Abstract
To improve the conductivity of nematocidal emamectin benzoate (EB) in pine trees (Pinus massoniana Lamb.), EB-inclusive nanocapsules (EB-NCs) were developed as trunk injections and spraying agents. Microscopy revealed that the EB-NCs were spherical in shape (100–200 nm in diameter) and micro-agglomerated with an obvious core-shell structure. The EB-NCs formulation maintained high toxicity in Bursaphelenchus xylophilus pine wood nematodes (LC50 = 0.44 mg L−1). Moreover, the formulation had better EB slow-release and photostability. In addition, the EB-NCs formulation was effectively absorbed and transported in the pine trees via either trunk injection or spraying. In terms of trunk injection, the average residues of EB in the lower, middle, and upper branches of P. massoniana 30 days after application in summer were 8.11, 16.42, and 6.98 mg L−1. In terms of spraying treatment, the EB-NC formulation was successfully conducted from the cortical tissue to the cambium and the EB fluorescence intensity inside of the branches was better than that of the 2% EB micro-emulsion. The EB-NC formulation has suitable conductivity in pine trees; hence, this study provides a potential agent for the control of pine wilt disease in the field under all weather conditions.
1. Introduction
The pine wood nematode (Bursaphelenchus xylophilus, PWN) is an internationally important quarantine pest that causes millions of cases of pine wilt disease (PWD) annually. PWD was originally discovered in the United States of America, and then introduced to Japan, China, Republic of Korea, Portugal, and other countries [1]. In East Asia, Pinus massoniana Lamb., Pinus hunbergia Parl., and Pinus densiflora Siebold & Zucc. are the main PWD susceptible pine species and account for a large proportion of local forest resources. During the phytophagous stage, PWNs migrate to the xylem resin and ray tubes, where they feed on the thin-walled cells, resulting in pine tree wilting [2,3]. Consequently, it is a devastating disease that kills pine trees in a matter of weeks [4]. At present, PWD control measures mainly rely on quarantine, clearing of dead trees, forest renovation, and screening of naturally occurring nematocidal metabolites [5,6,7,8].
Emamectin benzoate (EB) is an effective semi-synthetic compound for the preventive control of PWD and significantly reduces the incidence of PWD 1–3 years after application via trunk injection [9,10,11]. EB is a derivative of abamectin, which is a 16-membered macrocyclic lactone produced by Streptomyces avermitilis and is prepared as a salt with benzoic acid. EB enhances the effects of neurotransmitters such as glutamate and γ-aminobutyric acid on nematode nerve cells and causes an influx of chloride ions into the nerve cells, thereby disrupting nerve conduction and causing paralysis and death [12]. The residual quantity of EB in most parts of a pine tree can be maintained long-term over the 95% inhibitory EB concentration against PWNs at a relatively low concentration of 0.05 mg L−1 after trunk injection [13]. Thus, it was successful in the prevention of early PWN invasion via long-horned beetle insect vectors feeding on pine branches. However, the application of EB via trunk injection is affected by woodland temperatures as secreted turpentine resin increases with ambient temperatures. Therefore, in late spring and summer, the pesticide bottle nozzles are blocked with turpentine; thus, the liquid agent cannot be absorbed by the tree trunk and transported into the branches. Hence, trunk injection can only be applied in winter. Considering that PWN-borne beetles are active during the spring and summer seasons, a new formulation with improved EB conductivity under all weather conditions is needed to protect pine trees from PWN infection.
Nano-formulation can improve the conductivity and release rate of active ingredients in plants, reduce residual pollution, and enhance the control efficiency [14,15,16,17,18]. The exploration of nano-formulations focuses on the control of pests in agriculture and few studies focus on their use in wood plant pest control [19,20]. In this study, emamectin benzoate nanocapsules (EB-NCs) were manufactured to improve the conductivity of the nematocidal ingredient EB in pine trees during spring and summer. This study provides a new EB nano-formulation for potential application in the control of PWD.
2. Materials and Methods
2.1. Preparation of EB-NC Formulation
The emulsion polymerization method was used to prepare the EB-NC formulation by forming a nanodrug-carrying system that encapsulated EB in a water-soluble carrier. The simple fabrication method yields EB-NCs that reduce UV degradation while increasing the dispersion between nanoparticles. Sodium benzoate (2 g), sodium dodecyl sulfate (1 g), and magnesium sulfate (1 g) were uniformly dissolved in 10 mL of deionized water using an ultrasonic apparatus to form the aqueous phase. To form the oil phase, 0.5 g of octylphenol polyoxyethylene ether (Zhejiang Shijia Science and Technology Co., Ltd., Zhejiang, China), 0.5 g of Tween 80, and 2 g of EB TC (79% EB; original drug; Zhejiang Qianjiang Biochemistry Co., Ltd., Jiaxing, China) were added to 5 mL of methanol in a beaker and placed in a constant-temperature magnetic stirrer (Zhengzhou Great Wall Science, Industry and Trade Co., Ltd., Zhengzhou, China) at low-speed stirring until homogenized [18]. To ultimately form the 4.3% (w/v) EB for the EB-NC formulation, the oil phase was then added dropwise to the aqueous phase, followed by low-speed magnetic stirring for 4 h until the mixture became transparent. All manipulations were performed under dark conditions at 25 °C [21,22]. In the present study, the ratio of the oil phase to the water phase was the oversaturation threshold for the EB-NC suspension, and it was not possible to increase the encapsulation rate any further, which would have resulted in liquid delamination and crystal precipitation, so it was inferred that the encapsulation process used 100% of the EB.
2.2. Determination of EB-NC Characteristics
2.2.1. Microscopy
A 10 μL aliquot of the EB-NC formulation was diluted in 1 mL of ethanol (analytically pure). An aliquot of 20 μL was added onto a conductive gel. After ultra-low-temperature treatment using a freeze-dryer (Martin Christ, Osterode, Germany) and gold plating, EB-NCs were observed using a cryo-electron microscope (SU8100 cryo-electron microscope, Hitachi, Tokyo, Japan). The EB-NCs were also observed using a JEM-1400 flash transmission electron microscope (Nippon Electronic Co., Ltd., Huizhou, China) [23,24,25].
2.2.2. EB-NC Photostability
The EB-NC formulation was diluted in ethanol (analytically pure) to prepare a solution of 100 mg L−1 of EB and a 5 mL aliquot was added to a disposable plastic Petri dish with a diameter of 9 cm. The solution was spread evenly on the bottom of the dish and placed in the dark to air dry at 25 °C. Another two treatments of EB TC and EB ME (2% EB micro-emulsion, Zhejiang Qianjiang Biochemistry Co., Ltd.) diluted to the same concentration and dried in dishes were prepared for comparison. The Petri dishes were then placed in a 350 W high-pressure xenon lamp (Shanghai Electro-Optical Devices Factory, Shanghai, China) at a light intensity of 1700 lux and an irradiance of 1000 W/m2. The dishes were sampled at 0, 2, 4, 6, 12, 24, 36, 48, 72, and 96 h, with three replicates in each treatment. The Petri dishes were washed with 5 mL of methanol using ultrasonic washing for 1 min. The washing solution was transferred to 10 mL centrifugal tubes, adding methanol to a final volume of 5 mL. The EB quantity in the solution was determined using high-performance liquid chromatography (HPLC) using a Waters 600 high-performance liquid chromatograph (Waters Corporation, Milford, MA, USA) [26].
2.2.3. EB-NC Slow-Release Properties
The release of EB from the nanocapsules was analyzed using dynamic dialysis. An aliquot of 1 mL of EB-NC formulation was mixed with 20 mL of 70% (v/v) ethanol and transferred to a dialysis bag with a molecular weight cut-off of 10,000 Da. The dialysis bag was immersed in 100 mL of 70% ethanol and shaken continuously at 25 °C and 200 rpm. At timepoints of 2, 4, 6, 12, 24, 36, 48, 72, 96, and 120 h, 1 mL of the solution outside of the dialysis bag was sampled to determine the released EB quantity. The EB release rates of the EB TC and EB ME formulations were also assessed under the same operating conditions. Each treatment included three dialysis bags as replicates [26,27].
2.3. Indoor EB-NC Toxicity Assay against PWNs
The PWN strain No. NB-6 was originally isolated from diseased P. massoniana in Ningbo City, Zhejiang Province, China and reared in the laboratory with Botrytis cinerea at a constant temperature of 25 °C in the dark.
The nematocidal effect of the EB-NC formulation in PWNs was determined using the dipping method. The EB-NC formulation was added to double-distilled water to prepare a solution of 100 mg L−1 of EB and then serially diluted to form 0.25, 0.5, 1, 2.5, and 5 mg L−1 EB solutions. Nematodes (1000) were added to 100 μL of each EB solution concentration in 24-well culture plates. Each concentration included three replicates. The nematocidal effects of the EB ME and EB TC formulations were also tested for comparison and sterile water was used as a blank control. After incubation at 25 °C for 24 h, the number of surviving (in motion) and dead nematodes were determined under a microscope. Abbott’s formula was used to determine the mortality rates in the treatments [8].
2.4. Conductivity Distribution Dynamics of EB-NC Trunk Injection in Pinus massoniana
Thirty Pinus massoniana trees with 10–8 cm DBH and in good health were randomly selected from Qingluo County in Lin’an District, Hangzhou City, China (119°46′1.506″ E, 30°17′44.747″ N) for trunk injection. The EB-NC and EB ME formulations were separately injected on 1st March and 1st June, respectively. Using a 7 mm drill bit, an injection hole was drilled downwards into the trunk to a depth of approximately 4 cm in the base of each pine tree at 45° diagonally. A bottle containing 20 mL of either EB-NC or EB ME formulations was inserted into the hole. Five trees were injected with each formulation on each injection date. The injection-without-EB pine trees were used as controls. The lateral branches from the lower, middle, and upper parts of the pine trees were sampled to determine the EB quantity every 30 days during the spring season of March–May and the summer season June–August in 2023.
Each sample was crushed and added to 50 mL of methanol/water (95:5, v/v), extracted by ultrasonic shaking for 1.5 h, and filtered. The sample was shaken another three times with methanol, and the filtrates were combined. The filtrate was concentrated to 1 mL in a rotary evaporator and transferred to a clean-up column equipped with octadecylsilane-bonded silica gel filler (Otsubo Co., Ltd., Tokyo, Japan) and eluted with methanol/water (95:5, v/v) several times. The first 5 mL of eluent was discarded, and the following 10 mL of the eluent was collected for HPLC analysis.
2.5. In Situ Fluorescent Detection of EB in Trunk-Injected Pine Trees
EB emits weak fluorescence and is difficult to observe directly under a fluorescence microscope. Hence, a fluorescent derivative of EB was synthesized as a fluorescent indicator to observe the distribution of EB in slices of P. massoniana branches and needles. Lateral branches and needles of P. massoniana trunks injected with EB-NC and EB ME formulations were separately sampled 3 months after trunk injection for transverse sectioning. The samples were sliced into 50 μm-thick slices after cleaning with water. For EB detection, 60 μL of derivatization reagent A (v/v: 1-methylimidazole/acetonitrile, 7/3) was evenly added to both the front and back sides of the slices for 15 s. Then, 60 μL of derivatization reagent B (v/v: trifluoroacetic anhydride/acetonitrile, 7/3) was evenly added onto the slices. After incubation in the dark for 20 min, the slices were observed under a Leica orthogonal fluorescence microscope and photographed to observe the EB distribution in the transverse sections of pine branches and needles [28]. The fluorescence intensity in the images was comparatively analyzed using Image J (v1.54d) software to obtain the values of the average fluorescence intensity (intensity/area). To exclude interference of the derivatization reagents and pine autofluorescence in the experiment, the corrected fluorescence intensity of EB was determined by deducting the intensity detected in the control [29].
2.6. Conductivity Properties of EB in EB-NC-Sprayed Pinus massoniana
2.6.1. Fluorescence Localization to EB after Spray Conductance
Pines without wilt symptoms and 4–6 cm DBH were selected for spray treatments with EB-NC and EB ME formulations. The EB-NC formulation was diluted in an aqueous solution to a concentration of 100 mg L−1 of EB, and uniformly sprayed onto pine branches and needles. Each tree was sprayed with 50 mL of aqueous solution. An aqueous solution of EB ME with the same concentration of EB was sprayed in the same manner as the EB-NCs onto the pine trees. Each treatment included 3 trees as replicates. Lateral branches and needles were sampled 3, 9, 15, and 30 days after spraying. Slices were prepared following the abovementioned procedures to evaluate the conductive properties of EB into the pine trees using the fluorescence intensity in the images.
2.6.2. Detection of EB Residues after Spray Conduction
Using the above method, pines were sprayed on days 0, 10, and 20. An aqueous solution of EB ME with the same concentration of EB was sprayed in the same manner as the EB-NCs onto the pine trees. Each treatment included five trees as replicates. On day 30, the whole tree was dug up and bark, pine needles and xylem were collected from the lateral branches, main trunk and roots. The EB content was determined according to the HPLC method described above.
2.7. Data Analysis
The photostability, slow-release rate, and fluorescence intensity of EB were separately differentiated using a two-factor analysis of variance (ANOVA with Fisher’s least significant difference test). The EB conductivity in pine trees after trunk injection was differentiated using a three-factor ANOVA. Toxicity regression analysis of the three formulations of EB-NCs, EB TC, and EB ME was performed along with a probability value analysis, and the median lethal concentration (LC50), the 90% lethal concentration (LC90), and their 95% confidence limits were calculated. All analyses were conducted using an updated version of DPS (7.05) software [30].
3. Results
3.1. Characteristics of EB-NCs
The physical properties of EB-NCs using cryo-electron and transmission electron microscopy revealed that the EB-NCs were spherical in shape, with a particle size of approximately 100–200 nm (Figure 1). The capsule walls were complete, well dispersed, and had an obvious core-shell structure, indicating that the homogenized nanocapsules were successfully manufactured.
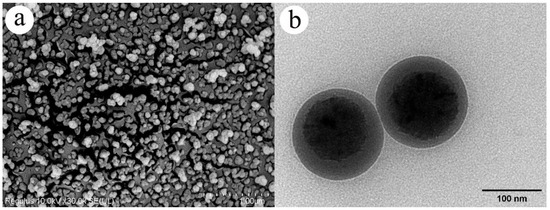
Figure 1.
Microscopic characteristics of emamectin benzoate-inclusive nanocapsules observed using (a) cryo-electron and (b) transmission electron microscopy.
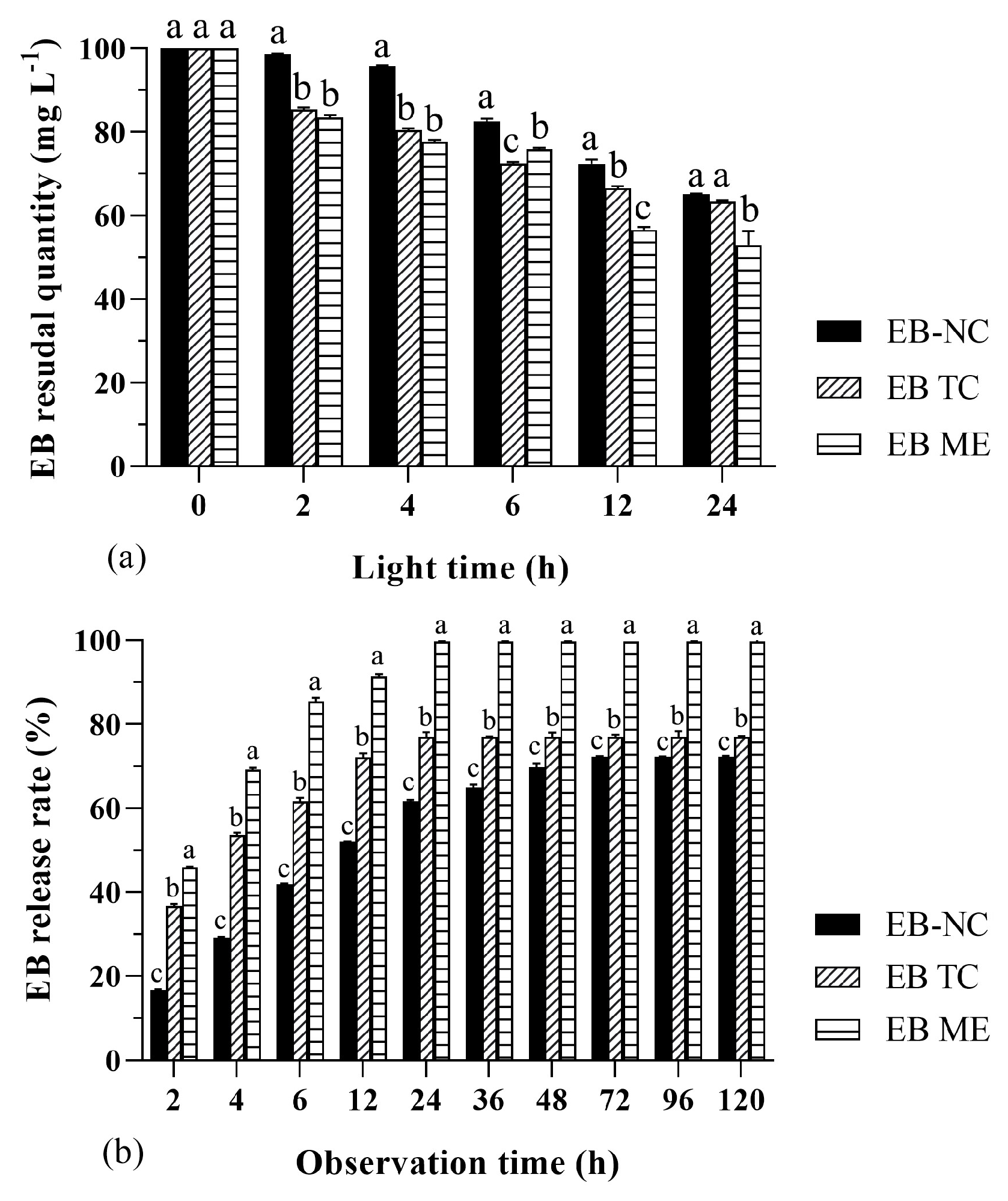
The photostability of the EB-NC formulation was significantly better than that of the EB TC and EB ME formulations (F2,36 = 10.4, p < 0.01), although the EB quantities in the three formulations decreased with increased light hours (F5,36 = 34.82, p < 0.01). After light treatment for the first 12 h, the EB quantity in the EB-NC formulation decreased by 27.65%, whereas the quantity in the EB TC and EB ME formulations decreased by 33.47% and 43.34%, respectively (Figure 2a). The results indicate that the nano-microcapsule formulation slowed the photodegradation rate of EB, thereby favoring the formulation stock and spray application in the field.
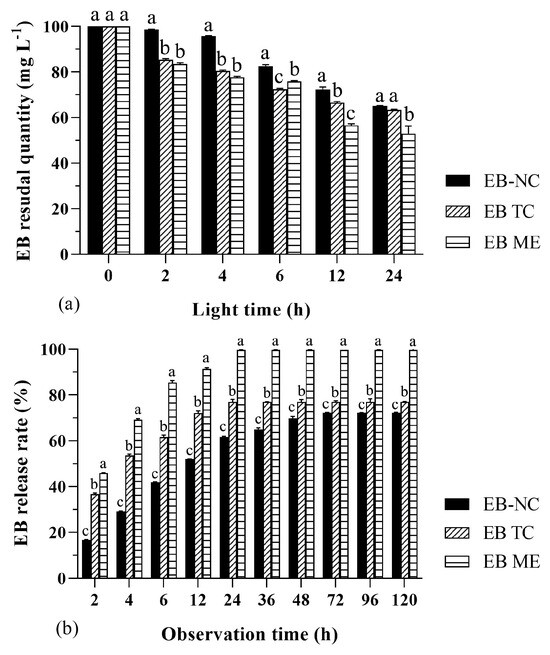
Figure 2.
Photostability and slow-release performance of emamectin benzoate-inclusive nanocapsules (EB-NCs). (a) Residual quantities of EB-NCs, original 79% EB drug (EB TC), and 2% emamectin benzoate micro-emulsion (EB ME) exposed to light for different total number of hours. (b) Cumulative release rate of EB-NCs, EB TC, and EB ME after various observation hours. All analyses were conducted using an updated version of DPS software. The error bars indicate standard deviation. Different lowercase letters on the bars indicate significant differences from one another [Fisher’s least significant difference (LSD); p < 0.05].
The release performance of the EB-NC formulation was significantly slower than that of the EB TC and EB ME formulations (F2,60 = 148.01, p < 0.001), although the released EB quantities increased with observation time (F9,60 = 44.94, p < 0.001). The largest release rates were 72.22%, 77.02%, and 99.70% for the EB-NC, EB TC, and EB ME formulations, respectively (Figure 2b), and the amount of time taken to reach the largest EB release rate was three-times longer for the EB-NC formulation than that for the EB TC and EB ME formulations. These results indicate that the nano-microcapsule formulation effectively slowed the release rate of EB, thereby favoring the full absorption of EB by trees and the avoidance of drug harm.
The LC50 and LC90 values for EB-NCs in PWNs after 24 h showed high toxicity (Table 1). There was no significant difference in the three formulations of EB-NCs, EB ME and EB TC, demonstrating that the procedure and other components of the nanocapsules did not affect the nematocidal activity of EB.

Table 1.
Toxicity of nanocapsules of emamectin benzoate (EB-NCs), original 79% EB drug (EB TC), and 2% emamectin benzoate micro-emulsion (EB ME) in pine wood nematodes.
3.2. EB Conduction Dynamics in Pinus massoniana with Trunk Injection
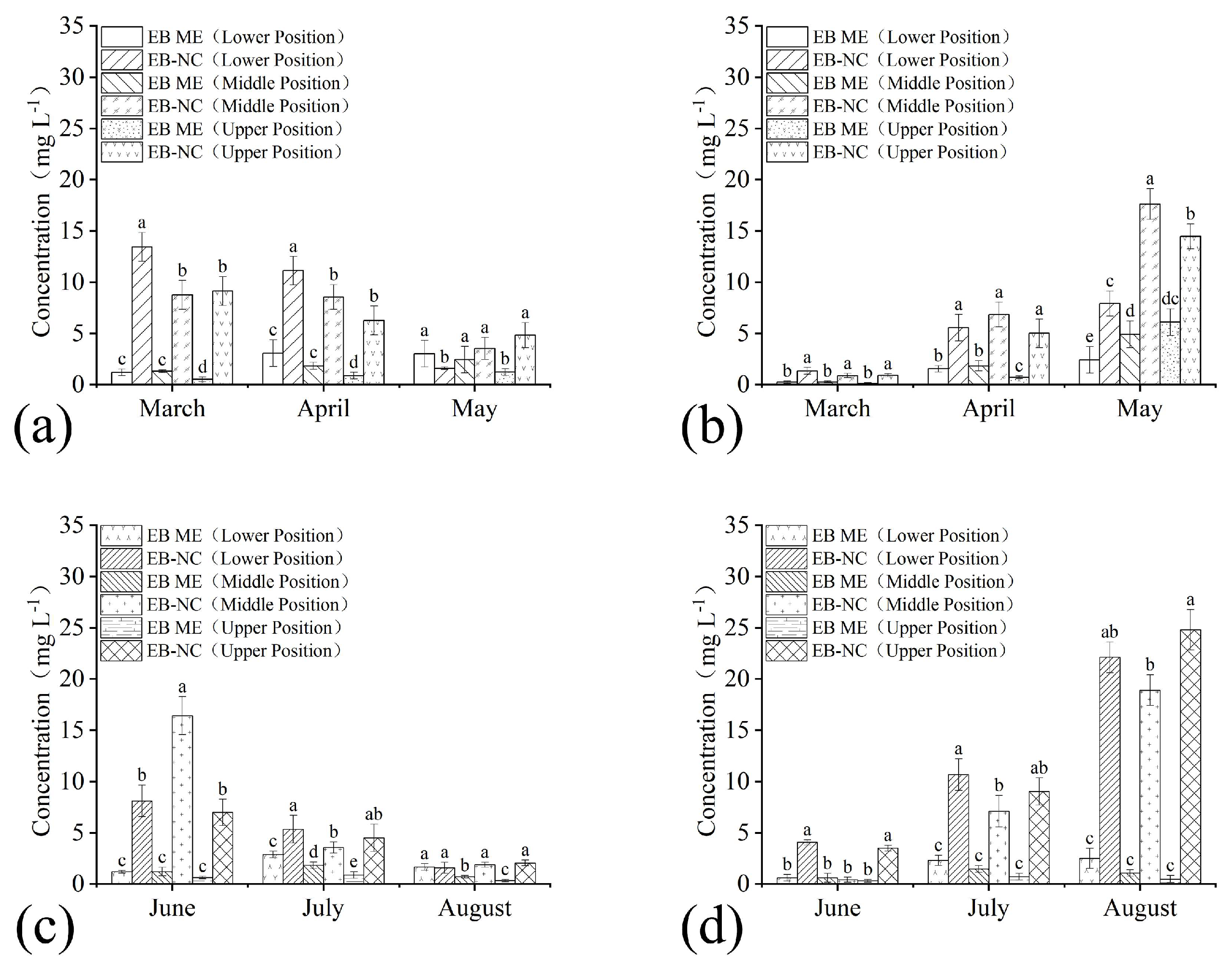
The residual amounts of EB in branches and needles sampled from the lower, middle, and upper parts of the pine trees varied during the months after trunk injection of EB-NC and EB ME formulations in the spring and summer seasons. There were significant differences in trunk injection formulations (branch: F1,36 = 76.84, p < 0.001 in spring and F1,36 = 227.29, p < 0.001 in summer; needle: F1,36 = 260.21, p < 0.001 in spring and F1,36 = 476.40, p < 0.001 in summer), month (branch: F2,36 = 7.09, p = 0.002 in spring and F2,36 = 78.23, p < 0.001 in summer; needle: F2,36 = 273.55, p < 0.001 in spring and F2,36 = 158.89, p < 0.001 in summer), and sampling parts (branch: F2,36 =0.96, p = 0.39 in spring and F2,36 = 11.65, p < 0.001 in summer; needle: F2,36 = 19.45, p < 0.001 in spring and F2,36 = 7.58, p = 0.002 in summer). The EB quantities in branches and needles after EB-NC formulation application were higher than those in branches injected with the EB ME formulation (Figure 3), indicating that EB-NC formulation injection had significantly higher conductivity and absorption efficiency in pine trees. Furthermore, it demonstrated that the nano-microcapsule formulation enabled EB conductivity in trees regardless of turpentine secretion. EB quantities in the branches decreased in the three months after trunk injection regardless of the sampling parts and seasons. The EB residual amounts were 1.58, 3.52, and 4.82 mg L−1 in the lower, middle, and upper parts in spring, and 1.57, 1.87, and 2.05 mg L−1 in summer (Figure 3a,c), respectively. Notably, these values were still higher than the LC50 values in PWNs. The EB quantities in the needles increased during the three months (Figure 3b,d), demonstrating that EB was transferred from the branches to the endpoint needles via transpiration.
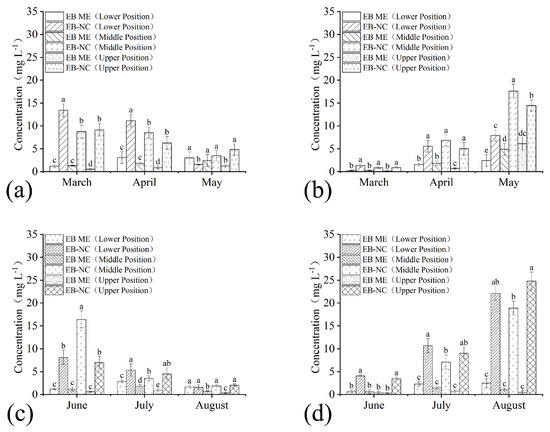
Figure 3.
Conductivity of emamectin benzoate-inclusive nanocapsules (EB-NCs) in Pinus massoniana. Emamectin benzoate (EB) quantities in the lower, middle, and upper (a,c) branches and (b,d) needles sampled in (a,b) spring and (c,d) summer seasons after trunk injection. The 2% emamectin benzoate micro-emulsion (EB ME) was trunk injected for comparison. The error bars indicate standard deviation. Different lowercase letters on the bars indicate significant differences from one another [Fisher’s least significant difference (LSD); p < 0.05].
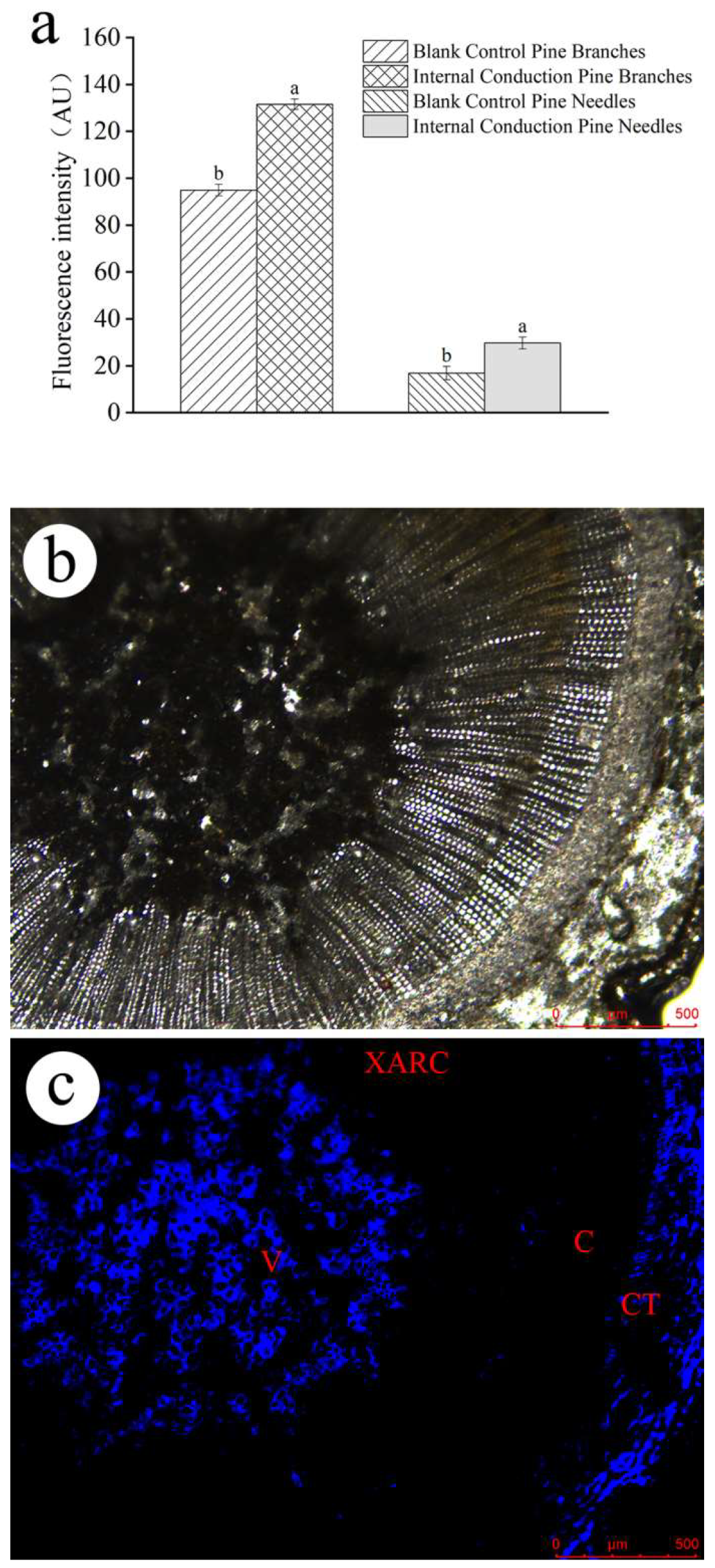
The residual EB quantities and conduction in P. massoniana after trunk injection were also observed using fluorescence imaging. There were faint autofluorescence signals in trees without injection (Figure S1). According to the distribution of the blue fluorescence, EB was mainly distributed in the cortical tissue, formation layer, ducts, resin tracts, and tubular cells of the xylem (Figure 4 and Figure S2), as well as in the ducts of pine needles after trunk injection with the EB-NC formulation (Figure S3). Moreover, the corrected fluorescence intensity was significantly higher in the branches (F1,4 = 113.73, p < 0.001) and needles (F1,4 = 105.36, p < 0.001) after EB-NC formulation injection than after the EB ME formulation injection.
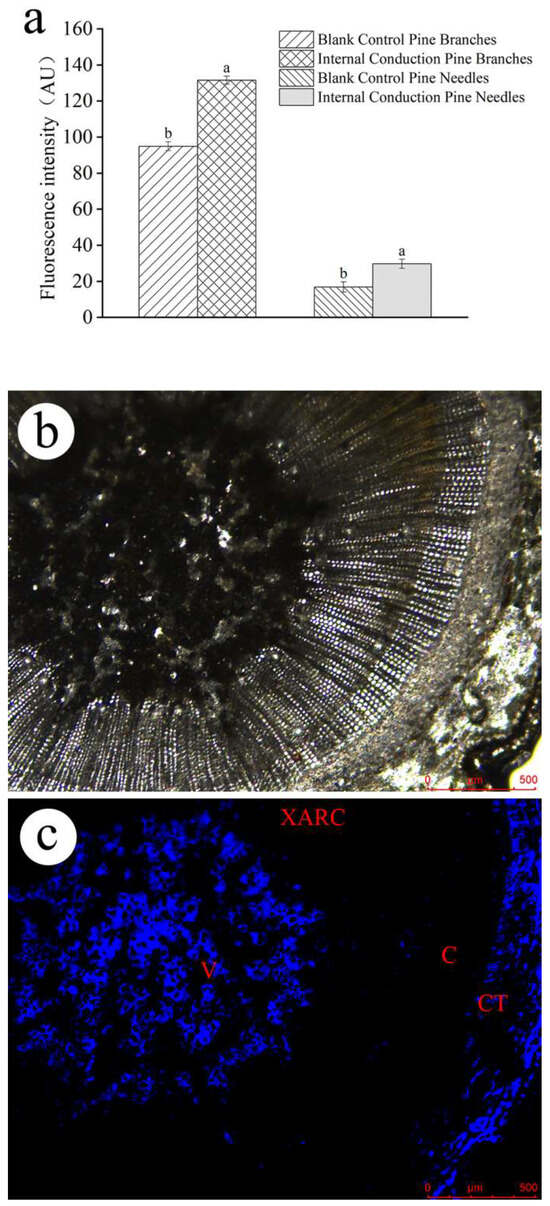
Figure 4.
Fluorescence intensity as an indicator of emamectin benzoate (EB) distribution in the branches of EB-NC formulation trunk-injected Pinus massoniana. (a) The corrected fluorescence intensity, (b) transverse section of branches under light microscopy and (c) EB distribution in transverse section. V, xylem duct; C, cambium region; CT, cortical tissue; and XARC, xylem axial resin duct. The error bars indicate standard deviation. Different lowercase letters on the bars indicate significant differences from one another [Fisher’s least significant difference (LSD); p < 0.05].
3.3. EB-NC Formulation Conductivity Properties in P. massoniana with Spray Application
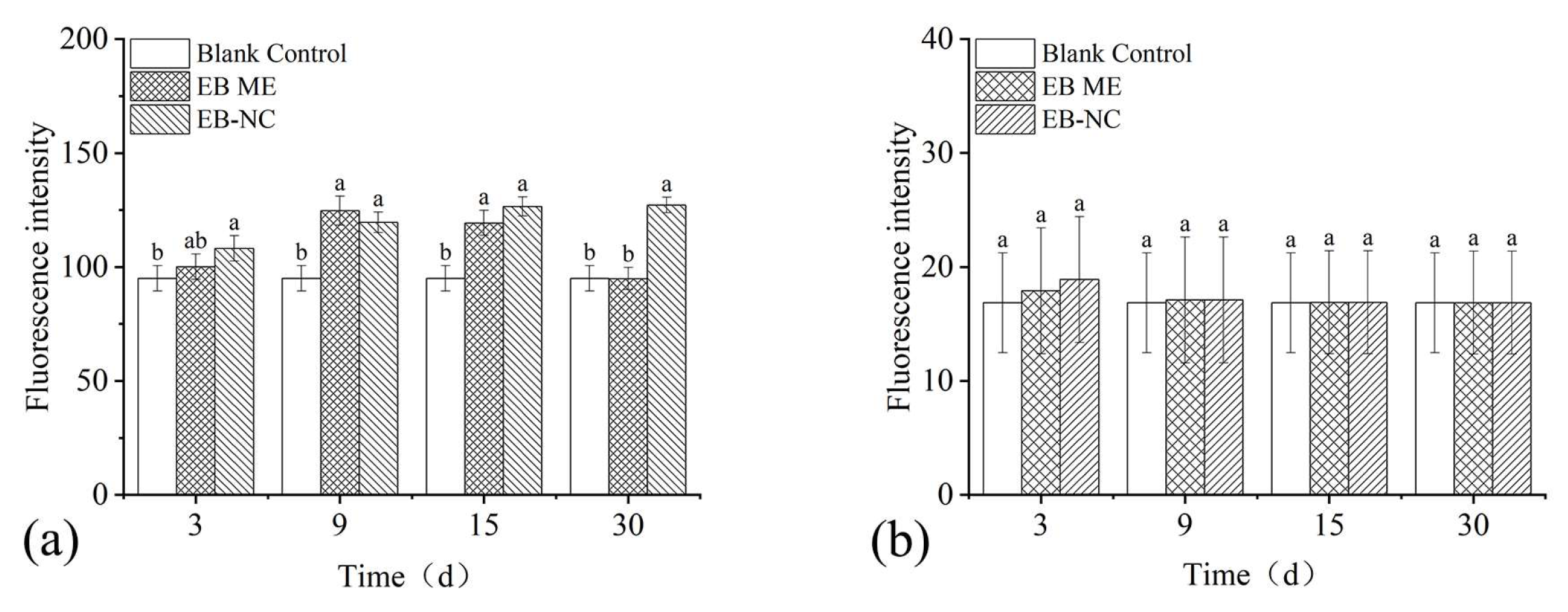
Considering the laborious task of EB application via trunk injection, spray applications were conducted to analyze the potential of EB absorbed from the surface into the branches of P. massoniana after spray application. Thirty days after spray application (Figure 5), there was no significant effect on the pine needles regardless of the formulations (F1,16 = 2.12, p = 0.24) and observation days (F3,16 = 4.16, p = 0.14). The fluorescence intensity in the transverse sections significantly differed in the branches (formulation: F1,16 = 9.46, p = 0.007; observation days: F3,16 = 7.86, p = 0.002).
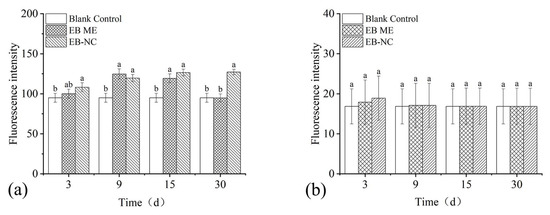
Figure 5.
Fluorescence intensity as an indicator of emamectin benzoate (EB) quantity in transverse sections of Pinus massoniana (a) branches and (b) needles after spraying with emamectin benzoate-inclusive nanocapsules (EB-NCs). The error bars indicate standard deviation. Different lowercase letters on the bars indicate significant differences from one another [Fisher’s least significant difference (LSD); p < 0.05].
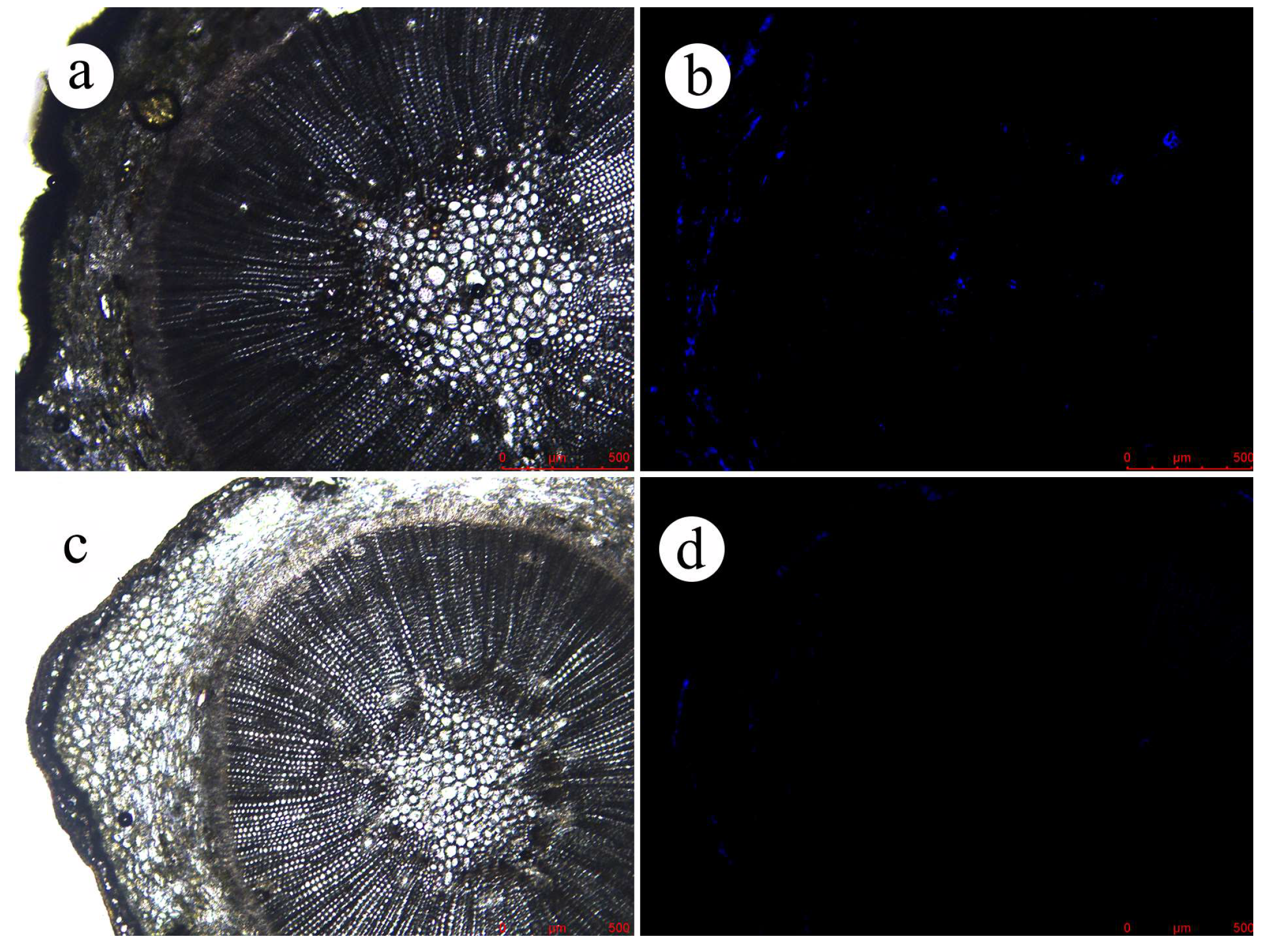
Further observation of EB distribution in the branches (Figures S4 and S5) revealed that the fluorescence was only detected on day 15 in the epidermis of the branches sprayed with the EB ME formulation. Moreover, on day 30 after spraying, little EB was observed, probably due to its degradation and rain washing. This indicates that little EB in the EB ME formulation was absorbed by the branches. In the EB-NC formulation spray treatment, fluorescence in the branches increased across the observation days (Figure S4). EB fluorescence was observed in the inner cortical tissues, formation layer, and axial resin tracts of the xylem of the branches, demonstrating improved conductivity of the EB-NC formulation from the epidermis to the core of the branches (Figure 6).
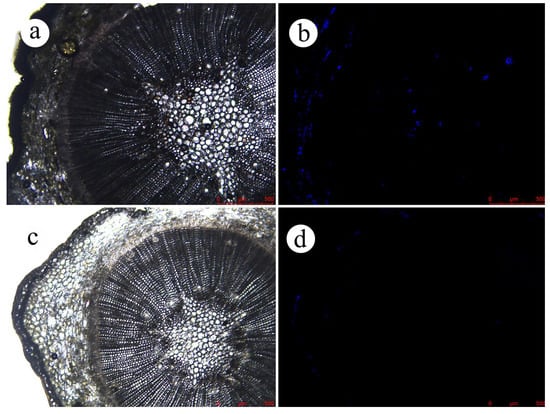
Figure 6.
Fluorescence intensity as an indicator of emamectin benzoate (EB) absorption by Pinus massoniana branches 30 days after spraying with diluted emamectin benzoate-inclusive nanocapsules (EB-NCs). (a) Optical microscope image of experimental group. (b) Fluorescence microscopic image of experimental group. (c) Optical microscope image of control group. (d) Fluorescence microscopic image of the control group.
After spraying with EB-NCs and EB ME three times within 30 days, there were significant differences in the EB residues detected in various parts of the whole plant of Pinus sylvestris. Among the EB-NC-treated pines, the highest EB residues were detected in the bark of the lateral branches at 11.61 mg L−1, followed by 3.35 mg L−1 in the xylem of lateral branches, and only 0.08 mg L−1 in the pine needles. The EB residues in the bark of the main trunk were 5.52 mg L−1, the xylem contained 2.43 mg L−1 and 2.82 mg L−1 was also detected in the roots. In EB ME-treated pines, 0.04 mg L−1 of EB residues were detected in the bark of lateral branches, 0.08 mg L−1 in the bark of the main trunk, and 0.01 mg L−1 in pine needles, while no EB residues were detected in the xylem and roots of the whole pine tree.
4. Discussion
The incidence of PWD has increased annually worldwide in the past decades. The disease has not been effectively controlled even though there has been an increase in the exploration of new nematocidal materials. One of the reasons is the complex structure and physiological characteristics of pine trees, such as turpentine secretion, which hinders the efficacy of agents, especially the conduction and retention of active ingredients in branches where PWNs invade. In our study, the nano-microcapsule formulation of emamectin benzoate rapidly conducted and fully distributed EB in trunk-injected pine trees regardless of turpentine impediment and was effectively absorbed through the branch surfaces after spraying.
EB was determined to be a suitable and effective ingredient of trunk injection agents for the control of PWD and guaranteed that pine forests were free from PWD for more than three years [9]. For example, a 9.7% EB-soluble liquid was injected at a dose of 0.3 mL per m3 into the seedlings of Pinus thunbergii, and none of the trees died within two years after treatment [31]. Similarly, after 2% EB ME trunk injections, the average EB residue measured after three years was 1.02 mg kg−1, which was higher than the LC50 and LC90 values of the indoor toxicity assay against PWNs [11]. However, trunk injection induces the secretion of turpentine in pine trees, resulting in a suitable application time only in winter when low temperatures decrease the secretion of turpentine. This causes the high costs of trunk injection application due to each pine tree being injected in the whole risk area of PWD occurrence. EB inhibits PWNs in the early phase of PWN infection [32]. Early monitoring of the PWN-borne vector insects is convenient and effective by using beetle attractants during the feeding and breeding periods in spring and summer. During field application, the EB-NC formulation can be combined with the attractant agents, thereby narrowing the application range and control costs based on the motion range of the PWN-borne beetles [33,34].
Another limitation of trunk injection is the topography of pine forests, many of which are distributed in steep mountains and inaccessible areas. Unmanned aerial vehicles have been widely used for spraying pesticides in agroforest systems [35,36]. However, previous formulations of EB, such as micro-emulsions, cannot be effectively absorbed by the surfaces of pine trees and are therefore unsuitable for spraying. In the present study, the EB-NC formulation was stable in light and was efficiently transported from the tree surface into the xylem 30 days after spraying, thereby demonstrating the potential of enlarging the application range of this novel formulation. A certain amount of EB remains in the needles of P. massoniana and affects insect populations in pine forests to some extent. Moreover, EB in the fallen pine needles may also affect arthropods in the soil [37]. Thus, the environmental risk of EB spraying should be evaluated before wider application. In addition, we will continue to focus on the specific effective control time of EB-NCs for pine wilt disease in field trials based on existing research. The release time of nanoparticles can be increased, the number of dosing times can be reduced, labor costs can be reduced, etc., in subsequent studies.
In this study, trifluoroacetic anhydride, 1-methylimidazole, and acetonitrile were employed to synthesize fluorescent probes combined with fluorescence microimaging for visualizing the distribution of EB within P. massoniana. Fluorescent probes are commonly used for detecting and labeling analytes as they can convert intermolecular interactions into a fluorescent signal. They have widespread applications in metal ions, pesticide residues, biomolecule content determination, biomolecule tracing, macromolecule labeling, as well as cellular and subcellular structure visualization [26,38]. Very few EBs have been studied in plants by fluorescence microimaging, but the use of derivatization liquid chromatography fluorescence detection for the detection of EB in plants is very common. Fluorescent derivatization reagents are used to produce fluorescent products from EB, and a fluorescence detector can be used to detect avermectin and its derivatives, such as emamectin benzoate and ivermectin, at specific excitation and emission wavelengths [39,40]. This is the same principle behind fluorescent probes, and means that fluorescent derivatives are able to quantitatively detect the analytes by fluorescence intensity at specific wavelengths, providing a theoretical basis for this study on the distribution of EB in trees.
Plants contain an abundance of autofluorescent molecules. The two most important autofluorescent molecules are chlorophyll and lignin, but many other molecules also autofluoresce on UV or visible light excitation, including components of the cytoplasm and cell wall. Autofluorescent molecules may interfere with detection using fluorescent staining or immunolocalization techniques. The excitation of chlorophyll occurs when exposed to UV, blue, or green light and results in a strong red emission. In contrast, lignin can be excited by either UV or blue light, with the fluorescence of UV-excited lignin being more sensitive to quenching molecules. Blue-light excitation of different lignified regions creates a pronounced contrast and is typically brighter due in part to the optical properties of microscope lenses [41,42]. In this study, we also employed blue light as the excitation wavelength; therefore, it is essential to eliminate autofluorescence interference from the pine tree’s own lignin. By comparing the fluorescence intensity of the fluorescent probe in branch sections of P. massoniana among the treatment group, control group, and blank control group using ImageJ software analysis, we can exclude autofluorescence stimulated by blue light from plants and further determine the EB distribution within the tree.
5. Conclusions
This study showed that EB-NCs have a particle size of 100–200 nm, and the water-soluble shell can effectively resist UV degradation with a slow-release property. The results of the conductivity distribution dynamics of EB-NCs trunk injection in P. massoniana showed that the EB-NCs could conduct in the P. massoniana more efficiently and without temperature limitation. Moreover, EB-NCs can enter the tree even if they are sprayed on the outside, and the high residual concentration far exceeds the LC50 for PWNs. In conclusion, the novel EB-NC formulation with suitable conductivity in pine trees provides a new strategy for PWD control, thereby presenting a potential agent for inhibiting early-phase PWN infection in spring and summer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15030444/s1. Figure S1. Fluorescence micrograph of transverse section of Pinus massoniana after trunk injection. Figure S2. Fluorescence micrograph of transverse section of Pinus massoniana pine needles after trunk injection. Figure S3. Fluorescence micrograph of transverse section of Pinus massoniana branches after trunk injection. Figure S4. Fluorescence micrograph of transverse section of Pinus massoniana branches after spraying. Figure S5. Fluorescence micrograph of transverse section of Pinus massoniana branches at 3, 9, 15 and 30 days after spraying.
Author Contributions
N.L. conceived and designed the research and finalized the manuscript, performed the experiments, performed the data analysis, and wrote the draft. X.S., X.Z., L.Z., A.C., J.H. and K.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31200487, 32271883) and jointly funded by the Zhejiang Basic Research Plan (LGN22C160004, 2022TS01-4).
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.S.; Anjo, S.I.; Fonseca, L.; Egas, C.; Manadas, B.; Abrantes, I. Bursaphelenchus xylophilus and B. mucronatus secretomes: A comparative proteomic analysis. Sci. Rep. 2016, 6, 39007. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, Y. Scanning Electron microscopy of pine seedling wood tissue sections inoculated with the pine wood nematode Bursaphelenchus xylophilus previously prepared for light microscopy. J. Nematol. 2012, 44, 255–259. [Google Scholar] [PubMed]
- Padmavathi, V.; Alagesan, K.; Alhowaity, A.; Khan, M.I.; Hamam, H.; Angayarkanni, M.; Govindan, V. Analysis and numerical simulation of fractional order pine wilt disease model. Int. J. Mod. Phys. B 2022, 36. [Google Scholar] [CrossRef]
- Iwahori, H.; Tsuda, K.; Kanzaki, N.; Izui, K.; Futai, K. PCR-RFLP and sequencing analysis of ribosomal DNA of Bursaphelenchus nematodes related to pine wilt disease. Fundam. Appl. Nematol. 1998, 21, 655–666. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Li, G.H.; Zhang, K.Q. Natural nematicidal metabolites and advances in their biocontrol capacity on plant parasitic nematodes. Nat. Prod. Rep. 2023, 40, 646–675. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.W.; Park, A.R.; Sung, M.; Kim, N.; Mannaa, M.; Han, G.; Kim, J.; Koo, Y.; Seo, Y.S.; Kim, J.C. Systemic Acquired Resistance-Mediated Control of Pine Wilt Disease by Foliar Application With Methyl Salicylate. Front. Plant Sci. 2022, 12, 812414. [Google Scholar] [CrossRef]
- Takai, K.; Suzuki, T.; Kawazu, K. Development and preventative effect against pine wilt disease of a novel liquid formulation of emamectin benzoate. Pestic. Manag. Sci. 2003, 59, 365–370. [Google Scholar] [CrossRef]
- Sousa, E.; Naves, P.; Vieira, M. Prevention of pine wilt disease induced by Bursaphelenchus xylophilus and Monochamus galloprovincialis by trunk injection of emamectin benzoate. Phytoparasitica 2013, 41, 143–148. [Google Scholar] [CrossRef]
- Pan, W.H.; Wu, J.L.; Jia, J.W.; Zhang, S.Y.; Ma, L.J.; Chen, A.L. Control effect of trunk injection of emamectin benzoate against pine wilt disease. Pestic. Dis. 2014, 33, 41–44. [Google Scholar]
- Ishaaya, I.; Kontsedalov, S.; Horowitz, A.R. Emamectin, a novel insecticide for controlling field crop pests. Pestic. Manag. Sci. 2002, 58, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pestic. Manag. Sci. 2001, 57, 463–466. [Google Scholar] [CrossRef]
- Elabasy, A.; Shoaib, A.; Waqa, S.M.; Jiang, M.; Shi, Z. Synthesis, characterization, and pesticidal activity of emamectin benzoate nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Molecules 2019, 24, 2801. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, Y.; Xie, J.; Sun, B.; Zhou, N.; Shen, H.; Shen, J. Carboxymethyl chitosan modified carbon nanoparticle for controlled emamectin benzoate delivery: Improved solubility, pH-responsive release, and sustainable pest control. ACS Appl. Mater. Interfaces 2019, 11, 34258–34267. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Mo, D.; Jiang, Y.; Gab, C.F.; Li, Q.G.; Wu, A.; Li, X.Y.; Xiao, J.A.; Hu, Q.; Yuan, H.Y.; et al. Fabrication, characterization, and insecticidal activity evaluation of emamectin benzoate–sodium lignosulfonate nanoformulation with pH-responsivity. Ind. Eng. Chem. Res. 2019, 58, 19741–19751. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, H.; Liao, G.; Chen, M.; Chen, X.; Qin, L.; Chen, C.; Chen, Z.; Wu, X.; Zhu, F. Distribution of emamectin benzoate granules in maize plants by broadcasting into maize leaf whorls. ACS Omege 2023, 8, 4209–4219. [Google Scholar] [CrossRef]
- Gao, F.; Cui, B.; Wang, C.; Li, X.; Li, B.; Zhan, S.; Shen, Y.; Zhao, X.; Sun, C.; Wang, C.; et al. Nano-EMB SP improves the solubility, foliar affinity, photostability and bioactivity of emamectin benzoate. Pestic. Manag. Sci. 2022, 78, 3717–3724. [Google Scholar] [CrossRef]
- Shen, Y.; An, C.; Jiang, J.; Huang, B.; Li, N.; Sun, C.; Wang, C.; Zhan, S.; Li, X.; Gao, F.; et al. Temperature-dependent nanogel for pesticide smart delivery with improved foliar dispersion and bioactivity for efficient control of multiple pests. ACS Nano 2022, 16, 20622–20632. [Google Scholar] [CrossRef]
- Wang, G.D.; Xiao, Y.Y.; Xu, H.H.; Hu, P.T.; Liang, W.L.; Xie, L.J.; Jia, J.L. Development of multifunctional avermectin poly(succinimide) nanoparticles to improve bioactivity and transportation in rice. J. Agric. Food Chem. 2018, 66, 11244–11253. [Google Scholar] [CrossRef]
- Saini, P.; Gopal, M.; Kumar, R.; Srivastava, C. Development of pyridalyl nanocapsule suspension for efficient management of tomato fruit and shoot borer (Helicoverpa armigera). J. Environ. Sci. Health Part. B 2014, 49, 344–351. [Google Scholar] [CrossRef]
- Yang, D.S.; Cui, B.; Wang, C.X.; Zhao, X.; Zeng, Z.H.; Wang, Y.; Sun, C.J.; Liu, G.Q.; Cui, H.X. Preparation and Characterization of Emamectin Benzoate Solid Nanodispersion. J. Nanomater. 2017, 2017, 6560780. [Google Scholar] [CrossRef]
- Nishino, Y.; Miyazaki, K.; Kaise, M.; Miyazawa, A. Fine cryo-SEM observation of the microstructure of emulsions frozen via high-pressure freezing. Microscopy 2022, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ranner, R.; Mimietz-Oeckler, S.; Nishino, Y.; Miyazawa, A. Development of a cryo-SEM system enabling direct observation of the cross sections of an emulsion adhesive in a moist state during the drying process. Microscopy 2015, 64, 459–463. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, H.; Qian, Y.; Lü, L.; Wang, B.; Qiu, X. Hollow lignin azo colloids encapsulated avermectin with high anti-photolysis and controlled release performance. Ind. Crops Prod. 2016, 87, 191–197. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B.; Wang, Y.; Lou, D. Polymer-controlled coreshell nanoparticles: A novel strategy for sequential drug release. RSC Adv. 2014, 4, 30430–30439. [Google Scholar] [CrossRef]
- Pang, Y.; Qin, Z.; Wang, S.; Yi, C.; Zhou, M.; Lou, H.; Qiu, X. Preparation and application performance of lignin-polyurea composite microcapsule with controlled release of avermectin. Colloid Polym. Sci. 2020, 298, 1001–1012. [Google Scholar] [CrossRef]
- Yagi, N.; Yoshinari, A.; Iwatate, R.J.; Isoda, R.; Frommer, W.B.; Nakamura, M. Advances in synthetic fluorescent probe labeling for live-cell imaging in plants. Plant Cell Physiol. 2021, 62, 1259–1268. [Google Scholar] [CrossRef]
- Lima-Faria, J.M.; Guimarães, L.N.; Silva, V.C.D.; Souza, I.D.C.; Fernandes, M.N.; Martinez, D.S.T.; Sabóia-Morais, S.M.T. Distribution and behavior of lipid droplets in hepatic cells analyzed by variations of cytochemical technique and scanning electron microscopy. MethodsX 2022, 9, 101769. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, H.R.; Kim, D.S.; Kwon, J.H.; Huh, M.J.; Park, I.K. Emamectin benzoate 9.7% SL as a new formulation for a trunk-injections against pine wood nematode, Bursaphelenchus xylophilus. J. For. Res. 2019, 31, 1399–1403. [Google Scholar] [CrossRef]
- Ni, A.; Yang, D.; Cheng, H.; Ye, J. Preliminary study on early diagnosis and rehabilitation treatment of pine wood nematode disease based on partial symptoms. Forests 2023, 14, 657. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.H.; Lee, S.M. Diel rhythmicity of field responses to synthetic pheromone lures in the pine sawyer Monochamus saltuarius. Insects 2021, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Jurc, M.; Hauptman, T.; Pavlin, R.; Borkovič, D. Target and non-target beetles in semiochemical-baited cross vane funnel traps used in monitoring Bursaphelenchus xylophilus (PWN) vectors in pine stands. Phytoparasitica 2016, 44, 151–164. [Google Scholar] [CrossRef]
- Ugawa, S.; Fukuda, K. Effect of aerial insecticide spraying on pine wilt disease in central Japan. Forest Pathol. 2007, 38, 16–28. [Google Scholar] [CrossRef]
- Maaß, O.; Kehlenbeck, H. Cost–Benefit Analysis of Monitoring Insect Pests and Aerial Spraying of Insecticides: The Case of Protecting Pine Forests against Dendrolimus pini in Brandenburg (Germany). Forests 2024, 15, 104. [Google Scholar] [CrossRef]
- Ouyang, X.; Fan, Q.; Chen, A.; Huang, J. Effects of trunk injection with emamectin benzoate on arthropod diversity. Pestic. Manag. Sci. 2023, 79, 935–946. [Google Scholar] [CrossRef]
- Warner, C.A.; Biedrzycki, M.L.; Jacobs, S.S.; Wisser, R.J.; Caplan, J.L.; Sherrier, D.J. An Optical Clearing Technique for Plant Tissues Allowing Deep Imaging and Compatible with Fluorescence Microscopy. Plant Physiol. 2014, 166, 1684–1687. [Google Scholar] [CrossRef]
- Yoshii, K.; Kaihara, A.; Tsumura, Y.; Ishimitsu, S.; Tonogai, Y. Simultaneous Determination of Residues of Emamectin and Its Metabolites, and Milbemectin, Ivermectin, and Abamectin in Crops by Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2001, 84, 910–918. [Google Scholar] [CrossRef]
- Lemos, M.A.T.; Matos, C.A.; de Resende, M.F.; Prado, R.B.; Donagemma, R.A.; Netto, A.D.P. Development, validation, and application of a method for selected avermectin determination in rural waters using high performance liquid chromatography and fluorescence detection. Ecotoxicol. Environ. Saf. 2016, 133, 424–432. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Wei, D.; Lv, S.; Zuo, J.; Zhang, S.; Liang, S. Recent advances research and application of lignin-based fluorescent probes. React. Funct. Polym. 2022, 178, 105354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).