Carbon Sequestration by Soils of Ash Dump Forest Areas in the Middle Urals (Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling and Analysis of Plant Communities
2.3. Sampling and Analysis of Soils
3. Results
3.1. Plant Community Parameters
3.2. Soil and Ash Substrate Parameters
3.3. Technosol Humus Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Monitoring Laboratory. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 21 October 2023).
- Land Code of the Russian Federation. Available online: https://rulaws.ru/Zemelnyy-kodeks/ (accessed on 15 September 2023).
- Makhonina, G.I. Ecological Aspects of Soil Formation in the Technogenic Systems of the Urals; Ural State University: Yekaterinburg, Russia, 2003; 356p. [Google Scholar]
- Ahirwal, J.; Maiti, K.S.; Reddy, M. Development of carbon, nitrogen and phosphate stocks of reclaimed coal mine soil within 8 years after forestation with Prosopis juliflora (Sw.) Dc. Catena 2017, 156, 42–50. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Niu, S.; Li, X.; Wang, Y.; Bai, Z. Reclamation promotes the succession of the soil and vegetation in opencast coal mine: A case study from Robinia pseudoacacia reclaimed forests, Pingshuo mine, China. Catena 2018, 165, 72–79. [Google Scholar] [CrossRef]
- Korznikov, K.A.; Popova, K.B. Plant communities on dumps of coal open-pit mining in Southern Sakhalin. Bull. Bot. Gard.-Inst. Far East. Branch RAS 2019, 21, 28–38. [Google Scholar]
- Soloviev, S.; Androkhanov, V.; Semina, I.; Shipilova, A. Restoration of vegetation cover in reclaimed areas with coal preparation waste in Kuzbass. In E3S Web of Conferences. “22nd International Scientific Conference on Energy Management of Municipal Facilities and Sustainable Energy Technologies, EMMFT 2020”; EDP Sciences: Les Ulis Cedex A, France, 2021; pp. 1–8. [Google Scholar]
- Gossen, I.N.; Gurkova, E.A.; Sokolov, D.A. Assessment of effectiveness of reclamation activities on coal dumps in Kuzbass. Ecol. Ind. Russ. 2023, 27, 33–39. [Google Scholar] [CrossRef]
- Yuen, Y.; Zhao, Z.; Zhang, P.; Chen, L.; Hu, T. Soil organic carbon and nitrogen pools in reclaimed mine soils under forests and cropland ecosystems in the loess plateau, China. Ecol. Appl. 2017, 102, 137–144. [Google Scholar] [CrossRef]
- Festin, E.S.; Chileshe, M.N.; Syampungani, S. Progresses in restoration of post-mining landscape in Africa. J. For. Res. 2018, 30, 381–396. [Google Scholar] [CrossRef]
- Nyenda, T.; Gwenzi, W.; Piyo, T.T.; Jacobs, S.M. Occurrence of biological crusts and their relationship with vegetation on a chronosequence of abandoned gold mine tailings. Ecol. Eng. 2019, 139, 105559. [Google Scholar] [CrossRef]
- Nyenda, T.; Gwenzi, W.; Gwata, C.; Jacobs, S.M. Leguminous tree species create islands of fertility and influence the understory vegetation on nickel-mine tailings of different ages. Ecol. Eng. 2020, 155, 105902. [Google Scholar] [CrossRef]
- Pasynkova, M.V. Ash of coal as a substrade for growing plants. In Plants and Industrial Environment; Ural Government University: Sverdlovsk, Russia, 1974; pp. 29–44. [Google Scholar]
- Maiti, S.K. Ecorestoration of the Coalmine Degraded Lands; Springer: New Delhi, India, 2013; 361p. [Google Scholar]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef]
- Kostic, O.; Mitrovic, M.; Knezevic, M.; Jaric, S.; Cajic, G.; Djurdjevic, L.; Pavlovic, P. The potential of four woody species for the revegetation of fly ash deposits from the ‘Nikola Tesla—A’ thermoelectric plant (Obrenovac, Serbia). Arch. Biol. Sci. 2012, 64, 145–158. [Google Scholar] [CrossRef]
- Mi trovic, M.; Jaric, S.; Kostic, O.; Gajic, G.; Karadzic, B.; Djurdjevic, L.; Oberan, L.; Pavlovic, D.; Pavlovic, M.; Pavlovic, P. Photosynthetic Efficiency of Four Woody Species Growing on Fly Ash Deposits of a Serbian ‘Nikola Tesla—A’ Thermoelectric Plant. Pol. J. Environ. Stud. 2012, 21, 1339–1347. [Google Scholar]
- Uzarowicz, U.; Zagorski, Z.; Mendak, E.; Bartminski, P.; Szara, E.; Kondras, M.; Oktaba, L.; Turek, A.; Ragozinski, R. Technogenic soils (Technosols) developed from fly ash and bottom ash from thermal power stations combusting bituminous coal and lignite. Part I. Properties, classification, and indicators of early pedogenesis. Catena 2017, 157, 75–89. [Google Scholar] [CrossRef]
- Uzarowicz, L.; Kwasowski, W.; Spiewak, O.; Switoniak, M. Indicators of pedogenesis of Technosols developed in an ash settling pond at the Belchatow thermal power station (central Poland). Soil Sci. Annu. 2018, 69, 49–59. [Google Scholar] [CrossRef]
- Uzarowicz, L.; Skibab, M.; Leuec, M.; Zagorskia, Z.; Gasinskid, A.; Trzcinskie, J. Technogenic soils (Technosols) developed from fly ash and bottom ash from thermal power stations combusting bituminous coal and lignite. Part II. Mineral transformations and soil evolution. Catena 2018, 162, 255–269. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Wos, B.; Pajak, M.; Wanic, T.; Krzaklewski, W.; Chodak, M. The impact of alders (Alnus spp.) on the physiko-chemical properties of technosols on a lignite combustion waste disposal site. Ecol. Eng. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Change 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Leff, J.W.; Wieder, W.R.; Taylor, P.G. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob. Change Biol. 2012, 18, 2969–2979. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef]
- Titlyanova, A.A.; Shibareva, S.V. Litter in Forest and Grass Ecosystems; Publishing House of SB RAS: Novosisbirsk, Russia, 2012; 137p. [Google Scholar]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2014; 315p. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Chibrik, T.S.; Lukina, N.V.; Filimonova, E.I.; Glazyrina, M.A.; Rakov, E.A.; Maleva, M.G.; Prasad, M.N.V. Biological recultivation of mine industry deserts: Facilitating the formation of phytocoenosis in the Middle Ural Region, Russia. In Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 389–418. [Google Scholar]
- Nekrasova, O.; Radchenko, T.; Filimonova, E.; Lukina, N.; Glazyrina, M.; Dergacheva, M.; Uchaev, A.; Betekhtina, A. Natural Forest colonization and soil formation on Ash Dump in southern taiga. Folia For. Pol. Ser. A For. 2020, 62, 306–316. [Google Scholar]
- Nekrasova, O.; Radchenko, T.; Filimonova, E.; Uchaev, A.; Dergacheva, M.; Petrova, T.; Betekhtina, A. Features of forest communities and soils formed on an Ash dump of the Middle Urals. For. Ideas 2022, 28, 88–99. [Google Scholar]
- Dergacheva, M.; Trunova, V.; Nekrasova, O.; Siromlya, T.; Uchaev, A.; Bazhina, N.; Radchenko, T.; Betekhtina, A. Assessment of the Macro- and Microelement Composition of Fly Ash From 50-Year-Old Ash Dumps in the Middle Urals (Russia). Metals 2021, 11, 1589. [Google Scholar] [CrossRef]
- Arynov, A.A. Coals of the Ekibastuz field. KarSU Bull. 2007, 1, 1–5. [Google Scholar]
- Climate-Data.org. Climate Data for Cities Worldwide. Available online: https://en.climate-data.org/asia/russian-federation/sverdlovsk-oblast/sredneuralsk-44950/ (accessed on 17 September 2023).
- Shakirov, A.V. Physical-Geographical Zoning of the Urals; UB RAC: Ekaterinburg, Russia, 2011; 618p. [Google Scholar]
- Chibrik, T.S.; Lukina, N.V. Characteristics of the processes of natural restoration of phytocenoses and transformation of phytocenosis cultures in ash dumps in different zonal and climatic conditions. In Ecological Foundations and Methods of Biological Reclamation of Ash Dumps of Thermal Power Plants in the Urals; Ural Branch of the Russian Academy of Sciences: Yekaterinburg, Russia, 2002; pp. 61–151. [Google Scholar]
- Ipatov, V.S.; Kirikova, L.A.; Mirin, D.M. Geobotany: Textbook; Publishing House of St. Petersburg University: St. Petersburg, Russia, 2010; 117p. [Google Scholar]
- Magarran, E. Ecological Diversity and Its Measurement; Mir: Moscow, Russia, 1992; 184p. [Google Scholar]
- Arinushkina, E.V. A Guide in Chemical Analysis of Soils; Moscow State University Publishers: Moscow, Russia, 1970; 487p. [Google Scholar]
- Vorobyova, L.A. Theory and Practice of Chemical Analysis of Soils; GEOS: Moscow, Russia, 2006; 400p. [Google Scholar]
- Vadyunina, A.F.; Korchagina, Z.A. Methods of Investigation of Soil Physical Properties; Agropromizdat: Moscow, Russia, 1986; 416p. [Google Scholar]
- Ponomareva, V.V.; Plotnikova, T.A. Methodical Instructions for Determining the Content and Composition of Humus in Soils; VASKhNIL: Leningrad, Russia, 1975; 105p. [Google Scholar]
- Quinn, G.; Keough, M. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; 537p. [Google Scholar]
- IUSS Working Group WRB; World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; 234p. [Google Scholar]
- Dushofur, F. Fundamentals of Soil Science. Soil Evolution; Progress: Moscow, Russia, 1970; 591p. [Google Scholar]
- Dergacheva, M.I. The humus Substance System as a Basis for the Diagnosis of Paleosols and Reconstruction of the Paleoenvironment; Russian Academy of Sciences, Siberian Branch, Institute of Soil Science and Agrochemistry; Izd-vo SB RAS: Novosibirsk, Russia, 2018; 294p. [Google Scholar]
- Nekrasova, O.A.; Uchaev, A.P. Ecological conditions of humus substance formation in the Middle and Southern Urals. In Soils Biosph; National Research Tomsk State University: Tomsk, Russia, 2018; Volume 1, pp. 342–344. [Google Scholar]
- Weber, J.; Straczynska, S.; Kocowicz, A.; Gilewska, M.; Bogacz, A.; Gwizdz, Z.M.; Debicka, M. Properties of soil materials derived from fly ash 11 years after revegetation of post-mining excavation. Catena 2015, 133, 250–254. [Google Scholar] [CrossRef]
- Uzarowicz, L.; Zagorski, Z. Mineralogy and chemical composition of technogenic soils (Technosols) developed from fly ash and bottom ash from selected thermal power stations in Poland. Soil Sci. Annu. 2015, 66, 82–91. [Google Scholar] [CrossRef]
- Tomaszewicz, T.; Chudecka, J. The assessment of chemical properties of soils made on base of ashes from hard coal after ten years of their functioning in environment. Environ. Eng. 2016, 163, 74–85. [Google Scholar]
- Kostic, O.; Jaric, S.; Gajic, G.; Pavlovc, D.; Pavlovic, M.; Mitrovic, M. Pedological properties and ecological implications of substrates derived 3 and 11 years after the revegetation of lignite fly ash disposal sites in Serbia. Catena 2018, 163, 78–88. [Google Scholar] [CrossRef]
- Mukul, S.A.; Halim, M.A.; Herbohn, J. Forest carbon stock and fluxes: Distribution, biogeochemical cycles, and measurement techniques. In Life on Land, Encyclopedia of the UN Sustainable Development Goals; Springer: Cham, Switzerland, 2020; pp. 365–380. [Google Scholar]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cecillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118–127. [Google Scholar] [CrossRef]
- Kolli, R.; Kauer, K.; Tonutare, T.; Lutter, R. Ecosystem carbon stocks and their annual sequestration rate in mature forest stands on the mineral soils of Estonia. Forest 2022, 13, 784. [Google Scholar] [CrossRef]
- Gasparini, P.; Cosmo, L.D.; Floris, A.; Laurentis, D.D. Italion National Forest Inventory—Methods and Results of the Third Survey; Springer Tracts in Civil Engineering: Cham, Switzerland, 2022; 598p. [Google Scholar]
- Osipov, A.F.; Startsev, V.V.; Prokushkin, A.S.; Dymov, A.A. Carbon stocks in forest soils of the Krasnoyarsk Region: Analysis of soil and tree species role. Theor. Appl. Ecol. 2023, 1, 67–74. [Google Scholar] [CrossRef]
- Firsova, V.P.; Dergacheva, M.I. Composition of organic matter of soils of Ural and Trans-Ural southern taiga forests. In Forest soils of the southern taiga of the Urals and Trans-Urals. Proceedings of the Institute of Plant and Animal Ecology of the UB of the USSR Academy of Sciences; Institute of Plant and Animal Ecology of the UB of the USSR Academy of Sciences: Sverdlovsk, Russia, 1972; Volume 85, pp. 130–145. [Google Scholar]
- Chestnykh, O.V.; Grabovsky, V.I.; Zamolodchikov, D.G. Soil carbon in forest regions of the European-Ural part of Russia. For. Sci. Issues 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Firsova, V.P.; Pavlova, T.S. Soil Conditions and Features of the Biological Cycle of Substances in Mountain Pine Forests; Nauka: Moscow, Russia, 1983; 165p. [Google Scholar]
- Wos, B.; Jozefowska, A.; Pajak, M.; Chodak, M.; Frouz, J.; Pietrzykowski, M. Carbon and macronutrient budgets in an alder plantation grown on a reclaimed combustion waste landfill. Forests 2020, 11, 430. [Google Scholar] [CrossRef]
- Allory, V.; Sere, G.; Ouvrard, S. A meta-analysis of carbon content and stocks in Technosols and identification of the main governing factors. Eur. J. Soil Sci. 2021, 73, e13141. [Google Scholar] [CrossRef]
- Orlov, D.S. Humic Acids of Soils and the General Theory of Humification; Publishing House of Moscow State University: Moscow, Russia, 1990; 325p. [Google Scholar]
- Vesterdal, L.; Raulund-Rasmussen, K. Forest floor chemistry under seven tree species along a soil fertility gradient. Can. J. For. Res. 1998, 28, 1636–1647. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Ineson, P.; Woods, C.; Piearce, T.G. Decomposition of Pinus sylvestris litter in litter bags: Influence of underlying native litter layer. Soil Biol. Biochem. 1998, 30, 47–55. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Reich, P.B.; Oleksyn, J.; Ogdahl, M.; Zytkowiak, R.; Hale, C.; Karolewski, P. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 2006, 87, 2288–2297. [Google Scholar] [CrossRef]
- Eriksson, K.-E.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1990; 416p. [Google Scholar]
- Aponte, C.; Garcia, L.V.; Maranon, T.; Gardes, M. Indirect host effect on ectomycorrhizal fungi: Leaf fall and litter quality explain changes in fungal communities on the roots of co-occurring Mediterranean oaks. Soil Biol. Biochem. 2010, 42, 788–796. [Google Scholar] [CrossRef]
- Persson, T.; Wessen, B.; Lundkvist, H.; Wiren, A.; Hyvonen, R. Effects of acidification and liming on carbon and nitrogen mineralization and soil organisms in mor humus. Water Air Soil Pollut. 1989, 45, 77–96. [Google Scholar] [CrossRef]
- Simmons, J.A.; Yavitt, J.B.; Fahey, T.J. Watersheb liming effects on the forest floor N cycle. Biogeochemistry 1996, 32, 221–244. [Google Scholar] [CrossRef]
- Andersson, S.; Nilsson, S.I.; Saetre, P. Leaching of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) in mor humus as affected by temperature and pH. Soil Biol. Biochem. 2000, 32, 1–10. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- EU Soil Strategy for 2030: Towards Healthy Soils for People and the Planet; Publications Office of the European Union: Brussels, Belgium, 2021; 25p.
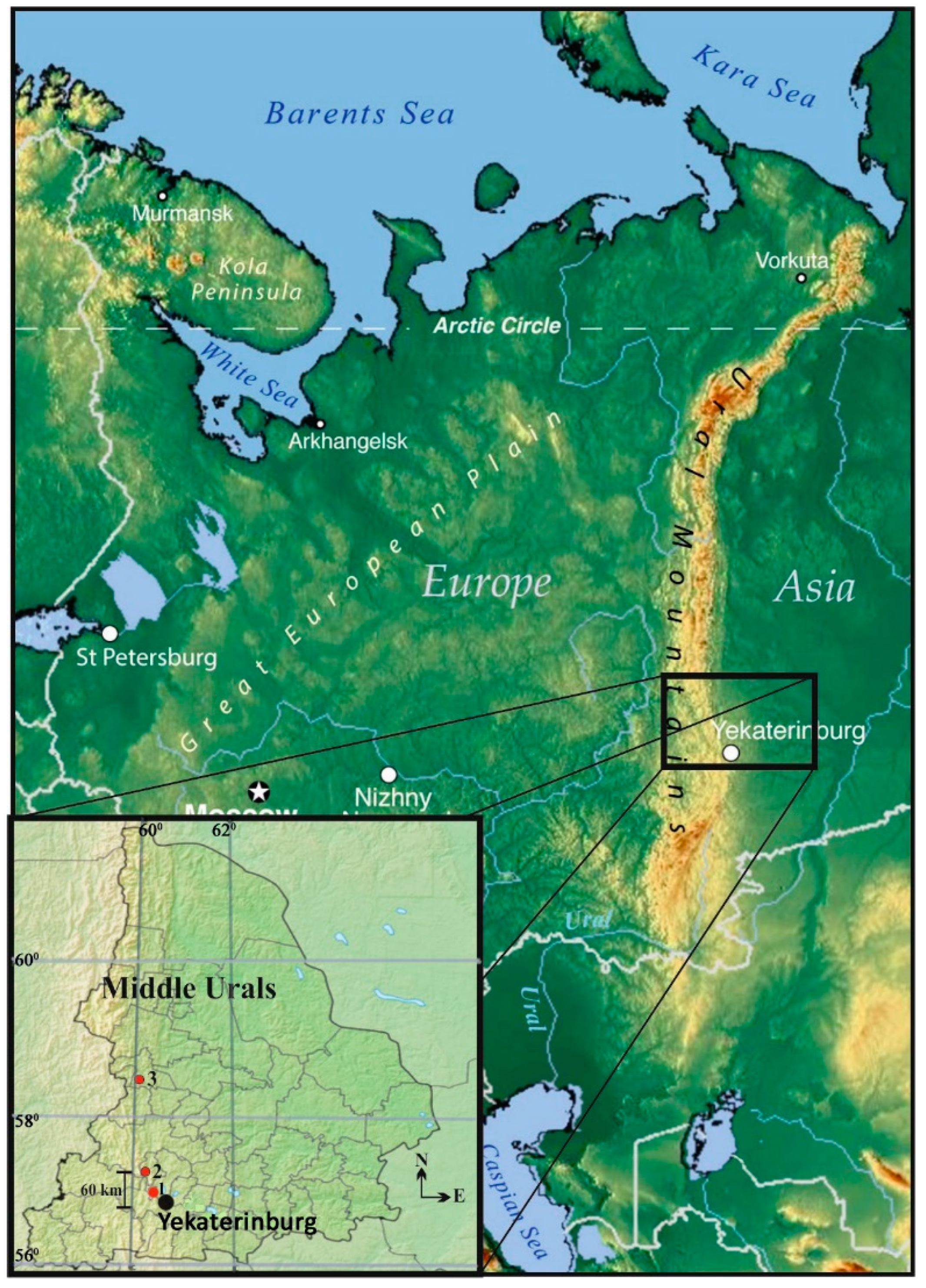
| Ash Dump Number | Power Plant | Coordinates | Coal Deposit | Ash Dump Area, ha |
|---|---|---|---|---|
| 1 | Sredneuralskaya (SUPP) | 57°00′ N, 60°27′ E | Ekibastuzskoe | 104 |
| 2 | Verkhnetagilskaya (VTPP) | 57°20′ N, 59°56′ E | Chelyabinsk and Bogoslovskoe | 125 |
| 3 | Nizhneturinskaya (NTPP) | 58°37′ N, 59°52′ E | Volchanskoe | 60 |
| Characteristics | Ash Dump | Background Forests | ||
|---|---|---|---|---|
| SUPP | VTPP | NTPP | ||
| Crown density | 0.40–0.60 | 0.45–0.65 | 0.70 | 0.40–0.60 |
| Tree height, m | 17.5–18.5 | 14.5–16.0 | 7.0–16.0 | 18–25.0 |
| Composition of the forest stand | 8Pp2B | 6B2PpSPn | 6Pp3BPn | 7B2PnS + Pc |
| Average diameter, cm | 16.8 | 16.1 | 13.7 | 25–30 |
| Age, years | 45–50 | 38–40 | 50–55 | 80–100 |
| Shrub layer cover, % | 15–40 | 5–30 | 45–50 | 20–30 |
| Coverage of the herb-shrub layer, % | 75–90 | 30–35 | 30–65 | 85–90 |
| Coverage of the moss-lichen layer, % | 5–10 | 3–5 | 0.5 | 25–35 |
| Shannon–Wiener index (H) | 3.13–3.19 | 2.55–2.80 | 2.8 | 3.27–3.60 |
| Species saturation, number of species per 0.25 m2, (min–max) | 9.1 (6–15) | 5.4 (3–9) | 7.8 (4–11) | 11.19 (4–19) |
| Floristic richness in the accounting area, (min–max) | 36.0 (31–42) | 37.0 (35–38) | 28.0 (30–24) | 66.0 (60–72) |
| Biomorphs | Group Name | Species Number | Dominant and Codominant Species (Proportion of Herbaceous by Weight up to 4%) |
|---|---|---|---|
| SUPP ash dump | |||
| Woody | Trees | 6 | Populus tremula, Betula pendula |
| Shrubs | 7 | Sorbus aucuparia, Chamaecytisus ruthenicus, Padus avium, Viburnum opulus | |
| Dwarf shrubs and semishrubs | 3 | Rubus saxatilis | |
| Herbs | Cereals | 5 | Agrostis tenuis, Festuca rubra,Deschampsia cespitosa |
| Legumes | 4 | Vicia sylvatica, Lathyrus pratensis | |
| Forbs | 17 | Equisetum pratensis,Fragaria vesca | |
| VTPP ash dump | |||
| Woody | Trees | 5 | Betula pendula, Populus tremula, Pinus sylvestris Salix caprea |
| Shrubs | 5 | Sorbus aucuparia, Padus avium, Salix myrsinifolia, Chamaecytisus ruthenicus, Rosa acicularis, | |
| Dwarf shrubs and semishrubs | 3 | Pyrola rothundifolia, Orthilia secunda | |
| Herbs | Cereals | 5 | Agrostis tenuis, Calamagrostis arundinacea, Poa pratensis, Festuca rubra, Deschampsia cespitosa |
| Legumes | 6 | Amoria repens, Lathyrus pratensis; Trifolium medium, Trifolium pratense, Vicia cracca | |
| Forbs | 12 | Alchemilla vulgaris, Equisetum pratense, Plantago media, Fragaria vesca, Platanthera bifolia | |
| NTPP ash dump | |||
| Woody | Trees | 4 | Betula pendula, Populus tremula |
| Shrubs | 5 | Chamaecytisus ruthenicus, Rosa acicularis, Sorbus aucuparia | |
| Dwarf shrubs and semishrubs | 1 | Rubus saxatilis | |
| Herbs | Cereals | 4 | Agrostis tenuis, Poa pratensis |
| Legumes | 3 | Vicia sepium | |
| Forbs | 13 | Chamerion angustifolium, Fragaria vesca | |
| Background sites | |||
| Woody | Trees | 5 | Betula pendula, Populus tremula, Pinus sylvestris |
| Shrubs | 8 | Chamaecytisus ruthenicus, Rosa acicularis, Rosa majalis, Sorbus aucuparia, Padus avium, Viburnum opulus | |
| Dwarf shrubs and semishrubs | 4 | Rubus saxatilis, Orthilia secunda | |
| Herbs | Cereals | 8 | Agrostis tenuis, Brachypodium pinnatu, Calamagrostis arundinace, Deschampsia cespitosa |
| Legumes | 5 | Lathyrus vernus | |
| Forbs | 39 | Aegopodium podagraria, Alchemilla vulgaris, Fragaria vesca, Geum rivale, Ranunculus acris, Ranunculus polyanthemos, Trollius europaeus, Veronica chamaedrys | |
| Sedges | 1 | Carex macroura | |
| Indicators | Ash Dump | Background Forests | ||
|---|---|---|---|---|
| SUPP | VTPP | NTPP | ||
| Aboveground phytomass, g/0.25 m2 | 21.73 ± 1.98 | 20.43 ± 3.17 | 7.95 ± 3.35 | 105.75 ± 6.61 |
| Limits of variation | 10.18–35.21 | 5.87–62.55 | 3.70–14.00 | 71.55–144.26 |
| Replications | Percentage Share of Fraction (mm) | ||||||
|---|---|---|---|---|---|---|---|
| 1.00–0.25 | 0.25–0.05 | 0.05–0.01 | 0.01–0.005 | 0.005–0.001 | <0.001 | <0.01 | |
| 1 | 2.0 | 65.3 | 27.0 | 1.3 | 4.0 | 0.4 | 5.7 |
| 2 | 0.2 | 54.5 | 36.0 | 7.5 | 0.9 | 0.9 | 9.3 |
| 3 | 1.0 | 60.4 | 29.5 | 8.7 | 0.4 | 0.0 | 9.1 |
| Ash Dump | Average Content, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | P2O5 | |
| STPP | 51.0 | 30.9 | 5.88 | 4.73 | 1.32 | 1.36 | 3.91 | 0.71 | 0.22 |
| VTPP | 40.30 | 14.94 | 8.16 | 3.54 | 2.32 | 1.01 | 2.52 | 1.02 | 0.23 |
| NTPP | 40.5 | 32.4 | 5.5 | 7.8 | 0 | nd * | nd | nd | nd |
| Ash Dump | pH | TOC | TN | Ca2+ | Mg2+ | P2O5 | K2O |
|---|---|---|---|---|---|---|---|
| % | mmol/100 g | mg/100 g | |||||
| STPP | 4.58–5.28 | 2.51–3.33 | 0.03–0.04 | 0.3–0.5 | 0.4–0.5 | 17.6–26.8 | 1.5–1.9 |
| VTPP | 5.85–8.23 | 0.70–2.54 | 0.03–0.07 | 0.5–8.1 | 0.3–0.7 | 11.5–23.8 | 1.6–8.9 |
| NTPP | 7.15–8.41 | 0.38–2.23 | 0.03–0.04 | 1.2–4.0 | 0.6–2.0 | 17.2–21.5 | 2.0–9.0 |
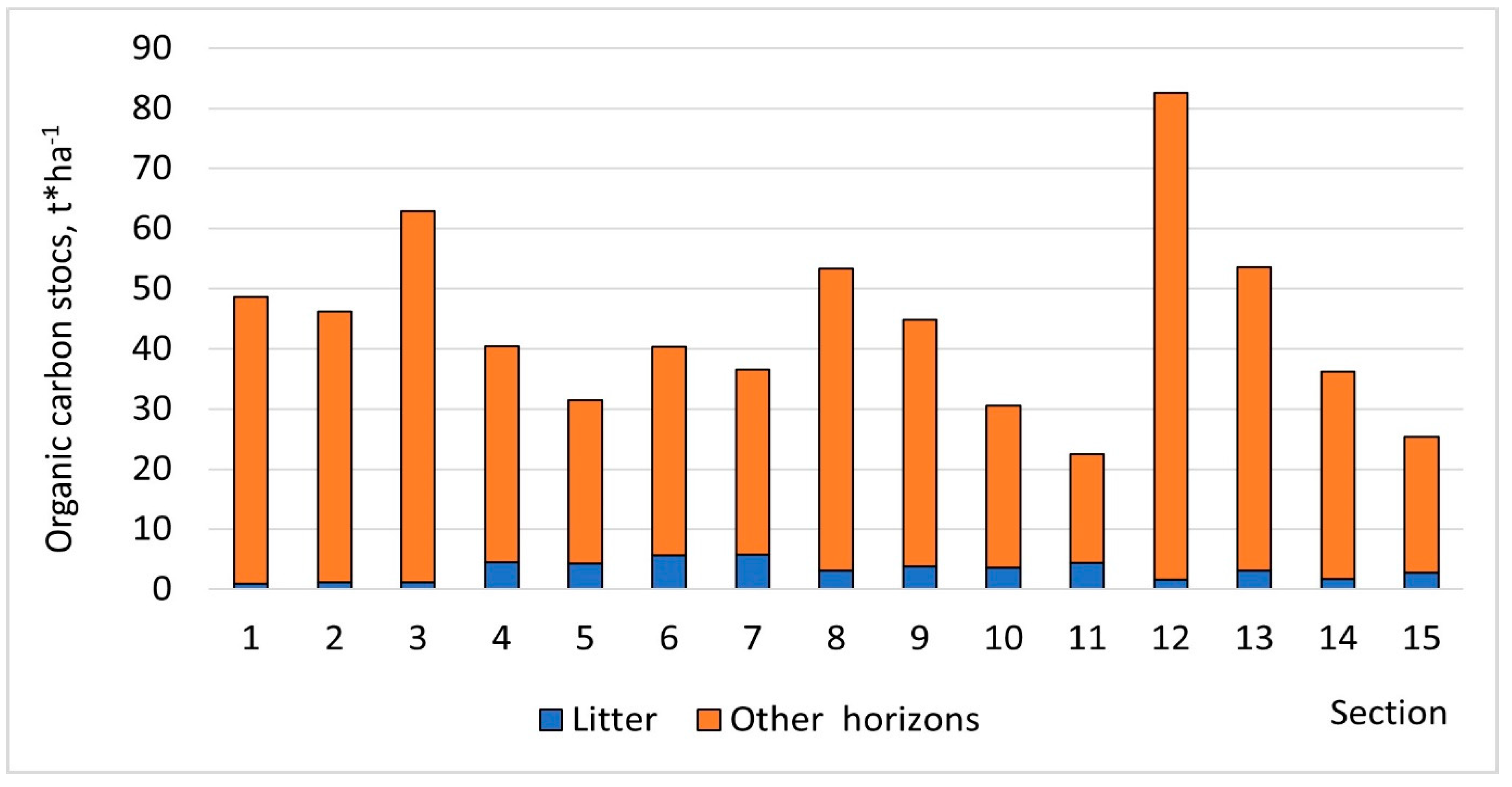
| Ash Dump | Section | pH | TOC | TN | Ca2+ | Mg2+ | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|
| % | mmol/100 g | mg/100 g | ||||||
| SUPP | 1 | 5.12 | 4.16 | 0.13 | 1.9 | 1.0 | 21.0 | 7.6 |
| 2 | 5.50 | 3.96 | 0.11 | 2.9 | 0.9 | 23.8 | 10.1 | |
| 3 | 5.29 | 5.76 | 0.16 | 2.6 | 1.5 | 14.7 | 8.5 | |
| VTPP | 4 | 5.83 | 5.59 | 0.24 | 0.8 | 0.4 | 22.9 | 27.6 |
| 5 | 6.15 | 4.64 | 0.32 | 0.8 | 0.4 | 26.3 | 21.3 | |
| 6 | 6.09 | 6.02 | 0.21 | 0.7 | 0.6 | 10.8 | 2.3 | |
| 7 | 5.92 | 5.91 | 0.18 | 0.8 | 0.6 | 19.2 | 2.3 | |
| 8 | 6.53 | 5.17 | 0.18 | 7.7 | 0.9 | 10.6 | 19.3 | |
| 9 | 6.45 | 5.10 | 0.16 | 8.7 | 0.9 | 9.6 | 12.0 | |
| 10 | 7.13 | 4.12 | 0.16 | 7.0 | 0.9 | 12.9 | 19.3 | |
| 11 | 7.03 | 4.26 | 0.26 | 3.1 | 1.5 | 24.1 | 17.4 | |
| NTPP | 12 | 7.38 | 6.22 | 0.31 | 5.2 | 1.1 | 11.4 | 15.4 |
| 13 | 7.38 | 5.35 | 0.23 | 5.2 | 1.1 | 11.4 | 15.4 | |
| 14 | 6.73 | 3.28 | 0.24 | 3.4 | 0.8 | 19.2 | 6.1 | |
| 15 | 6.75 | 3.39 | 0.28 | 2.5 | 0.9 | 21.2 | 6.2 | |
| n | pH | TOC | TN | Ca2+ | Mg2+ | P2O5 | K2O |
|---|---|---|---|---|---|---|---|
| % | mmol/100 g | mg/100 g | |||||
| 7 | 4.47–5.15 | 4.08–7.97 | 0.18–0.29 | 1.7–2.7 | 0.7–1.5 | 0.6–2.0 | 3.1–5.8 |
| Ash Dump | Section Number | Thickness | pH | TOC | TN | C:N | Ca2+ | Mg2+ | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | % | mmol/100 g | mg/100 g | |||||||
| SUPP | 1 | 0.5 | 6.40 | 28.55 | 1.13 | 25.3 | 12.5 | 10 | 80.7 | 33.1 |
| 2 | 0.5 | 6.56 | 34.61 | 1.10 | 31.5 | 20.0 | 12.5 | 50.9 | 33.3 | |
| 3 | 0.5 | 6.55 | 34.33 | 1.18 | 29.1 | 25.0 | 7.5 | 40.1 | 31.7 | |
| VTPP | 4 | 2 | 6.39 | 31.95 | 1.43 | 22.3 | 4.8 | 2.0 | 98.2 | 184 |
| 5 | 2 | 6.39 | 30.69 | 1.87 | 16.4 | 4.8 | 1.9 | 99.5 | 127.8 | |
| 6 | 2 | 5.40 | 40.30 | 1.76 | 22.9 | 4.7 | 2.0 | 54.1 | 8.3 | |
| 7 | 2 | 6.16 | 41.30 | 1.49 | 27.7 | 5.0 | 2.3 | 69.7 | 7.4 | |
| 8 | 2 | 6.78 | 22.56 | 0.99 | 22.8 | 10.3 | 1.5 | 22.7 | 112.8 | |
| 9 | 2 | 5.90 | 27.46 | 0.88 | 31.2 | 10.0 | 1.7 | 25.1 | 85.7 | |
| 10 | 2 | 5.98 | 25.71 | 1.07 | 24.0 | 8.7 | 1.7 | 22.4 | 115.1 | |
| 11 | 2 | 6.87 | 31.84 | 1.79 | 17.8 | 5.8 | 1.8 | 97.6 | 106.7 | |
| NTPP | 12 | 1 | 5.91 | 23.64 | 1.49 | 15.9 | 5.3 | 2.3 | 82.5 | 24.2 |
| 13 | 1 | 5.99 | 44.11 | 1.63 | 27.1 | 5.3 | 1.2 | 85.4 | 25.9 | |
| 14 | 1 | 5.85 | 24.46 | 1.57 | 15.6 | 9.0 | 3.8 | 101.2 | 19.4 | |
| 15 | 1 | 5.99 | 39.78 | 1.86 | 21.4 | 9.7 | 3.0 | 114.3 | 18.2 | |
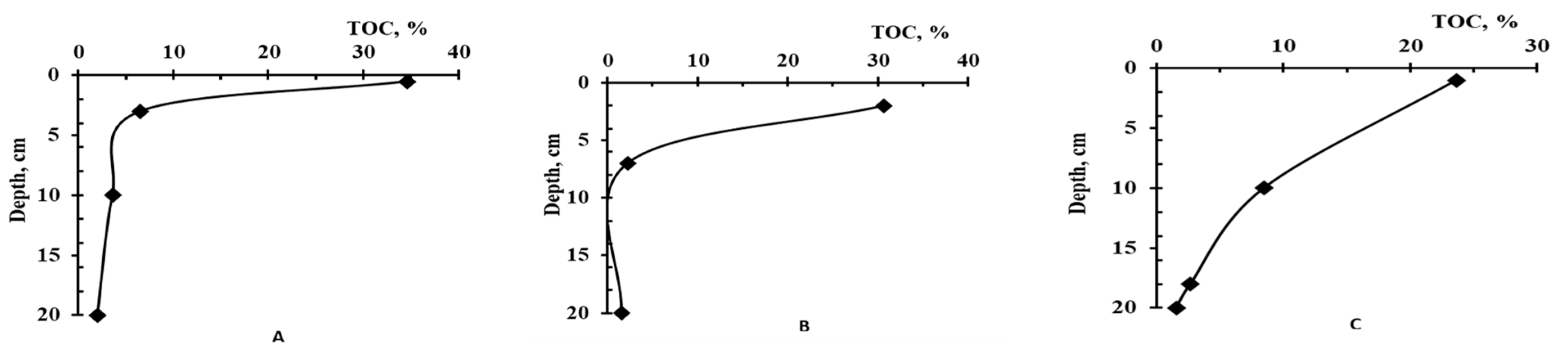
| Horizon | Depth, cm | TOC, % | Humic Acid Fractions | ∑HA | ∑FA | Humins | CHA:CFA | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||
| Technosol of SUPP ash dump | |||||||||
| O | 0–0.5 | 28.55 | 8.3 | 0.3 | 5.2 | 13.8 | 16.1 | 70.1 | 0.86 |
| AT | 0.5–3 | 9.57 | 8.6 | 0.6 | 9.7 | 18.9 | 25.3 | 55.8 | 0.75 |
| A | 3–10 | 3.36 | 3.0 | 0.1 | 0.8 | 3.9 | 12.7 | 83.4 | 0.31 |
| C | 10–20 | 2.14 | 0.6 | 0.2 | 0.2 | 1.0 | 6.4 | 92.6 | 0.16 |
| Technosol of VTPP ash dump | |||||||||
| O | 0–2 | 30.69 | 5.9 | 0.2 | 4.2 | 10.3 | 12.4 | 77.3 | 0.83 |
| A | 2–7 | 2.23 | 5.9 | 2.4 | 4.6 | 12.9 | 23.9 | 63.2 | 0.54 |
| C | 7–20 | 1.39 | 0.7 | 3.2 | 1.2 | 5.1 | 12.8 | 82.1 | 0.40 |
| Sod-podzolic soils of the Middle Urals (n = 24) [47] | |||||||||
| A | 4.08 | 16.9 | 2.2 | 4.5 | 23.6 | 32.5 | 43.9 | 0.73 | |
| s | 1.46 | 6.2 | 2.1 | 3.3 | 6.2 | 9.6 | 16.2 | 0.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasova, O.; Radchenko, T.; Betekhtina, A.; Petrova, T.; Uchaev, A.; Dergacheva, M. Carbon Sequestration by Soils of Ash Dump Forest Areas in the Middle Urals (Russia). Forests 2023, 14, 2178. https://doi.org/10.3390/f14112178
Nekrasova O, Radchenko T, Betekhtina A, Petrova T, Uchaev A, Dergacheva M. Carbon Sequestration by Soils of Ash Dump Forest Areas in the Middle Urals (Russia). Forests. 2023; 14(11):2178. https://doi.org/10.3390/f14112178
Chicago/Turabian StyleNekrasova, Olga, Tatiana Radchenko, Anna Betekhtina, Tatiana Petrova, Anton Uchaev, and Maria Dergacheva. 2023. "Carbon Sequestration by Soils of Ash Dump Forest Areas in the Middle Urals (Russia)" Forests 14, no. 11: 2178. https://doi.org/10.3390/f14112178






