- Lebrija-Trejos, E.; Perez-Garcia, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef]
- Bochet, E.; Garcia-Fayos, P. Factors controlling vegetation establishment and water erosion on motorway slopes in Valencia, Spain. Restor. Ecol. 2004, 12, 166–174. [Google Scholar] [CrossRef]
- Canton, Y.; Del Barrio, G.; Sole-Benet, A.; Lazaro, R. Topographic controls on the spatial distribution of ground cover in the Tabemas badlands of SE Spain. Catena 2004, 55, 341–365. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.B.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.E.; Fagan, W.; Schade, J.; et al. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef]
- Zhu, D.H.; Hui, D.F.; Wang, M.Q.; Yang, Q.; Yu, S.X. Light and competition alter leaf stoichiometry of introduced species and native mangrove species. Sci. Total Environ. 2020, 738, 140304. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Galford, A.E.; Chrzanowski, T.H.; Findlay, D.L.; Mills, K.H.; Paterson, M.J.; Stainton, M.P.; Schindler, D.W. Pelagic C:N:P stoichiometry in a eutrophied lake: Responses to a whole-lake food-web manipulation. Ecosystems 2000, 3, 293–307. [Google Scholar] [CrossRef]
- Karpinets, T.V.; Greenwood, D.J.; Sams, C.E.; Ammons, J.T. RNA: Protein ratio of the unicellular organism as a characteristic of phosphorous and nitrogen stoichiometry and of the cellular requirement of ribosomes for protein synthesis. BMC Biol. 2006, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Herben, T.; Goldberg, D.E. Community assembly by limiting similarity vs. competitive hierarchies: Testing the consequences of dispersion of individual traits. J. Ecol. 2014, 102, 156–166. [Google Scholar] [CrossRef]
- Kang, S.; Niu, J.; Zhang, Q.; Li, D.; Ren, H.; Ren, J.; Zhang, X.; Dong, J. Environmental filtering does not necessarily prevent trait divergence: A case study of the Xilin River Basin in Inner Mongolia, China. J. Plant Ecol. 2017, 10, 497–509. [Google Scholar] [CrossRef]
- Bernard-Verdier, M.; Navas, M.L.; Vellend, M.; Violle, C.; Fayolle, A.; Garnier, E. Community assembly along a soil depth gradient: Contrasting patterns of plant trait convergence and divergence in a Mediterranean rangeland. J. Ecol. 2012, 100, 1422–1433. [Google Scholar] [CrossRef]
- Suter, M.; Edwards, P.J. Convergent succession of plant communities. is linked to species’ functional traits. Perspect. Plant Ecol. 2013, 15, 217–225. [Google Scholar] [CrossRef]
- Jalonen, R.; Valette, M.; Boshier, D.; Duminil, J.; Thomas, E. Forest and landscape restoration severely constrained by a lack of attention to the quantity and quality of tree seed: Insights from a global survey. Conserv. Lett. 2018, 11, e12424. [Google Scholar] [CrossRef]
- Reubens, B.; Moeremans, C.; Poesen, J.; Nyssen, J.; Tewoldeberhan, S.; Franzel, S.; Deckers, J.; Orwa, C.; Muys, B. Tree species selection for land rehabilitation in Ethiopia: From fragmented knowledge to an integrated multi-criteria decision approach. Agrofor. Syst. 2011, 82, 303–330. [Google Scholar] [CrossRef]
- Kulpa, S.M.; Leger, E.A. Strong natural selection during plant restoration favors an unexpected suite of plant traits. Evol. Appl. 2013, 6, 510–523. [Google Scholar] [CrossRef]
- Pywell, R.F.; Bullock, J.M.; Roy, D.B.; Warman, L.I.Z.; Walker, K.J.; Rothery, P. Plant traits as predictors of performance in ecological restoration. J. Appl. Ecol. 2003, 40, 65–77. [Google Scholar] [CrossRef]
- Rinella, M.J.; James, J.J. A modelling framework for improving plant establishment during ecological restoration. Ecol. Model. 2017, 361, 177–183. [Google Scholar] [CrossRef]
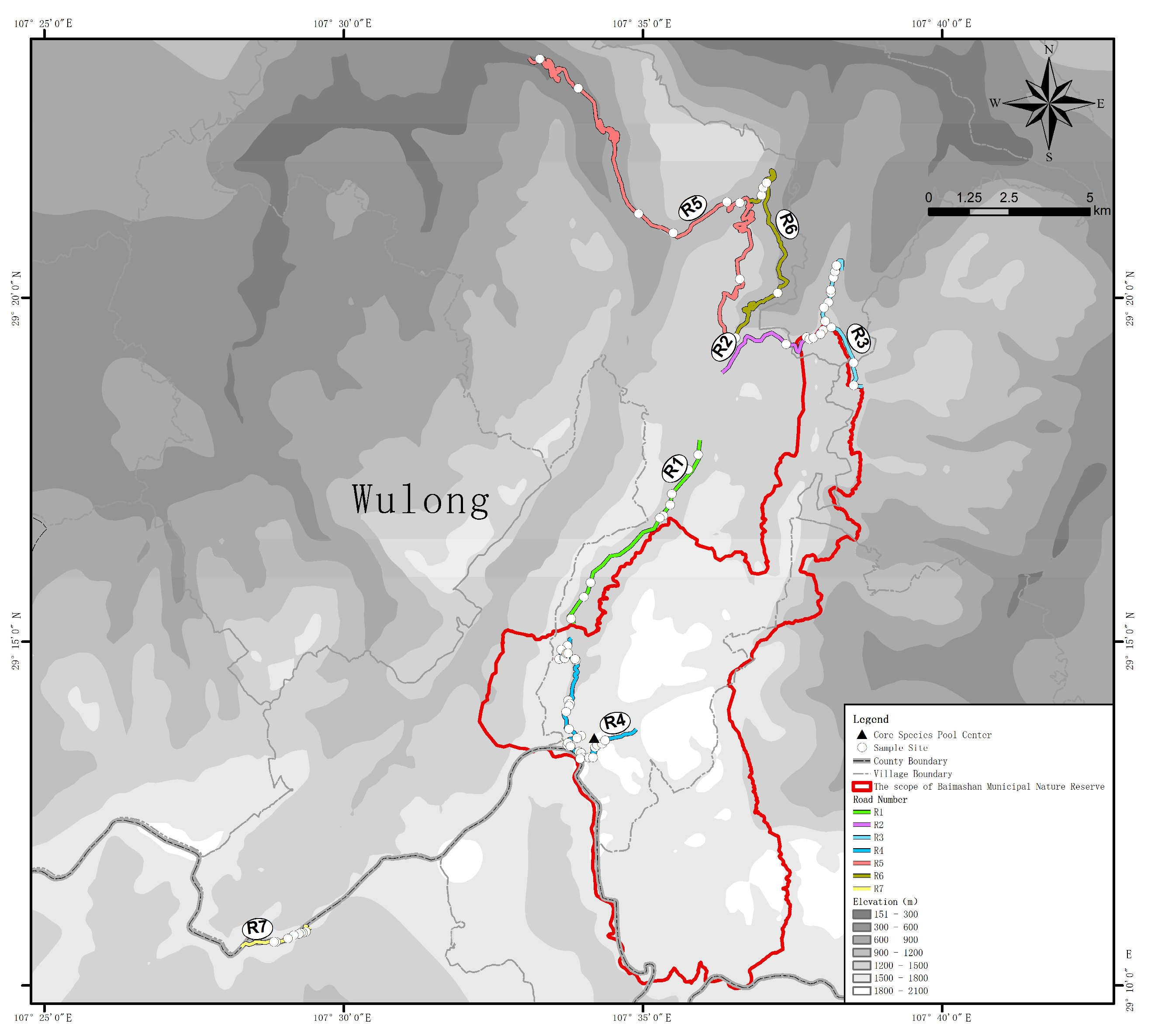

| Road Number | Construction Time (Year) | Length (km) | Elevation (mean ± SD, m) | Vegetation 1 | Slope (°) | Sample Points | DSP 2 (km) |
|---|---|---|---|---|---|---|---|
| R1 | 2013 | 6.51 | 1361 ± 129 | VEGthe | 63.83 ± 16.82 | 9 | 5.61 |
| R2 | 2014 | 4.24 | 1283 ± 202 | VEGthe | 65.23 ± 10.99 | 9 | 12.18 |
| R3 | 2016 | 4.63 | 1219 ± 133 | VEGcon | 67.86 ± 14.18 | 8 | 12.86 |
| R4 | 2000 | 7.38 | 1588 ± 169 | VEGbro | 69.14 ± 13.96 | 31 | 1.17 |
| R5 | 2017 | 21.22 | 795 ± 261 | VEGbro | 82.68 ± 5.63 | 6 | 14.73 |
| R6 | 2006 | 8.57 | 1060 ± 98 | VEGthe | 77.5 ± 5.56 | 5 | 13.51 |
| R7 | 2017 | 2.45 | 1570 ± 24 | VEGbro | 58.54 ± 9.61 | 8 | 9.82 |
| Trait Category | Trait | Scale and Unit | Abbreviation | Ecological Relevance | Information Compilation Source |
|---|---|---|---|---|---|
| General plant traits | Total nitrogen content of leaves | Continuous (mg g−1) | LNC | Seedling growth | Field harvesting and laboratory measurements |
| Total phosphorus content in leaves | Continuous (mg g−1) | LPC | Seedling growth | Field harvesting and laboratory measurements | |
| Total carbon content of leaves | Continuous (mg g−1) | LCC | Seedling growth | Field harvesting and laboratory measurements | |
| Total potassium content in leaves | Continuous (mg g−1) | LKC | Seedling growth, Response to stress | Field harvesting and laboratory measurements | |
| Specific Leaf Area | Continuous (mm2 mg−1) | SLA | Seedling growth, Response to stress | Field harvesting and laboratory measurements | |
| specific root length | Continuous (mm mg−1) | SRL | Seedling growth, Response to stress | Field harvesting and laboratory measurements | |
| Seed-related traits | Seed mass | Continuous (mg) | SM | Dispersal, Germinate, Seedling growth | Field harvesting and laboratory measurements |
| Seed eccentricity index | Continuous (dimensionless, ≥1) | FI | Settle, Response to stress | Field harvesting and laboratory measurements, and calculations | |
| Seed flatness index | Continuous (dimensionless, ≥1) | EI | Settle, Response to stress | Field harvesting and laboratory measurements, and calculations | |
| Seed accessory functional organs | Classified, including seeds with wings, seeds with hairs, seeds that secrete mucus, Seeds without appendages, and Spores of Pteridophyte | SSF.wing | Dispersal | Field harvesting and laboratory measurements | |
| SSF.hair | Dispersal, Germinate | Field harvesting and laboratory measurements | |||
| SSF.sucilage | Settle, Response to stress | Field excavations and observations, and literature | |||
| SSF.spore | Dispersal | Field excavations and observations, and literature | |||
| SSF.no | Dispersal | Field excavations and observations, and literature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, K.; Qin, H.; Wang, Z.; Lin, L.; Zhu, H.; Wang, H. Illuminating Plant Community Assembly on Karst Mountain Road Slopes through Plant Traits and Environmental Filters. Forests 2023, 14, 1990. https://doi.org/10.3390/f14101990
Qin K, Qin H, Wang Z, Lin L, Zhu H, Wang H. Illuminating Plant Community Assembly on Karst Mountain Road Slopes through Plant Traits and Environmental Filters. Forests. 2023; 14(10):1990. https://doi.org/10.3390/f14101990
Chicago/Turabian StyleQin, Kunrong, Hua Qin, Zizhuo Wang, Li Lin, Haoxiang Zhu, and Haiyang Wang. 2023. "Illuminating Plant Community Assembly on Karst Mountain Road Slopes through Plant Traits and Environmental Filters" Forests 14, no. 10: 1990. https://doi.org/10.3390/f14101990
APA StyleQin, K., Qin, H., Wang, Z., Lin, L., Zhu, H., & Wang, H. (2023). Illuminating Plant Community Assembly on Karst Mountain Road Slopes through Plant Traits and Environmental Filters. Forests, 14(10), 1990. https://doi.org/10.3390/f14101990





