Effects of Bacillus cereus NJSZ-13 on Fatty Acid Metabolism of Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Nematodes and Strains
2.2. Obtaining of the Bacterial Suspension and Fermentation Filtrate
2.3. NJSZ-13 Treatment of B. xylophilus
2.4. Observation of Lipid Droplets of B. xylophilus Treated with Strain NJSZ-13
2.5. Determination of Fatty Acid Content in B. xylophilus by Strain NJSZ-13
2.6. qRT-PCR Detection of Genes Related to Fatty Acid Synthesis Pathways in Nematodes
2.6.1. Extraction and Detection of Total RNA from B. xylophilus
2.6.2. Reverse Transcription of RNA
2.6.3. Real Time Fluorescent Quantitative PCR
3. Results
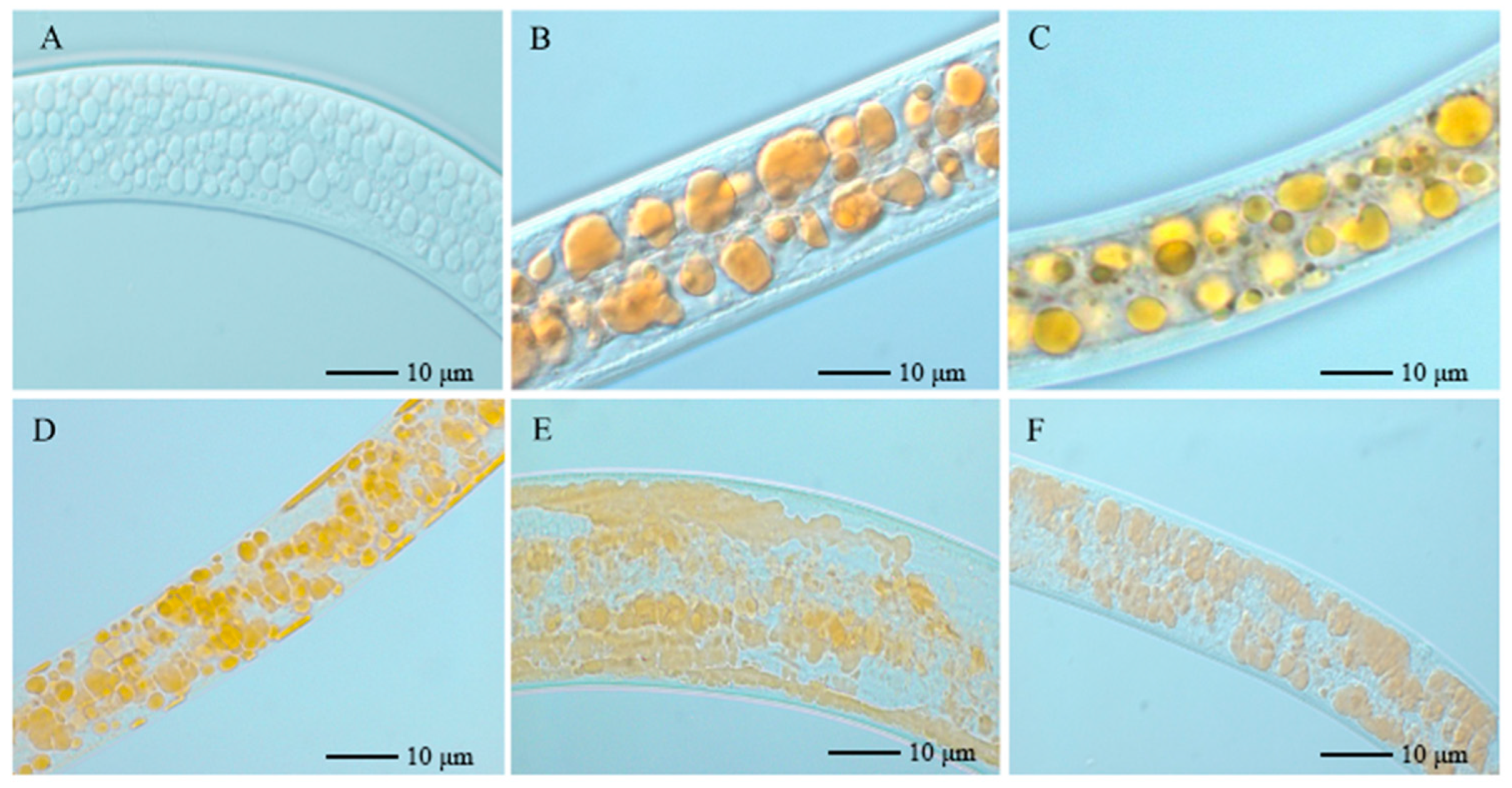
3.1. Effect of Strain NJSZ-13 on Lipid Droplets of B. xylophilus
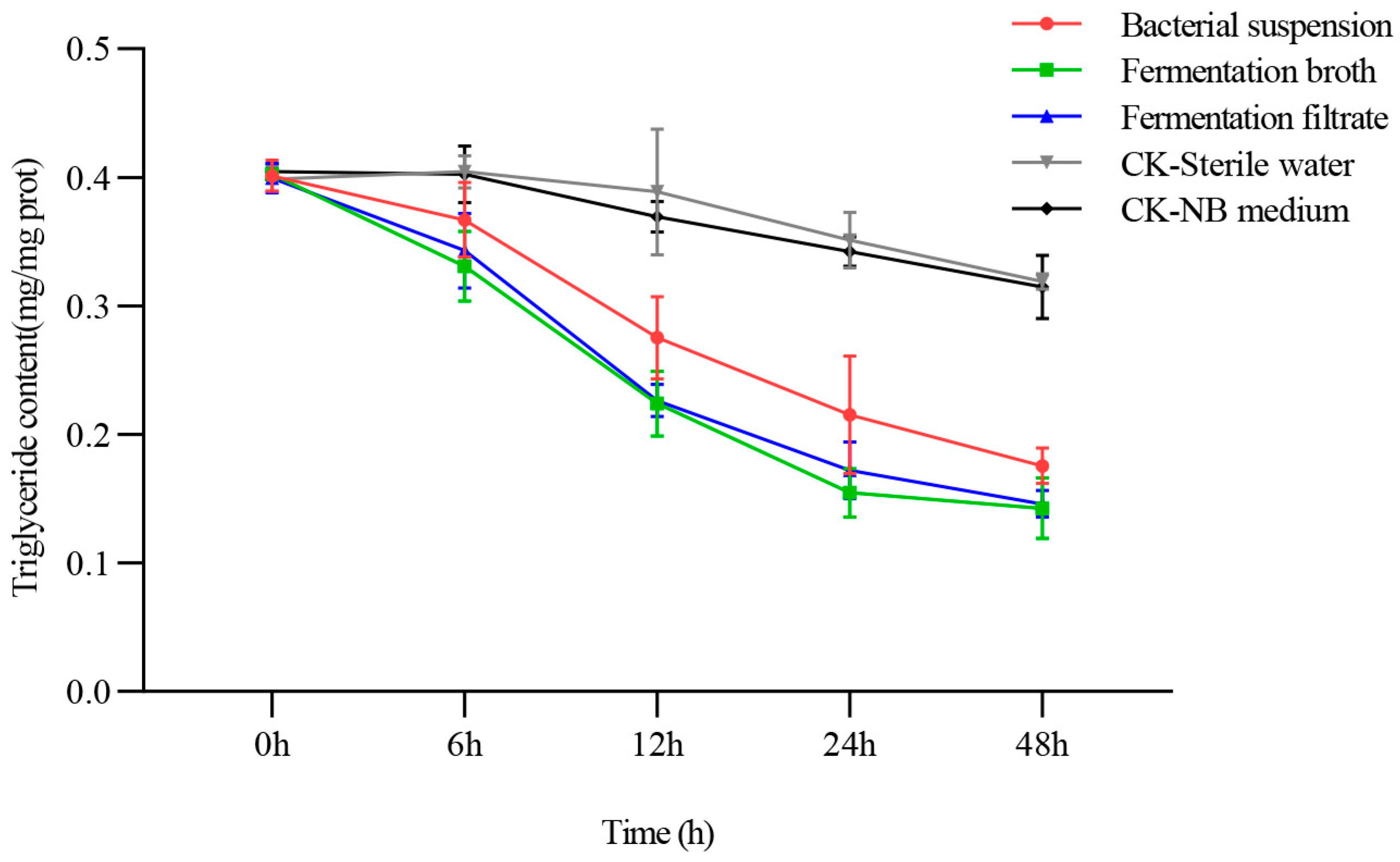
3.2. Effect of NJSZ-13 on Triglyceride Content in B. xylophilus
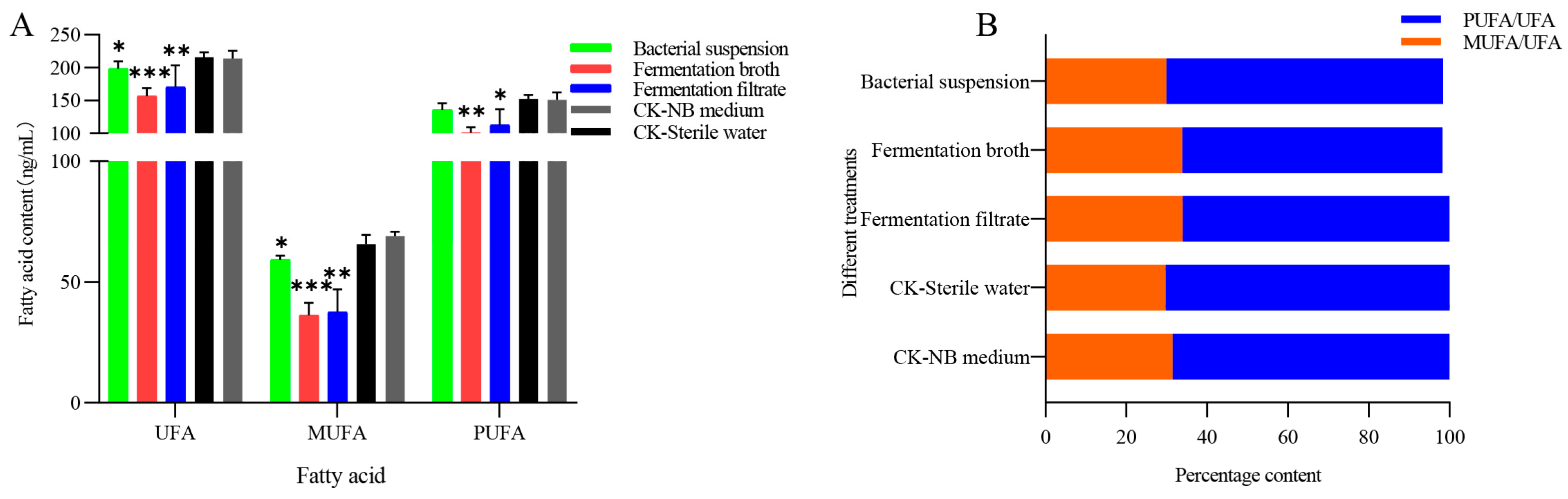
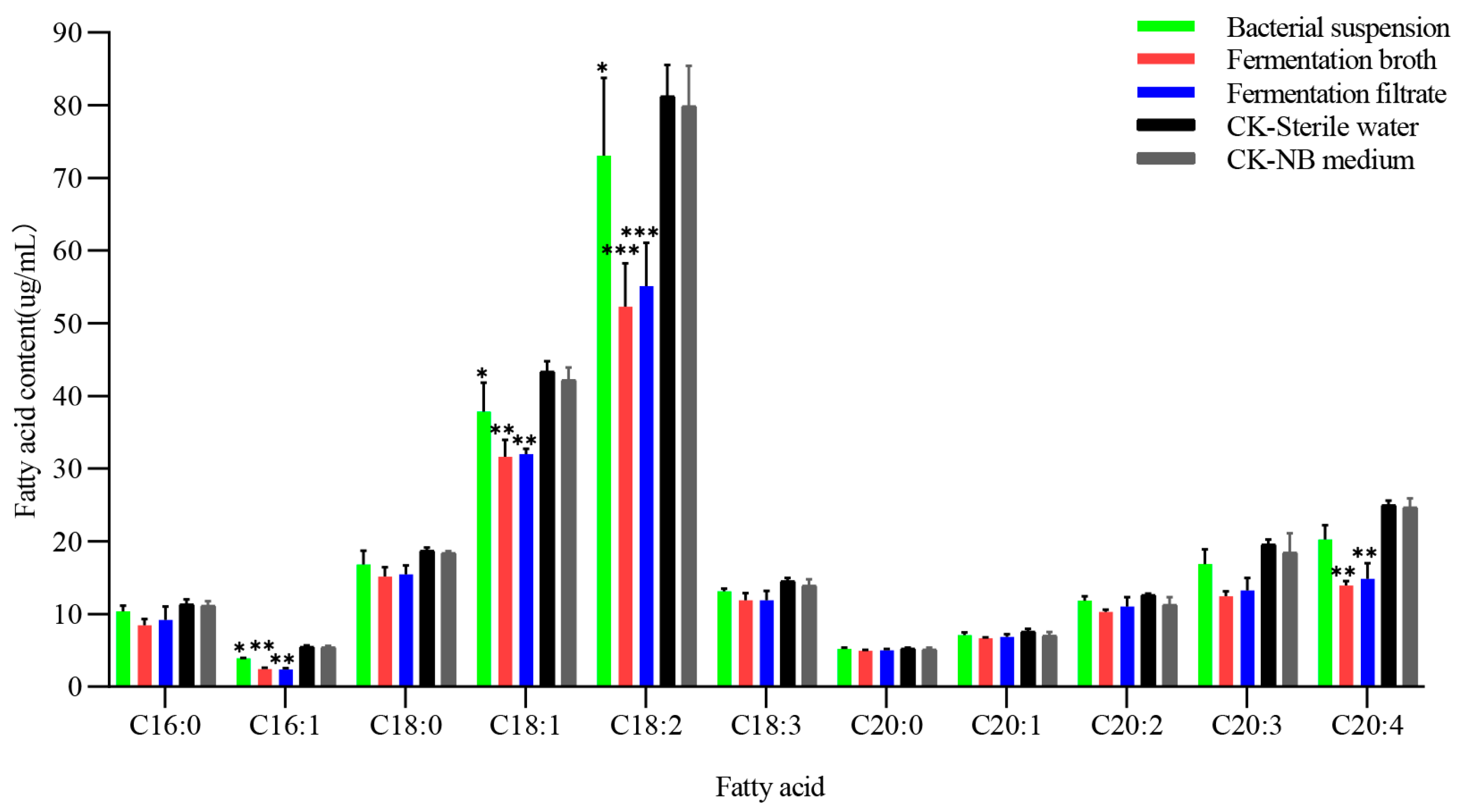
3.3. Effect of NJSZ-13 on Fatty Acid Content in B. xylophilus
3.4. Effect of Strain NJSZ-13 on Bx-SCD Expression in B. xylophilus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, N.; Jeon, H.W.; Mannaa, M. Induction of resistance against pine wilt disease caused by Bursaphelenchus xylophilus using selected pine endophytic bacteria. Plant Pathol. 2019, 68, 434–444. [Google Scholar] [CrossRef]
- Wang, L.C.; Su, S.R.; Chen, F.M.; Dong, X.Y.; Tian, C.L.; Wang, Y. Preliminary investigations of vector beetle and nematode species in Pinus massoniana forest in Huangshan City. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 29–35. [Google Scholar]
- Ding, X.L.; Zhang, Y.; Lin, S.X.; Ye, J.R. An overview of high-throughput sequencing techniques applied on Bursaphelenchus xylophilus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 1–7. [Google Scholar]
- Chen, J. Study on the Control of Bursaphelenchus xylophilus. J. Jilin For. Sci. Technol. 2019, 48, 41–48. [Google Scholar]
- Yin, Y.N.; Tan, J.J.; Li, M.W.; Xu, J.L.; Hao, D.J. A study on the biocontrol of pine wilt disease by Bacillus cereus NJSZ-13. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 152–158. [Google Scholar]
- Wu, J.W.; Yin, Y.N.; Tan, J.J.; Hao, D.J. A preliminary study on resistance of Pinus massoniana induced by Bacillus cereus NJSZ-13 strain to Bursaphelenchus xylophilus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 53–58. [Google Scholar]
- Xu, J.L.; Tan, J.J.; Hao, D.J. Effect of Bacillus cereus NJSZ-13 strain on oviposition and reproduction of Bursaphelenchus xylophilus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 209–214. [Google Scholar]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Byberg, L.; Kilander, L.; Lemming, E.W.; Michalsson, K.; Vessby, B. Cancer death is related to high palmitoleic acid in serum and to polymorphisms in the SCD-1 gene in healthy Swedish men. Am. J Clin. Nutr. 2014, 99, 551–558. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef]
- Strittmatter, P.; Spatz, L.; Corcoran, D.; Rogers, M.J.; Setlow, B.; Redline, R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc. Natl. Acad. Sci. USA 1974, 71, 4565–4569. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.J.; Browse, J.; Watts, J.L. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2006, 2, e108. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.; Lee, S.D.; Lopez, I.; Arnold, R.S.; Lambeth, J.D.; Suh, P.G.; Ryu, S.H. Selective activation of phospholipase D2 by oleic acid. FEBS Lett. 1999, 454, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, K.; Cameron, S.; Liling, Z.; Tracy, T.; Mariella, R.; Allen, V. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 2006, 147, 3398–3407. [Google Scholar]
- Karaskov, E.; Scott, C.; Zhang, L.; Teodoro, T.; Ravazzola, M.; Volchuk, A. Diet, cancer and the lipidome. Endocrinology 2006, 147, 3398–3407. [Google Scholar] [CrossRef]
- Wang, L.; Folsom, A.R.; Eckfeldt, J.H. Plasma fatty acid composition and incidence of coronary heart disease in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.A.; Hey, S.J.; Lacey, D.J.; Shewry, P.R. Identification of a Caenorhabditis elegans Δ6-fatty-acid-desaturase by heterologous expression in Saccharomyces cerevisiae. Biochem. J. 1998, 330, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.W. Molecular Mechanism of Low Temperature Response of Pine Wood Nematode. Doctor’s Thesis, Northeast Forestry University, Harbin, China, 2007. [Google Scholar]
- Pan, M.; Xu, J.L.; Tan, J.J.; Sun, Y.F.; Ye, J.R. Effects of Bacillus cereus NUSZ-13 on physiology and biochemical indexesof Bursaphelenchus xylophilus. J. Northeast For. Univ. 2022, 51, 134–138. [Google Scholar]
- Shen, L.; Lin, X.; Liu, F.; Huang, Y.; Ye, J.; Tan, J. Early Diagnosis of Pine Wood Nematode Disease Based on Chlorophyll Fluorescence Parameters and Organic Acids. Forests 2023, 14, 1582. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Y.R.; Tan, J.J.; Liu, F.; Hu, J.F. Optimization of fermentation conditions for Bacillus pumilus LYMC-3 to antagonize Sphaeropsis sapinea. Fermentation 2023, 9, 482. [Google Scholar] [CrossRef]
- Ashrafi, K. Obesity and the regulation of fat metabolism. WormBook 2007, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Du, Y.L.; Xu, S.M.; Liu, P.S. Stearoyl-CoA desaturase-1 and lipid metabolism. Chin. Bull. Life Sci. 2011, 23, 1101–1105. [Google Scholar]
- Watts, J.L.; Browse, J. A palmitoyl-CoA-specific Δ9 fatty acid desaturase from Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2000, 272, 263–269. [Google Scholar] [CrossRef]
- Liang, H.M. Study on Pseudomonas aeruginosa ATCC27853 Strain Toxicity in Caenorhaditis elegans. Master’s Thesis, Lanzhou University, Lanzhou, China, 2015. [Google Scholar]
- Martin, S.; Parton, R.G. Lipid droplets: A unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef]
- Friedman, J.M. Obesity: Causes and control of excess body fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Yalan, D.U.; Wang, Y. Lipid droplet—A cellular organelle for lipid metabolism. Acta Biophys. Sin. 2010, 26, 97–105. [Google Scholar]
- Zhang, J.J. Study on the Molecular Mechanism of Zinc Regulation of Fat Metabolism in Caenorhabditis elegans. Doctor’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2016. [Google Scholar]
- Ding, H.Y. Study on the Inhibition of SREBP and SCD by Pu’er Tea in Reducing Fat Storage of Caenorhabditis elegans. Master’s Theis, University of Chinese Academy of Sciences, Beijing, China, 2014. [Google Scholar]

| Treatment | 48 h Bx-SCD Gene Expression |
|---|---|
| Bacterial suspension | 0.9525 B |
| Fermentation broth | 0.5737 C |
| Fermentation filtrate | 0.5165 C |
| CK-Sterile water | 1 A |
| CK-NB medium | 1 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Xu, J.; Han, S.; Sun, Y.; Tan, J. Effects of Bacillus cereus NJSZ-13 on Fatty Acid Metabolism of Bursaphelenchus xylophilus. Forests 2023, 14, 2065. https://doi.org/10.3390/f14102065
Pan M, Xu J, Han S, Sun Y, Tan J. Effects of Bacillus cereus NJSZ-13 on Fatty Acid Metabolism of Bursaphelenchus xylophilus. Forests. 2023; 14(10):2065. https://doi.org/10.3390/f14102065
Chicago/Turabian StylePan, Min, Jialin Xu, Shengjie Han, Yufeng Sun, and Jiajin Tan. 2023. "Effects of Bacillus cereus NJSZ-13 on Fatty Acid Metabolism of Bursaphelenchus xylophilus" Forests 14, no. 10: 2065. https://doi.org/10.3390/f14102065




