Abstract
Soil extracellular enzymes play a key role in mediating the degradation of soil organic matter, but little is understood as to how the pattern of soil extracellular enzymes could be altered by nitrogen (N) addition. In this study, the effects of N addition (started from 2006, four treatments: control, 0 g N·m–2·yr–1; low N addition, 2.5 g N·m–2·yr–1; high N addition, 5.0 g N·m–2·yr–1) on soil extracellular enzymes and microbial biomass in a natural secondary forest of Northeastern China. The results showed that the activity of urease, sucrase, peroxidase and N-acetyl-β-D-glucosidase decreased with N addition, and the activity of acid phosphatase and leucine aminopeptidase increased significantly with N addition. Soil total N, temperature at 5 cm soil depth, pH value, microbial biomass carbon and microbial biomass N were the key factors affecting soil enzyme activity. In summary, the enzyme activity related to soil organic matter degradation shows a decreasing trend under N addition. The results suggest that the increase in N deposition will slow down the degradation of soil organic matter in natural secondary forest, which is more conducive to the accumulation of organic matter in the soil.
1. Introduction
Nitrogen (N) deposition significantly impacts soil organic matter decomposition and storage [1,2,3], and enzymes play a crucial role in the decomposition of soil organic matter [4,5]. Soil enzymes are vital participants in all biochemical reactions within the soil, facilitating the decomposition and mineralization of soil organic matter. Their activity can reflect changes in soil environment, alterations in substrate effectiveness, and the energy and nutrient requirements of microorganisms [6]. In recent years, extensive attention has been given to the impact of N deposition on soil microorganisms and extracellular enzyme activity.
The activities of peroxidase (POD) and polyphenol oxidase (PPO) are closely linked to the formation of soil humus, and the availability of N in the soil can affect the activity of lignin-degrading enzymes, thereby influencing the decomposition of plant residues and litter. Dong et al. found that N deposition inhibited the activity of lignin-degrading enzymes, leading to the accumulation of recalcitrant organic carbon in grassland soils [7]. Similar conclusions were drawn by Waldrop et al., indicating that N deposition can alter the degradation of soil organic matter by changing the N content in the soil [8]. Activities of hydrolytic enzymes such as urease (UE), leucine aminopeptidase (LAP), N-acetyl-β-D-glucosaminidase (NAG), sucrase (SC) and acid phosphatase (ACP) can reflect the cycling status of nutrients such as nitrogen (N), carbon (C) and phosphorus (P) in the soil (Table 1). Research on hydrolytic enzymes has yielded varying results across different ecosystems globally. Studies in northern forest ecosystems have shown that N addition can suppress the activities of hydrolytic enzymes associated with C and N cycling [9], aligning with the findings of Zhou et al. in desert ecosystems [10]. In contrast, Saiya-Cork et al.’s research in predominantly maple forest ecosystems suggested a significant increase in hydrolytic enzyme activity (e.g., UE and SC) due to N addition [11]. Song et al.’s study in evergreen broadleaf forests also discovered that N addition inhibited the activity of some hydrolytic enzymes [12]. In summary, N deposition has distinct effects on the activity of hydrolytic and oxidative enzymes in various forest types. Natural secondary forests, as a predominant forest type in China, differ significantly in structure, dynamics, growth, productivity and stand environment from natural forests and plantations [13]. However, the response of soil extracellular enzymes in natural secondary forests to N deposition remains not fully understood. Furthermore, according to the economic theory of microbial metabolism (resource allocation theory) [14], enzyme production increases when nutrients are limited [15]. Conversely, when nutrients are no longer limiting, enzyme production decreases [16]. Therefore, the activity of soil enzymes related to the C and N cycles typically reflects microbial demand for energy and nutrients [17]. After N addition, soil nutrient availability may shift toward P limitation, potentially leading to the inhibition of soil enzymes associated with C and N cycles and an increase in soil enzymes related to P cycling.

Table 1.
Types, targets and substrates of soil enzymes.
Soil microorganisms that regulate enzyme activity also play a crucial role in nutrient cycling within ecosystems. Geisseler et al.’s research in grassland ecosystems has demonstrated a significant reduction in soil microbial biomass with N addition, consistent with the findings of Zhang et al. [18,19]. Wang et al. observed that long-term N addition substantially decreased microbial biomass carbon (MBC) and N (MBN), reducing bacterial abundance while enhancing the activity of specific enzymes involved in C, N and P mineralization [20]. Dong et al.’s study revealed that N addition elevated the activity of degradation enzymes, shifting temperate grasslands from N limitation to phosphorus limitation [7]. Cui et al. suggested that when soil microorganisms are nutrient-limited, they increase the supply of carbon sources, such as enzymes, for energy production, leading to an increased soil carbon release and influencing soil carbon sequestration [21]. Furthermore, the form of N (e.g., NH4NO3 or urea) may also be a potentially important factor influencing the response of soil microorganisms to N addition. NH4NO3 and urea are among the most commonly used N fertilizers in N addition experiments. Yue et al. (2016) observed, in their experiments using urea as the N source, an increase in bacterial biomass upon urea addition [22]. This effect could possibly be attributed to the augmented C input resulting from urea addition, which may introduce interference with the effects of N addition. Hence, it is imperative to investigate the impact of N deposition on soil nutrient dynamics through soil enzyme activity, to understand whether N deposition induces relative nutrient limitation in the microorganisms of Changbai Mountain’s natural secondary forests, and to explore the mechanisms by which N deposition alters carbon decomposition and storage through changes in enzyme activity.
In this study, we conducted research on the impact of simulated N deposition on soil microorganisms and enzyme activity based on a long-term N addition experiment in the natural secondary forests of Changbai Mountain in Northeast China. Our hypotheses were as follows: (1) N addition may potentially suppress enzyme activities associated with carbon and N cycling while promoting enzyme activities related to phosphorus cycling. (2) N addition might alleviate N limitation in natural secondary forests, consequently altering soil microbial biomass. (3) N deposition could potentially decrease soil carbon decomposition and increase soil carbon storage by inhibiting enzyme activity.
2. Materials and Methods
2.1. Research Area Overview
The test site is located in the forestry bureau of Lushuihe (42°24′9″ N, 128°5′45″ E), Jilin province, Northeast China. The experimental sample plot is 920 m above sea level, the slope is less than 5°, and the area has a typical monsoon climate; cold and dry in winter, warm and humid in summer. The mean annual temperature in this area is 2.7 °C, and the mean annual precipitation is 871.6 mm. According to the monitoring results of the nearby forest monitoring station, the average atmospheric N deposition in this area is 2.45 g N m−2·yr−1, of which about 75% is wet N [23]. The experimental forest was cut down in the early 1970s to form a natural secondary forest, and its dominant species were, at the time, Betula platyphylla and Populus davidiana, and the average tree age was about 45 years. Main shrub species were Sorbaria sorbifolia, Philadelphus schrenkii, Schisandra chinensis, Deutzia scabra and Euonymus alatus.
2.2. Nitrogen Addition and Soil Sampling
The N addition experiment has been carried out since May 2006. Nine 30 m × 30 m plots were set up in the natural secondary forest. A buffer band of 20 m was set between each plot to prevent N interference. Three N addition gradients were established: control group (CK, without N addition), low N addition (LN, 2.5 g N m–2·yr–1) and high N addition (HN, 5.0 g N m–2·yr–1). Three random position repetitions were set per treatment. Started from 2006, N addition was conducted monthly during annual growing season (May to October). The specified amount of NH4NO3 was dissolved into 40 L of deionized water and evenly sprayed over the surface with a sprayer. Control plots were sprayed with 40 L of deionized water to ensure no difference in precipitation. Soil samples were collected in spring (19 May and 19 June ), summer (19 July and 19 August) and autumn (19 September and 19 October) of 2017 to determine the effects of seasonal changes, five soil columns (0–10 cm) were randomly selected from each plot using a soil drill (inner diameter 5 cm, length 10 cm); then, the soil samples (the sieve is 2 mm) were sifted to remove stones and roots.
2.3. Analysis of Soil Physical and Chemical Properties
Soil temperature and moisture data were directly determined by the soil temperature and using the humidity probe (EM50; Decagon Devices, Pullman, Washington, WA, USA). Dry soil samples were tested for soil total carbon (TC), total nitrogen (TN), total phosphorus (TP) and pH value. Soil TC and TN were measured using Multi N/C 3000 analyzer (Analytik Jena AG, Jena, Germary); soil TP was measured via spectrophotometer with molybdenum–antimony antibody colorimetry. pH values of the sample were measured using a pH meter (Sartorius PB-10, Gottingen, Germany). The basic physicochemical properties of all samples under different N additions are summarized in Table 2.

Table 2.
Effects of nitrogen addition, season and their interaction on abiotic soil variables.
2.4. Determination of Soil Microbial Biomass C (MBC) and N (MBN)
Soil samples were treated with chloroform fumigation according to the following steps: (1) Take fresh soil using a vacuum dryer and put in a beaker with 30 mL chloroform, a beaker with NaOH (absorb CO2) and a beaker with a little water (to retain the humidity). (2) Cover the vacuum dryer and use a vacuum pump to remove the air. (3) Let chloroform boil for 5 min. (4) Close the vacuum dryer valve, incubate at 25 °C in the dark for 24 h, and then remove the soil and place it in a ventilated space for 2–3 h. Soil samples without fumigation were taken using another vacuum dryer, and water was used instead of chloroform as the control. Soil samples without and with fumigation were taken and K2SO4 (0.5 mol/L) solution was added in a ratio of 1:4 (soil/K2SO4). The solution was shaken for 30 min before filtering. MBC and MBN values were measured via Multi N/C 3000 analyzer (Analytik Jena AG, Jena, Germary).
2.5. Determination of Soil Enzyme Activity
The PPO and POD activity in soil was measured by the number of mg of purplish cholic acid produced by substrate culture at 30 °C for one hour. The activity of UE was indicated by the number of NH3-N mg produced by 1 g soil over 24 h. Soil SC activity was determined via 3, 5-dinitrosalicylic acid colorimetry. Production of 1 mg of reducing sugar per g of soil per day represented one unit of enzyme activity. Soil ACP activity was determined via phenyl disodium phosphate colorimetry. Production of 1 μmol phenol per gram of soil per day represented one unit of enzyme activity. For soil LAP and NAG activity, the production of 1 μmol p-nitroaniline or p-nitrophenol per g of soil per day represented an enzyme activity unit.
2.6. Data Processing and Analysis
First, we analyzed the statistical distribution of all data for normality using the Shapiro–Wilk test. According to the result of this test, the difference of soil enzyme activity, soil microbial biomass and soil physical and chemical properties were analyzed via one-way ANOVA. The significance level was p < 0.05. Then, the Least-Significant Difference (LSD) was used to compare multiple treatments (SPSS 19.0). Lastly, principal coordinate analysis (PCoA) and redundancy analysis (RDA) were used to determine the relationship between environmental factors and soil enzyme activity (R 3.3.3).
3. Results
3.1. Effects of Nitrogen Addition on Soil Physicochemical Properties
Soil total nitrogen (TN), total phosphorus (TP) and pH values were significantly affected by N addition. Throughout the growing period, the largest increase in soil TN occurred in August, which was increased by 26.11% compared with CK (Table S1). From May to September, the addition of N increased soil TP and significantly reduced soil pH values. During the entirety of all the growing seasons, the addition of N had no effect on the temperature and humidity at a soil depth of 5 cm (Table S1). Seasonal changes significantly affected soil TN, TP, pH W5cm and T5cm values (Table S1). Soil temperature was significantly higher in summer (July and August) than in spring and autumn. Soil moisture in autumn (September and October) was significantly lower than that in spring and summer. Soil pH values were higher in summer than in spring and fall. The values of TN and TP increased significantly from August to October. In addition, there was a significant interaction between the amount of N addition that affected TN, TP and pH values and seasonal changes (Table 2).
3.2. Effects of Nitrogen Addition on Soil Microbial Biomass
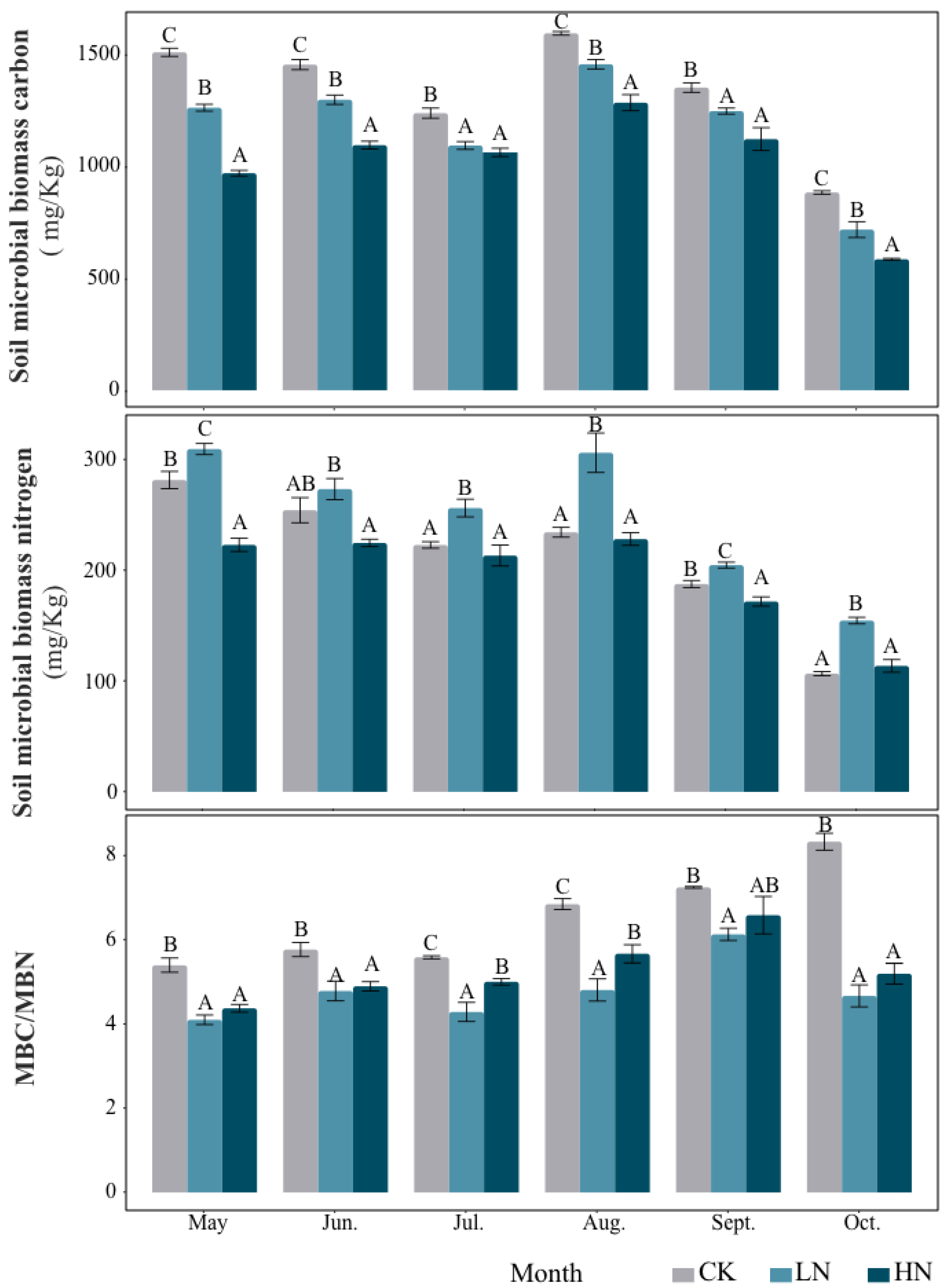
In general, the addition of N reduced microbial biomass carbon (MBC) and carbon/nitrogen ratio (MBC/MBN) (Figure 1). During the whole growing season, the soil MBC decreased significantly with the increase in N application rate. Under LN treatments, MBC decreased by 16.40%, 10.77%, 12.67%, 9.67%, 8.78% and 19.77% compared to CK from May to October, respectively. Under HN treatments, MBC decreased by 35.77%, 24.72%, 14.16%, 19.44%, 17.04% and 33.75% compared to CK from May to October, respectively. The general trend of soil microbial nitrogen (MBN) that was affected by N addition was inhibited under HN treatments and promoted under LN treatments. The addition of N reduced the MBC/MBN ratio during all of the growing seasons.
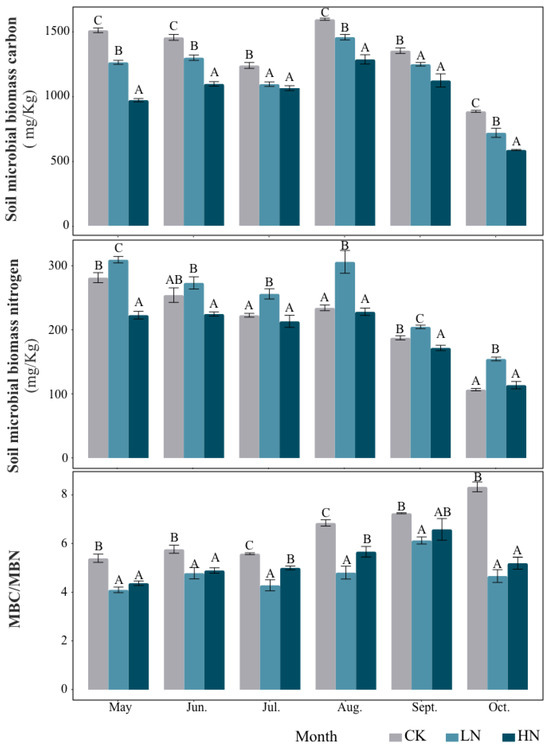
Figure 1.
The contents of MBC and MBN under different treatments (mg kg−1, mean ± SD, n = 5). MBC/MBN represents the ratio of soil microbial biomass carbon to nitrogen. The white bar graph represents CK (do not add N); The gray bar graph is LN (2.5 g N·m–2·yr–1); The black bar graph represents HN (5 g N·m–2·yr–1). Different capital letters indicate significant differences between treatment groups.
Seasonal changes also had a significant effect on microbial biomass (Table 3). Under the condition of native soil, the value of MBC/MBN showed an obvious upward trend during the six months of observation, while the change of MBN showed a general downward trend. In addition, soil microbial biomass was also affected by the interaction of seasonal change and N addition (Table 3). Under the condition of N addition, MBC and MBN decreased significantly in autumn.

Table 3.
Effects of nitrogen addition, season and their interaction on soil microbial C, N and ratio of MBC/MBN.
3.3. Effects of Nitrogen Addition on Soil Enzyme Activities
N addition and seasonal change significantly affected the activities of UE, LAP, POD, SC, NAG and ACP (Table 4), but had no significant effect on PPO, and their interaction only had significant effects on POD, LAP and NAG.

Table 4.
Effects of nitrogen addition, season and their interaction on soil enzyme activities.
3.3.1. Effects of Nitrogen Addition on Soil Enzyme Activities Associated with N Cycling
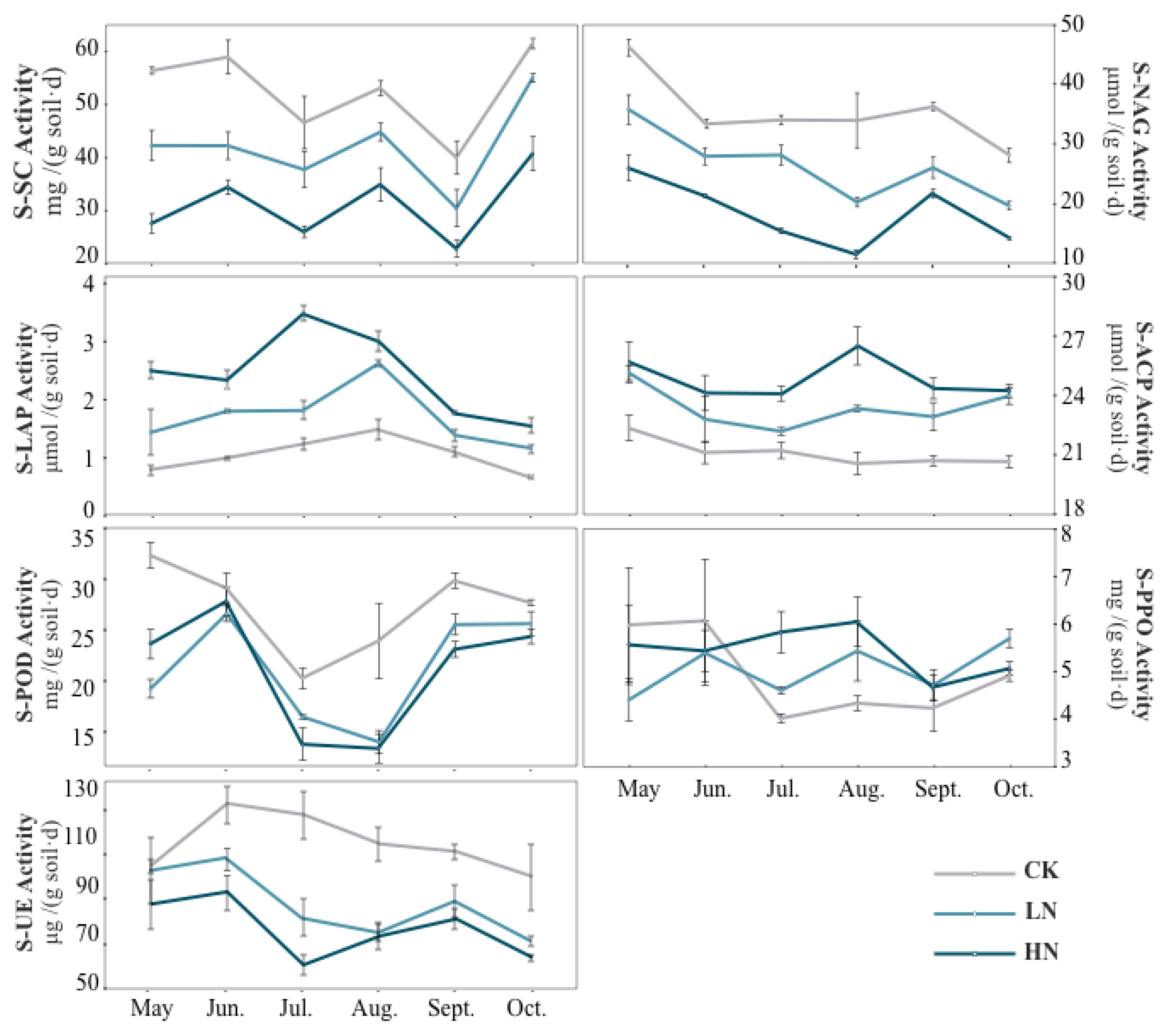
The activities of UE, LAP and NAG showed different trends under different N treatments (Figure 2). During the entirety of all the growing seasons, the addition of exogenous N reduced UE activity, and the difference was significant between July and October (p < 0.05). The UE activity was inhibited much more strongly with the increase in N application. Unlike UE, N addition significantly increased LAP activity, which increased significantly by 224.01%, 140.47%, 187.10%, 104.23%, 62.06% and 139.03% compared with the control from May to October under HN treatments, respectively. The NAG activity was inhibited by N addition throughout the growing seasons.
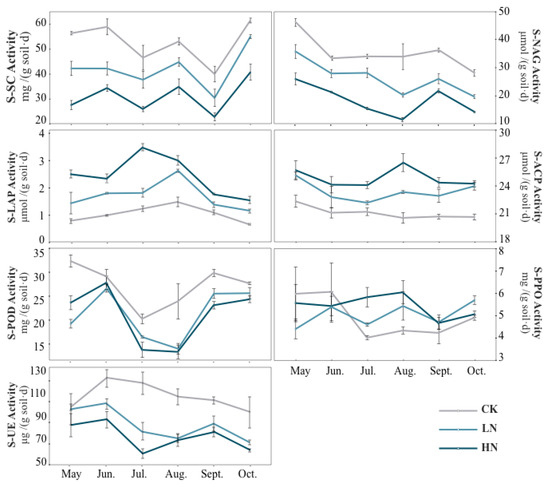
Figure 2.
A line chart showing the response patterns of soil enzyme activity to nitrogen addition and seasonal changes. CK, do not add N; HN, high level of N (5.0 g N·m–2·yr–1); LN, low level of N (2.5 g N·m–2·yr–1). Enzyme abbreviations are listed in Table 1.
From the perspective of seasonal variation, the UE and NAG activities under N addition treatment were significantly lower than those in summer and autumn than in spring. Under HN treatment, the activities of these two enzymes reached the lowest in summer (Figure 2). The LAP activity in summer was significantly higher than that in spring and autumn, and was significantly affected by the interaction of nitrogen addition and seasonal change (Table 4).
3.3.2. Effects of Nitrogen Addition on Soil Enzyme Activities Associated with Carbon Cycling
The activities of SC were significantly reduced by N addition (Figure 2). From May to October, the activities of SC decreased by 24.97%, 28.27%, 18.94%, 15.54%, 23.80% and 10.49% compared to the control under LN treatment, respectively. For HN treatment, The activities of SC decreased by 50.93%, 41.51%, 44.05%, 34.14%, 42.77% and 33.75% from May to October, respectively. Under HN treatment, the activities of SC were significantly different from the control (p < 0.05).
3.3.3. Effects of Nitrogen Addition, Seasonal Change and Their Interactions on Soil Enzyme Activities Associated with Phosphorous Cycling
In our study, we found that N addition had a positive effect on soil acid phosphatase (ACP) (Figure 2). ACP activity did not show significant seasonal fluctuations throughout the growing seasons, but increased under both LN and HN treatments, there were significant differences except in June (p < 0.05). ACP activity increased the most under HN treatment in August, with a 0.22-fold increase compared with the control. In general, seasonal changes significantly affected the ACP activity (Table 4), but the ACP activity did not change significantly during all of the growing seasons under the control (Figure 2).
3.3.4. Effects of Nitrogen Addition, Seasonal Change and Their Interactions on Soil Enzyme Activities Related to Humus Formation
The effects of N addition on peroxidase (POD) and polyphenol oxidase (PPO) had seasonal changes (Figure 2). N addition inhibited PPO activity in spring, but its inhibition effect was not significant. In summer and autumn, PPO activity increased under N addition, especially under HN treatments in July and August, which increased significantly (p < 0.05) by 0.38 and 0.43 times compared with the control, respectively. With the exception of June, N addition significantly reduced the POD activity (p < 0.05).
There were significant seasonal variations concerning POD activity, which demonstrated a higher activity in spring and autumn than that in summer (Figure 2). Under the control, the PPO activity showed a high trend in spring and low in summer and autumn.
3.4. Effects of Environmental Factors on Soil Microbial Biomass and Soil Enzyme Activity
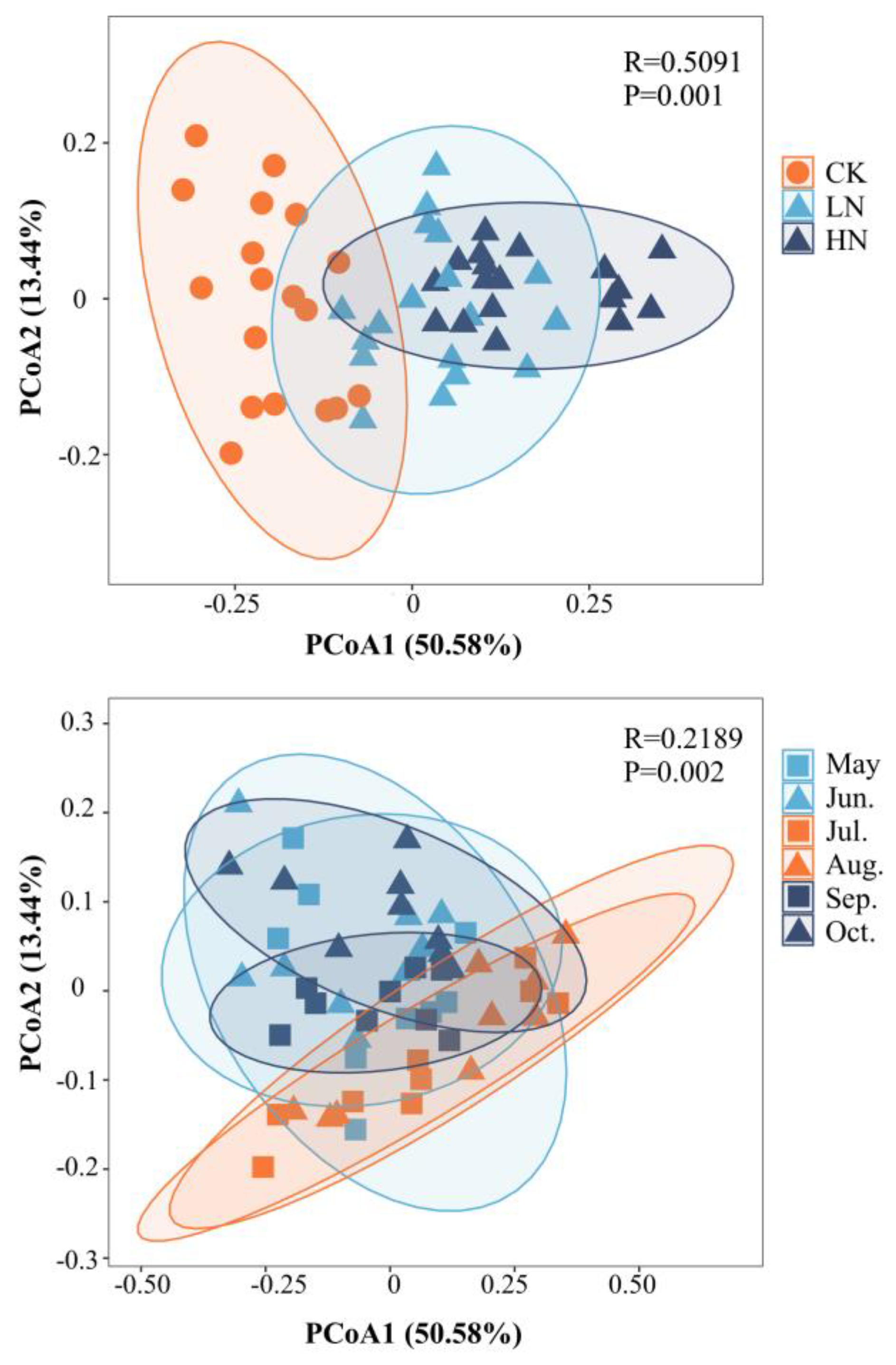
The principal co-ordinates analysis (PCoA) could reveal the differences among the samples (Figure 3), which could explain 64.02% of the total difference. PCoA1 coordinate could explain 50.58% of the difference. In Figure 3, soil enzyme activity after N addition showed obvious distribution characteristics in the pCoA1 ordination axis. It could be seen that the addition of N significantly affected soil enzyme activity (p < 0.05). Seasonal change as a grouping condition, soil enzyme activity in summer has obvious distribution characteristics in the pCoA2 sorting axis (Figure 3). Seasonal changes also significantly affected soil enzyme activity (p < 0.05).
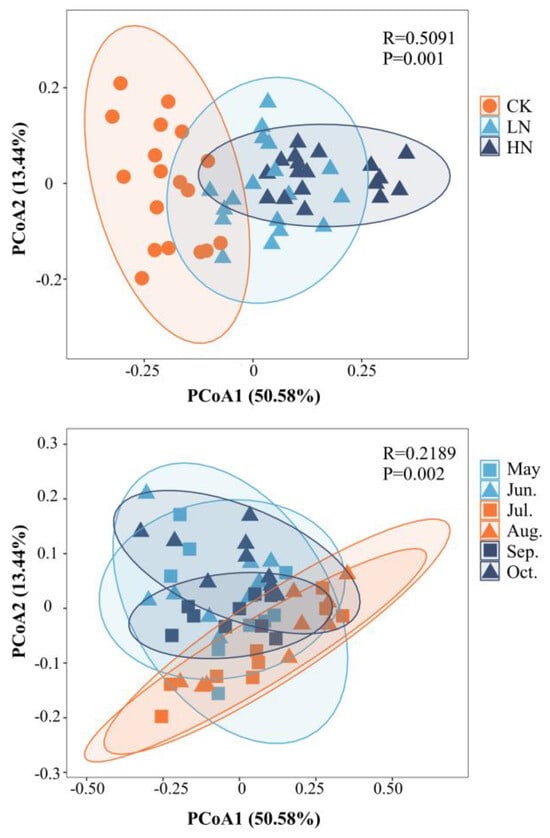
Figure 3.
Principal co-ordinates analysis (PCoA) charts to determine whether the soil enzyme activities are significantly affected by nitrogen addition and seasonal changes. CK, do not add N; HN, high level of N (5.0 g N·m−2·yr−1); LN, low level of N (2.5 g N·m−2·yr−1).
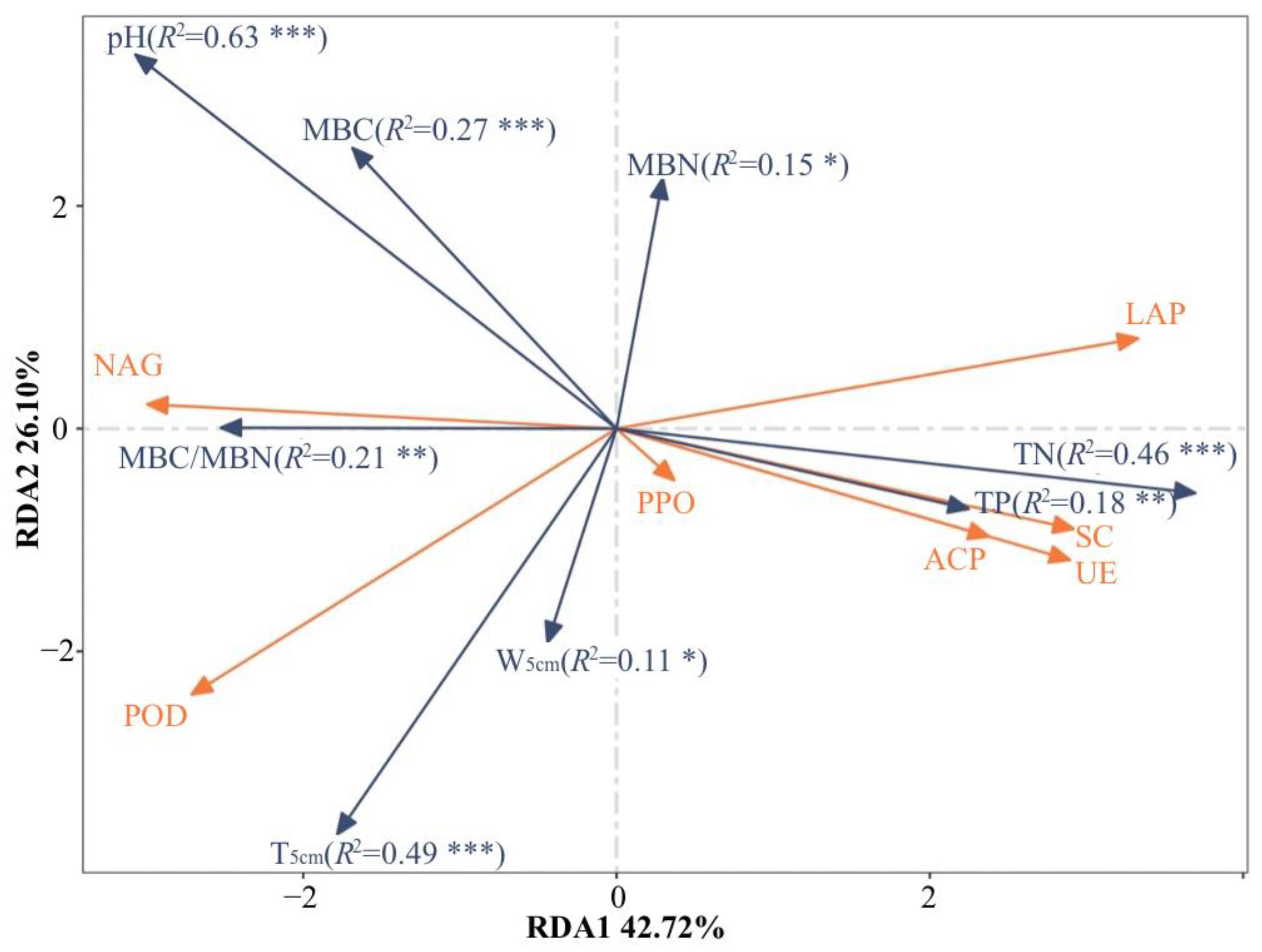
Redundancy analysis (RDA) could demonstrate the relationship between soil enzyme activity and soil physical and chemical properties (Figure 4). RDA1 and RDA2 axes could explain 42.72% and 26.10% of the total difference, respectively. Monte Carlo permutation test was used to test the significance of environmental factors associated with enzyme activity. Among the eight environmental factors, soil enzyme activity was significantly affected by TN (R2 = 0.46, p < 0.001), TP (R2 = 0.18, p < 0.01), T5cm (R2 = 0.49, p < 0.001), W5cm (R2 = 0.11, p < 0.05), pH (R2 = 0.63, p < 0.001), MBC (R2 = 0.27, p < 0.01), MBN (R2 = 0.15, p < 0.05) and MBC/MBN (R2 = 0.215, p < 0.01).
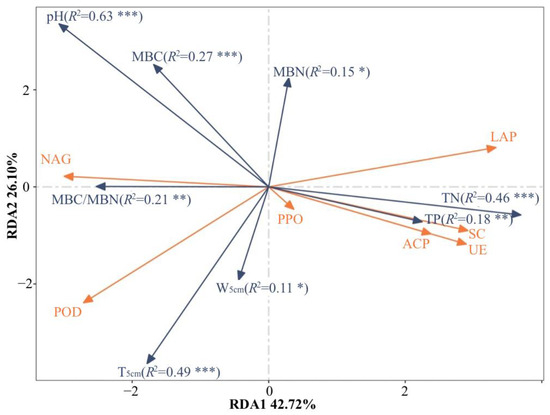
Figure 4.
Redundancy analyses (RDA) of the soil enzyme activities, environmental factors and microbial communities. Enzyme abbreviations are listed in Table 1. Monte Carlo permutation test was used to test the significance of environmental factors associated with enzyme activity, simulating permutation 999 times. TN represents soil total nitrogen; TP represents soil total phosphorus; T5cm represents soil temperature at 5 cm soil depth; W5cm represents soil volume water content at 5 cm soil depth; MBC represents soil microbial biomass carbon; MBN represents soil microbial biomass nitrogen. The * indicates the degree of significances (*: p < 0.05; **: p < 0.01; ***: p < 0.001).
4. Discussion
N addition can change soil microbial biomass and extracellular enzyme activity, and thus exert an important influence on soil organic matter degradation and litter decomposition process. However, the changes were regulated by a variety of ecological factors and had great complexity and uncertainty in a natural secondary forest.
In our study, we observed that N addition exhibited a certain inhibitory effect on MBC (Figure 1), consistent with findings in several previous studies [19,24,25,26,27]. The reasons behind the impact of N addition on MBC in field studies are multifaceted. This complexity arises because N addition can directly or indirectly affect the availability of C and N resources required for microbial growth. Additionally, N addition-induced soil acidification may also inhibit microbial growth [28]. Furthermore, N addition leads to an increase in soil inorganic nitrogen content, which can react with soil organic matter, leading to the accumulation of recalcitrant compounds that may hinder microbial growth [29,30]. Studies by Yue et al. (2016) using urea as an N source yielded results indicating an increased bacterial biomass upon urea addition, possibly due to the an enhanced C input, resulting from urea addition, which may interfere with the effects of N addition [21]. Tu et al. (2011) found in experiments conducted in artificial forest ecosystems that N addition could potentially lead to an increase in soil microbial biomass [31]. However, the research by McCrackin et al. on urban and desert ecosystems did not reveal any significant impact of nitrogen deposition on soil microbial biomass [32]. These differing conclusions could be attributed to variations in the physicochemical properties of forest soils across different ecosystem types or variations in microbial requirements for nitrogen sources.
Moreover, we found that throughout the growing seasons, MBN levels generally showed an LN-promoted, HN-suppressed trend. This may be due to high doses of N additions that cause soil N to become oversaturated, thereby inhibiting the supply of soil nutrients [33]. Soil acidification caused by N addition will reduce the C/N value of soil microorganisms [34], which is consistent with our results. This suggests that the microbial transition to a bacteria-dominated symbiosis will change the soil enzyme activity and contribute to the increase in soil carbon reserves [35,36].
Long-term N addition, seasonal change and their interaction significantly affected soil enzyme activities. N addition can change the physical and chemical properties of soil and lead to soil acidification. In our study, we also found that N application significantly reduced the pH value of soil and changed the total N of soil (Table 2). On the one hand, these changes directly affect soil extracellular enzyme activity, and on the other hand, indirectly cause changes in extracellular enzyme activity by affecting soil microbial biomass. In addition, changes in climate, precipitation and other environmental factors due to seasonal changes further increase the uncertainty and complexity of soil enzyme activities in temperate forests.
Although urease (UE) and leucine aminopeptidase (LAP) are important soil enzymes related to N cycle, their responses to N addition are not consistent. Our results showed that the addition of N reduced UE activity during the entirety of all the growing seasons, and the difference was significant from July to October. Zhou et al. showed that increasing N significantly reduced the activity of soil urease [10]. Ajwa et al. in tall steppe in the United States also showed that N addition had an inhibitory effect on soil UE activity [37], and these results were consistent with our study. UE-related enzymatic reaction substrate is urea, which is extremely specific, with an optimal pH of 7.8. The reason for this inhibitory response may be that the addition of NH4NO3 leads to an increase in the amount of N ions directly absorbed by plants, so there is less dependence on the conversion of enzymatic reactions, leading to a decrease in UE activity. Some studies had also shown that the amount of N applied was positively correlated with UE activity [11,12]. This may be due to the difference in the duration of N addition. It is generally believed that when N is not the limiting factor of plant growth, long-term N application may have an inhibitory effect on forest soil enzyme activity, and this inhibitory effect may be more obvious for our test site with ten consecutive years of N addition.
In our study, N addition significantly increased LAP activity. The results of meta-analysis by Xiao et al. were also consistent with our conclusions [38]. LAP is a key enzyme in the N cycle that converts organic N into inorganic N that can be used by plants and microbes. Seasonal change and the interaction between season and N addition also significantly affected LAP activity. LAP activity in summer was significantly higher than that in spring and autumn. It is different from the UE, where LAP mainly comes from microorganisms, and the activity increases may be related to the changes of microbial community structure.
NAG activity is highly correlated with the biomass of fungi, which is considered as an important quantitative indicator of the biomass of soil fungi [39]. In our study, N addition reduced NAG activity, which may be related to a significantly lower soil pH value. Xu et al. found that NAG activity was positively correlated with soil pH value [40]. In addition, the change of soil fungi biomass also affects NAG activity. NAG can provide large amounts of C or N to the soil microbiota by degrading the chitin cell wall material of insects and most soil fungi [41,42], and the decrease in microbial biomass C/N indicates the decrease in fungi biomass in microbial communities, which may be another reason for the decrease in NAG activity. Allison et al. have also found that in boreal forest soils, the activity of NAG enzymes decreases as N emissions increase [43]. This inhibition of N circulatory enzyme can cause negative feedback through N deposition, thus improving the utilization rate of N. Like LAP, the activity of NAG is also affected by N addition, seasonal change and their interaction. The activity of NAG in spring was higher than that in summer and autumn. Both LAP and NAG are soil enzymes closely related to microorganisms, and the influence of seasonal change on them may be more due to the variation of soil microbial community.
POD and PPO are the main lignin decomposition enzymes, and the response of PPO activity to N deposition is affected by seasonal changes. PPO activity decreased slightly in spring and increased in summer and autumn. The overall trend of POD activity affected by N addition was a slight decrease, which was lower in summer than those in spring and autumn. The changes of oxidases were often highly dynamic, and they had obvious seasonal change trends compared with hydrolytic enzymes [44,45]. In the study of soil enzyme activities, Saiya et al. found that N addition reduced soil phenolic oxidase, but had little effect on peroxidase [11]. Deforest et al. showed that the activity of lignin hydrolytic enzyme decreased under the influence of N addition, which was consistent with our conclusion in spring [46]. Bowden et al. found that N addition reduced soil POD activity in long-term N addition experiments in North America [27]. This is in agreement with our conclusion. Currently, there are two explanations for this result: (1) lignin-decomposing enzymes are mainly produced from white rot fungi, and N application inhibits the biomass and activities of microorganisms, resulting in the decrease in enzyme activity; (2) the addition of N can significantly inhibit PPO and POD activity, and the addition of NO3- improves the decomposition of unstable plant fall products, but inhibits the decomposition of lignin. It can be seen that the factors affecting the activity of oxidase are diverse. If the environmental factors that affected the changes are determined via statistical methods, it may require a longer time and a more detailed data analysis.
Soil total phosphorus is the most important soil nutrient to determine the difference of soil organic carbon [41]. ACP is able to mineralize organophosphorus, which well reflects the phosphorus conversion efficiency. Our results showed that ACP activity was significantly increased with N addition. This is consistent with our expectation that when N is no longer a limiting factor for forests, and even when excess levels increase, ecosystems tend to increase the conversion efficiency of less abundant elements. The addition of N may have aggravated the phosphorus restriction in our test site, stimulated the demand for phosphorus by microorganisms and plants and enhanced the activity of phosphorus-related invertase. The results of different ecosystem studies were consistent with our findings [47,48,49,50], which demonstrated that N application could promote ACP activity. However, some studies had shown that N addition significantly reduced ACP activity [10]. The reason for this divergence may be the different accumulation duration of N deposition and different types of forest vegetation.
SC is an important indicator of soil carbon cycle speed. In this study, the effect of N addition on SC activity was significantly reduced. The substrate of SC enzymatic reaction is sucrose and the hydrolyzed product is glucose. Therefore, the change of SC activity can directly affect the glucose content in the soil, and then affect the microbial group based on glucose. From another perspective, the decrease in SC activity also indicates that N application reduces the degradation rate of organic matter in the natural secondary forest of Changbai Mountains and contributes to the accumulation of soil organic matter. Liu et al. showed that N addition inhibited SC activity of an artificial pine forest in Taiyue Mountains in China, which was consistent with our results [51].
Soil enzymes are an important link in the nutrient cycling and energy flow of the ecosystem and are easily changed by micro-environment influences. Previous studies had shown that soil enzyme activity was mainly affected by temperature, moisture, microbial biomass, pH, available nutrients and ecosystem types [52,53,54]. In our study, TN, TP, T5cm, W5cm, pH values, MBC, MBN and MBC/MBN values were found to be key factors affecting soil enzyme activity. NAG, POD and LAP activity are more susceptible to changes in environmental factors (Figure 4). Studies had shown that nutrients such as MBC could significantly affect soil enzyme activity [55], this is consistent with our conclusion. Through RDA analysis, we found that pH played the most important role in the sample distribution that affected enzyme vitality among the eight main environmental factors (R2 = 0.63 p < 0.001). Liu et al. came to the same conclusion in related studies, emphasizing that N addition affected soil enzyme activity patterns by altering soil pH, rather than by changing the composition of plants and microbiomes [56]. This shows that soil acidification caused by N addition plays a key role in soil organic matter decomposition and nutrient cycle in the natural secondary forest ecosystem of Changbai Mountains. However, in our study, soil microbial biomass and community structure also have a relatively important impact on soil enzyme activity patterns. Therefore, we are more inclined to think that soil enzyme activity patterns are the result of environmental factors, plant communities and microbiome communities. Kandeler et al. found that the increase in water content would lead to the increase in enzyme activity, which might be related to the increased mobility of enzymes and the contact area of the substrate [57]. Hackl et al. also believed that soil moisture content was an important factor driving the change of soil enzyme activity [58]. The effect of N deposition on soil enzyme activity is complex, and the change of soil enzyme activity is the result of environmental factors and ecological processes in temperate forests.
5. Conclusions
In general, N addition changed TN and TP contents in the soil of natural secondary temperate forests, resulting in a decline in the ratio of carbon and N of soil microorganisms. The soil UE, SC, POD and NAG activity decreased with N addition, while the soil ACP and LAP activity increased significantly under N addition. TN, TP, T5cm, W5cm, pH values, MBC, MBN and MBC/MBN values are the key factors affecting soil enzyme activity. The decrease in the key enzyme activity of carbon and N cycles caused by N application could reduce the degradation rate of organic compounds, which indicates that N addition contributes to the increase in soil C storage of natural secondary temperate forest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14102049/s1, Table S1: Effects of nitrogen addition on abiotic soil variables in different seasons (mean ± SD); Table S2: Correlation Analysis of Environmental Factors and Soil Enzyme Activity; Table S3: Partial Correlation Analysis of Total Nitrogen on Soil Enzyme Activity Controlling for pH.
Author Contributions
Q.W. and G.Y. designed the study, were awarded funding, supervised data collection and contributed to and edited manuscripts. Q.W., Y.H., T.L., Y.X. and G.Y. contributed to the whole manuscript preparation and design and wrote the main manuscript text. Q.W., Y.H., Y.X., G.Y. and T.L. prepared all the figures. Q.W., Y.H., T.L., Y.X., G.Y. and G.L. prepared all tables and collected the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (42230703, 41773075).
Data Availability Statement
Data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Turner, B.L.; Zhou, G.; Mo, J. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef]
- Zak, D.R.; Argiroff, W.A.; Freedman, Z.B.; Upchurch, R.A.; Entwistle, E.M.; Romanowicz, K.J. Anthropogenic N deposition, fungal gene expression, and an increasing soil carbon sink in the Northern Hemisphere. Ecology 2019, 100, e02804. [Google Scholar] [CrossRef]
- Wang, J.J.; Bowden, R.D.; Lajtha, K.; Washko, S.E.; Wurzbacher, S.J.; Simpson, M.J. Long-term nitrogen addition suppresses microbial degradation, enhances soil carbon storage, and alters the molecular composition of soil organic matter. Biogeochemistry 2019, 142, 299–313. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lv, Y.N.; Liu, X.Y.L.; Wang, L. Ecological effects of atmospheric nitrogen deposition on soil enzyme activity. J. For. Res. 2013, 24, 109–114. [Google Scholar] [CrossRef]
- McGuire, K.L.; Zak, D.R.; Edwards, I.P.; Blackwood, C.B.; Upchurch, R. Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest. Oecologia 2010, 164, 785–795. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Hu, C.; Wu, L.; Duan, Y.; Zhang, W.; Aziz, T.; Cai, A.; Abrar, M.M.; Xu, M. Soil and microbial biomass stoichiometry regulate soil organic carbon and nitrogen mineralization in rice-wheat rotation subjected to long-term fertilization. J. Soils Sediments 2020, 20, 3103–3113. [Google Scholar] [CrossRef]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L. Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol. Biochem. 2004, 36, 1443–1451. [Google Scholar] [CrossRef]
- Deforest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci. Soc. Am. J. 2004, 68, 132–138. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Y.M.; Tao, Y.; Zhang, B.C. Responses of soil enzymes activities and microbial biomass N to simulated N deposition in Gurbantunggut Desert. Acta Ecol. Sin. 2011, 31, 3340–3349. [Google Scholar]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Song, X.G.; Hu, T.X.; Xian, J.R. Soil enzymes activities and its response to simulated nitrogen deposition in an evergreen broad-leaved forest, southern Sichuan. Acta Ecol. Sin. 2009, 29, 1234–1240. [Google Scholar]
- Zhu, J. A review on fundamental studies of secondary forest management. J. Appl. Ecol. 2002, 13, 1689–1694. [Google Scholar]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Shukla, G.C., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Turner, B.L.; Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Geisseler, D.; Lazicki, P.A.; Scow, K.M. Mineral nitrogen input decreases microbial biomass in soils under grasslands but not annual crops. Appl. Soil Ecol. 2016, 106, 1–10. [Google Scholar] [CrossRef]
- Zhang, N.; Wan, S.; Li, L.; Bi, J.; Zhao, M.; Ma, K. Impacts of urea n addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 2008, 311, 19–28. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Nie, Y.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Yue, K.; Peng, Y.; Peng, C.; Yang, W.; Peng, X.; Wu, F. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: A meta-analysis. Sci. Rep. 2016, 6, 19895. [Google Scholar] [CrossRef]
- Hu, Y.L.; Han, S.J.; Li, X.F. Responses of soil available nitrogen of natural forest and secondary forest to simulated n deposition in changbai mountain. J. Northeast For. Univ. 2009, 37, 36–38. [Google Scholar]
- Zheng, L.; Zhao, Q.; Lin, G.; Hong, X.; Zeng, D. Nitrogen addition impacts on soil phosphorus transformations depending upon its influences on soil organic carbon and microbial biomass in temperate larch forests across northern China. Catena 2023, 230, 107252. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Mcnulty, S.; Fernandez, I.J.; Boggs, J.; Schlesinger, W.H. Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For. Ecol. Manag. 2006, 222, 459–468. [Google Scholar] [CrossRef]
- Bowden, R.D.; Wurzbacher, S.J.; Washko, S.E.; Wind, L.; Lajtha, K. Long-term nitrogen addition decreases organic matter decomposition and increases forest soil carbon. Soil Sci. Soc. Am. J. 2019, 83, S82–S95. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef]
- Tu, L.H.; Dai, H.Z.; Hu, T.X.; Zhang, J.; Luo, S.H. Effects of simulated nitrogen deposition on soil respiration in a Bambusa pervariabilis × Dendrocala mopsi plantation in Rainy Area of West China. Chin. J. Appl. Ecol. 2011, 22, 829–836. [Google Scholar]
- Mccrackin, M.L.; Harms, T.K.; Grimm, N.B.; Hall, S.J.; Kaye, J.P. Responses of soil microorganisms to resource availability in urban, desert soils. Biogeochemistry 2008, 87, 143–155. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.T.; Yang, L.B.; Xu, N.; Chai, C.R.; Wang, J.F.; Fu, X.L. Effect of simulation nitrogen depositions on bacterial diversity of Deyeuxia angustifoliain wetland of Sanjiang Plain. Pratacultural. Sci. 2016, 33, 589–598. [Google Scholar]
- Yu, X.-Y.; Zhu, Y.-J.; Wang, B.; Liu, D.; Bai, H.; Jin, L.; Wang, B.-T.; Ruan, H.-H.; Mao, L.; Jin, F.-J.; et al. Effects of nitrogen addition on rhizospheric soil microbial communities of poplar plantations at different ages. For. Ecol. Manag. 2021, 494, 119328. [Google Scholar] [CrossRef]
- Guo, P.; Yang, L.; Kong, D.; Zhao, H. Differential effects of ammonium and nitrate addition on soil microbial biomass, enzymatic activities, and organic carbon in a temperate forest in North China. Plant Soil 2022, 481, 595–606. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Dell, C.J.; Rice, C.W. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Parham, J.A.; Deng, S.P. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol. Biochem. 2000, 32, 1183–1190. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzymes activity and stoichiometry in forest ecosystems along the north-south transect in eastern china (nstec). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Ameur, D.; Zehetner, F.; Johnen, S.; Jöchlinger, L.; Pardeller, G.; Wimmer, B.; Rosner, F.; Faber, F.; Dersch, G.; Zechmeister-Boltenstern, S.; et al. Activated biochar alters activities of carbon and nitrogen acquiring soil enzymes. Pedobiologia 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling n pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in alaskan boreal forest. Glob. Chang. Biol. 2010, 14, 1156–1168. [Google Scholar] [CrossRef]
- Amonette, R.; Gallo, M.E.; Lauber, C.; Zak, D.R.; Sinsabaugh, R.L. Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb. Ecol. 2004, 48, 218–229. [Google Scholar]
- Dong, L.; Berg, B.; Gu, W.; Wang, Z.; Sun, T. Effects of different forms of nitrogen addition on microbial extracellular enzyme activity in temperate grassland soil. Ecol. Process. 2022, 11, 36. [Google Scholar] [CrossRef]
- Deforest, J.L. Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol. Biochem. 2004, 36, 965–971. [Google Scholar] [CrossRef]
- Alarcón-Gutiérrez, E.; Bruno, C.; Christopher, A.; Virgile, C.; Stéven, C. Effects of nitrogen availability on microbial activities, densities and functional diversities involved in the degradation of a mediterranean evergreen oak litter (Quercus ilex L.). Soil Biol. Biochem. 2008, 40, 1654–1661. [Google Scholar]
- Wang, Q.K.; Liu, S.L. Responses to n and p fertilization in a young eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Appl. Soil Ecol. 2008, 40, 484–490. [Google Scholar] [CrossRef]
- Li, S.; Du, Y.; Guo, P.; Guo, L.; Qu, K.; He, J. Effects of different types of n deposition on the fungal decomposition activities of temperate forest soils. Sci. Total Environ. 2014, 497–498, 91–96. [Google Scholar] [CrossRef]
- Ma, S.; Chen, G.; Tang, W.; Xing, A.; Chen, X.; Xiao, W.; Fang, J. Inconsistent responses of soil microbial community structure and enzyme activity to nitrogen and phosphorus additions in two tropical forests. Plant Soil 2021, 460, 453–468. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhao, X. Effects of simulated nitrogen deposition on the soil enzymes activities in a Pinus tabulaeformis forest at the Taiyue Mountain. Acta Ecol. Sin. 2015, 35, 4613–4624. [Google Scholar]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Bell, T.H.; Klironomos, J.N.; Henry, H.A.L. Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Sci. Soc. Am. J. 2010, 74, 820–828. [Google Scholar] [CrossRef]
- Stark, S.; Männistö, M.K.; Eskelinen, A. Nutrient availability and ph jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Gao, J.; Wang, E.; Ren, W.; Liu, X.; Yang, Y. Effects of simulated climate change on soil microbial biomass and enzyme activities in young chinese fir (Cunninghamia lanceolata) in subtropical china. Acta Ecol. Sin. 2017, 37, 272–278. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S. Nitrogen addition shapes soil enzymes activity patterns by changing ph rather than the composition of the plant and microbial communities in an alpine meadow soil. Plant Soil 2019, 440, 11–24. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Hackl, E.M.; Pfeffer, C.; Donat, G.; Bachmann, S. Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Biol. Biochem. 2005, 37, 661–671. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).