Study on Desiccation Tolerance and Biochemical Changes of Sassafras tzumu (Hemsl.) Hemsl. Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants Materials
2.2. Seed Desiccation Treatment
2.3. Determination of Seed Viability
2.4. Determination of Relative Electrical Conductivity
2.5. Determination of Antioxidant System Index
2.6. Data Processing and Analysis
3. Results
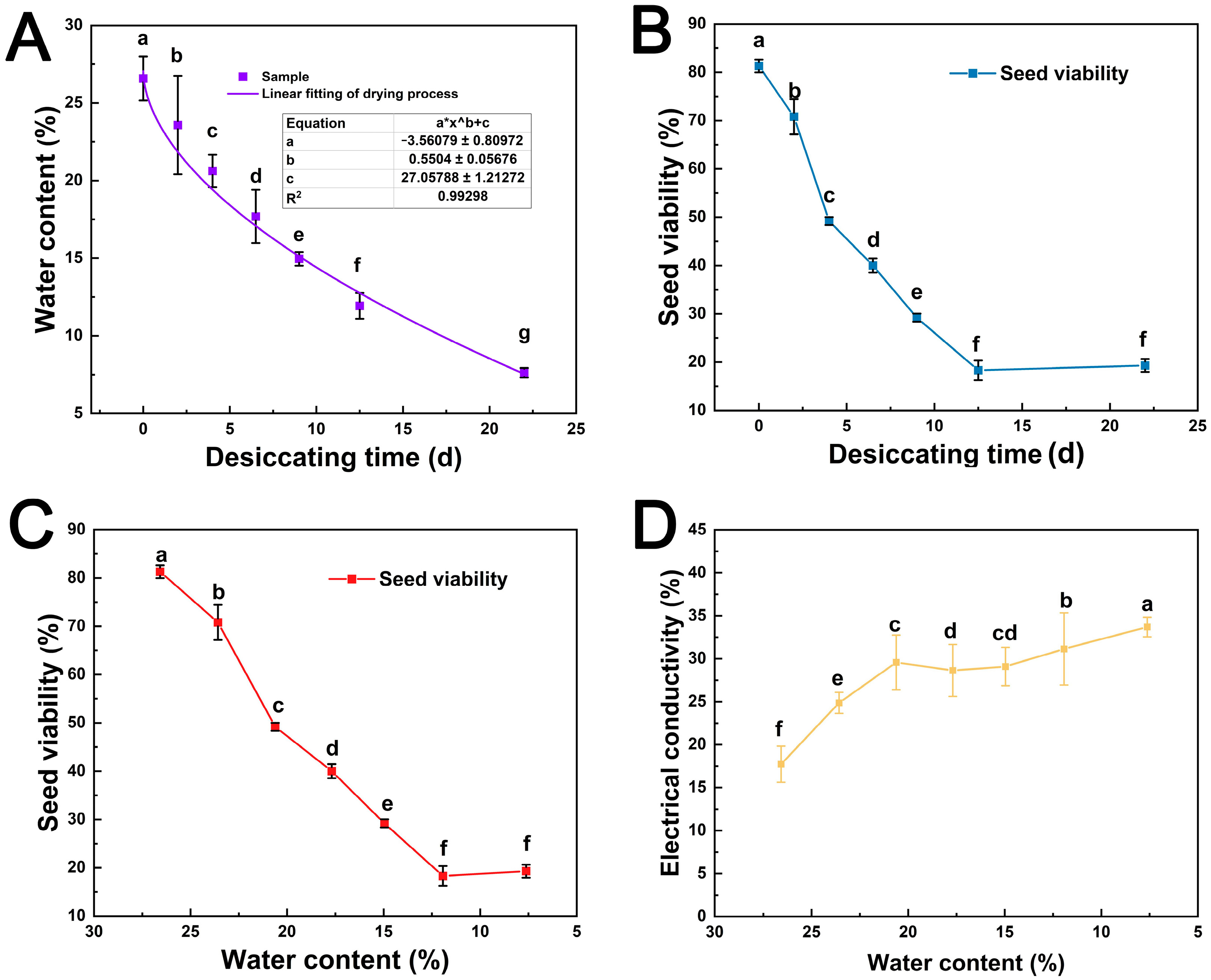
3.1. Effects of Desiccation on Viability and Relative Electrical Conductivity of S. tzumu Seed
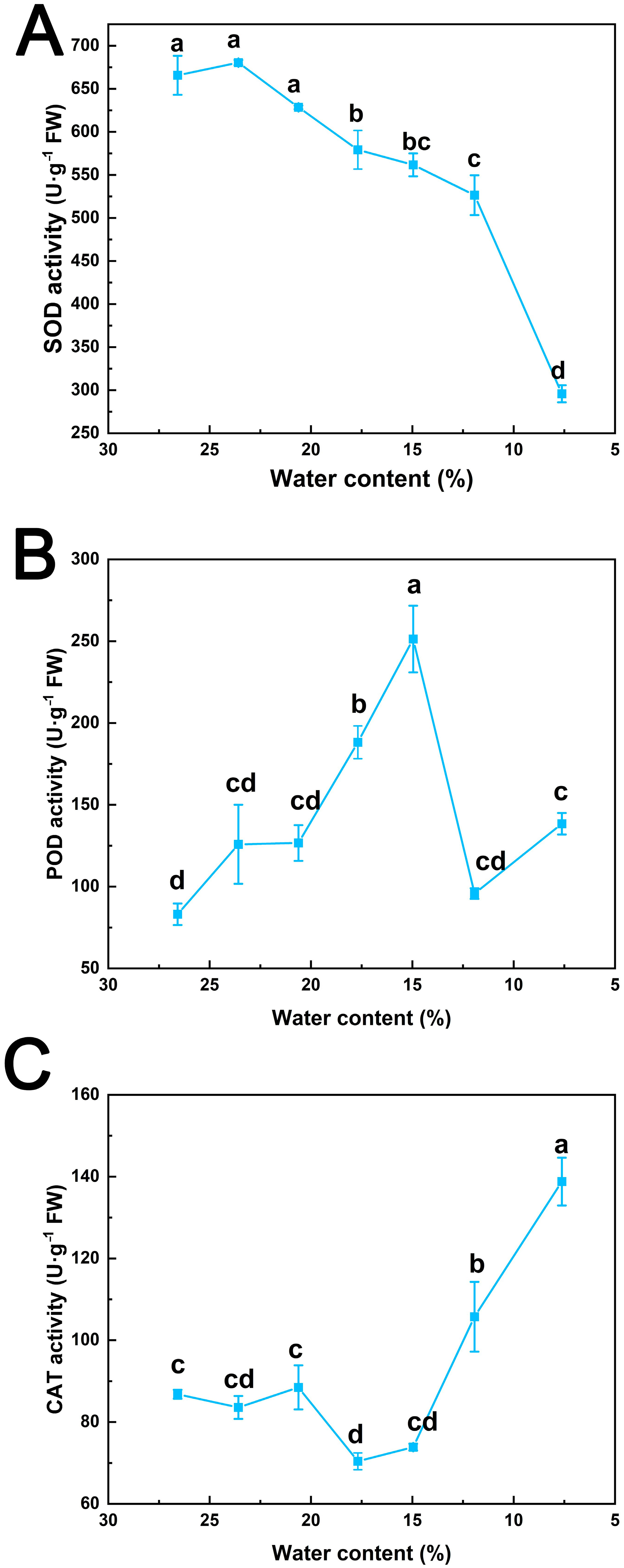
3.2. Effects of Desiccation on Antioxidant Enzyme System of S. tzumu Seed
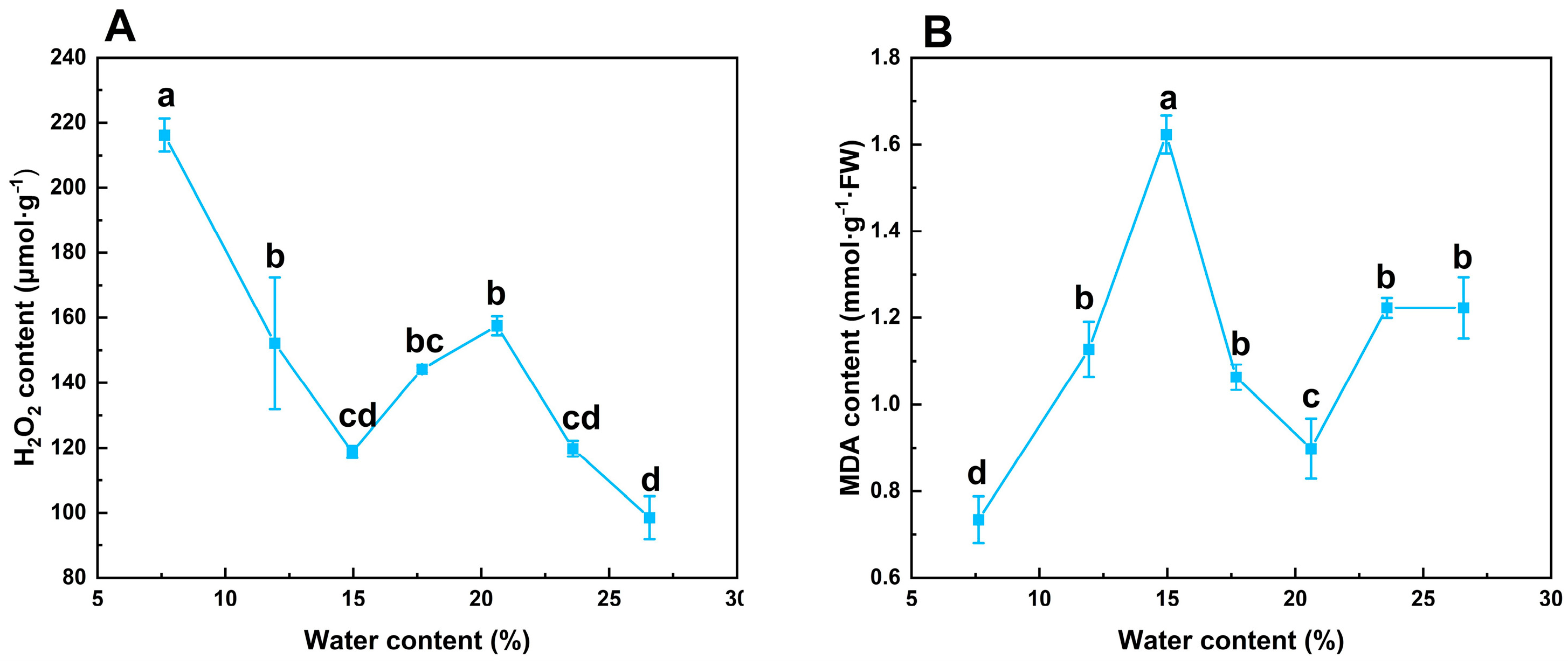
3.3. Effects of Desiccation on Oxidative Stress of S. tzumu Seeds
3.4. Correlation Analysis of Physiological Changes in S. tzumu Seed during Desiccation
4. Discussion
4.1. Desiccation Tolerance of S. tzumu Seeds
4.2. Changes and Interactions of Physiological Indexes during Dehydration of S. tzumu Seeds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Zhang, Y.; Jia, D.; Tao, J. Species distribution modeling of Sassafras tzumu and implications for forest management. Sustainability 2020, 12, 4132. [Google Scholar] [CrossRef]
- Hou, Y.-L.; Chang, H.-S.; Wang, H.-C.; Wang, S.-Y.; Chen, T.-Y.; Lin, C.-H.; Chen, I.-S. Sassarandainol: A new neolignan and anti-inflammatory constituents from the stem of Sassafras randaiense. Nat. Prod. Res. 2015, 29, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Jin, C.; Tai, P.; You, J. Corrosion resistance of natural rot resistant wood and its chemical composition change during decay. Sci. Silvae Sin. 1989, 5, 447–452. [Google Scholar]
- Wu, P.; Luo, Z. Precious sassafras of Guizhou. Guizhou Forest. Sci. Technol. 1981, 3, 8–12. [Google Scholar]
- Gu, S.; Chen, J.; Han, H.; Wang, Z. Preliminary observation on morphological structure and development of fruit and fruit of Sassafras tzumu. Zhejiang For. Sci. Technol. 1991, 4, 16–22. [Google Scholar]
- Huang, B.; Zhu, P.; Fu, S. Tissue culture of Sassafras tzumu (Hemsl.) Hemsl. J. Jiangxi For. Sci. Technol. 2010, 4, 11–12. [Google Scholar]
- Chen, H.; Jiang, J.; Liu, J.; Tan, Z.; Li, Y. Physiological dormancy and dormancy release of Sassafras tzumu, a colored-leaf tree species with high landscape and economic value. Iforest-Biogeosci. For. 2022, 15, 349. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H.; Hong, T.; Roberts, E. An intermediate category of seed storage behaviour? I. Coffee. J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Kermode, A.R.; Finch-Savage, B.E. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In Desiccation and Survival in Plants: Drying without Dying; CABI Publishing: Wallingford, UK, 2002; pp. 149–184. [Google Scholar]
- Berjak, P.; Pammenter, N.W. Recalcitrant seeds. In Handbook of Seed Physiology; Food Products Press: New York, NY, USA, 2004; pp. 305–345. [Google Scholar]
- Vertucci, C.W.; Farrant, J.M. Acquisition and loss of desiccation tolerance. In Seed Development and Germination; Routledge: London, UK, 2017; pp. 237–271. [Google Scholar]
- Berjak, P.; Pammenter, N. Orthodox and Recalcitrant Seeds. In Tropical Tree Seed Manual; U.S. Department of Agriculture: Washington, DC, USA, 2002; pp. 137–147. [Google Scholar]
- Shih, M.-D.; Hoekstra, F.A.; Hsing, Y.-I.C. Late embryogenesis abundant proteins. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 48, pp. 211–255. [Google Scholar]
- Berjak, P.; Pammenter, N.W. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef] [PubMed]
- Umarani, R.; Aadhavan, E.K.; Faisal, M.M. Understanding poor storage potential of recalcitrant seeds. Curr. Sci. 2015, 108, 2023–2034. [Google Scholar]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, H.; Chengzhen, W.; Dongjin, H.; Xiaoli, B.; Shujun, Y. Biomass production and productivity of natural regenerated Sassafras tzumu trees. J. Trop. Subtrop. Bot. 2002, 10, 105–110. [Google Scholar]
- Zhuang, Z.; Shi, F.H.; Ding, Y.F.; Shen, Y.B. Study on dehydration tolerance of Citrus grandis × junos seed. Jiangsu Agric. Sci. 2015, 09, 229–231. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef] [PubMed]
- França-Neto, J.d.B.; Krzyzanowski, F.C. Use of the tetrazolium test for estimating the physiological quality of seeds. Seed Sci. Technol. 2022, 50, 31–44. [Google Scholar] [CrossRef]
- Vieira, R.; Paiva, J.; Perecin, A.; Perecin, D. Electrical conductivity and field performance of soybean seeds. Seed Technol. 1999, 21, 15–24. [Google Scholar]
- Oberley, L.W.; Spitz, D.R. [61] Assay of superoxide dismutase activity in tumor tissue. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 457–464. [Google Scholar]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 685–690. [Google Scholar]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, P.; Niu, Z.; Li, B.; Lv, Y.; Wei, S.; Hu, Y. Effects of artificial aging on physiological quality and cell ultrastructure of maize (Zea mays L.). Cereal Res. Commun. 2022, 51, 615–626. [Google Scholar] [CrossRef]
- Hong, T.; Ellis, R.H. A protocol to Determine Seed Storage Behaviour; Bioversity International: Rome, Italy, 1996. [Google Scholar]
- Feng, J.; Shen, Y.; Shi, F.; Li, C. Changes in seed germination ability, lipid peroxidation and antioxidant enzyme activities of Ginkgo biloba seed during desiccation. Forests 2017, 8, 286. [Google Scholar] [CrossRef]
- Pammenter, N.; Berjak, P. Some thoughts on the evolution and ecology of recalcitrant seeds. Plant Species Biol. 2000, 15, 153–156. [Google Scholar] [CrossRef]
- Wang, G.; Shen, W.; Zhang, Z.; Guo, S.; Hu, J.; Feng, R.; Zhao, Q.; Du, J.; Du, Y. The Effect of Neutral Salt and Alkaline Stress with the Same Na+ Concentration on Root Growth of Soybean (Glycine max (L.) Merr.) Seedlings. Agronomy 2022, 12, 2708. [Google Scholar] [CrossRef]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Chandel, K.; Chaudhury, R.; Radhamani, J.; Malik, S. Desiccation and freezing sensitivity in recalcitrant seeds of tea, cocoa and jackfruit. Ann. Bot. 1995, 76, 443–450. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Song, S.; Fu, J. Dehydration sensitivity and lipid peroxidation of seed of Clausena lansium. Chin. J. Plant Physiol. 1997, 2, 163–168. [Google Scholar]
- Li, Y.; Ma, Y.; Han, L. Relationship between superoxide dismutase and dehydration tolerance of Pachira glabra seeds. Sci. Silvae Sin. 2009, 5, 74–79. [Google Scholar]
- Li, L.; Sun, X.; Zhang, G.; Long, G.; Meng, Z.; Yang, S.; Chen, J. Effects of dehydration rate on dehydration sensitivity and antioxidant enzyme activity of Panax notoginseng seed. Seed 2014, 12, 1–5. [Google Scholar]
- Niedzwiedz-Siegien, I.; Bogatek-Leszczynska, R.; Côme, D.; Corbineau, F. Effects of drying rate on dehydration sensitivity of excised wheat seedling shoots as related to sucrose metabolism and antioxidant enzyme activities. Plant Sci. 2004, 167, 879–888. [Google Scholar] [CrossRef]
- Franchi, G.; Piotto, B.; Nepi, M.; Baskin, C.; Baskin, J.; Pacini, E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. J. Exp. Bot. 2011, 62, 5267–5281. [Google Scholar] [CrossRef]
- Pukacka, S.; Malec, M.; Ratajczak, E. ROS production and antioxidative system activity in embryonic axes of Quercus robur seeds under different desiccation rate conditions. Acta Physiol. Plant. 2011, 33, 2219–2227. [Google Scholar] [CrossRef]
- Duhan, S.; Kumari, A.; Lal, M.; Sheokand, S. Oxidative stress and antioxidant defense under combined waterlogging and salinity stresses. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; WILEY online Library: Hoboken, NJ, USA, 2019; pp. 113–142. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Qiao, X.; Ma, Q.; Zhao, T.; Sun, H.; Li, S. Study on the effect of water loss on seed quality of Quercus morgensis. Seed 2018, 5, 70–72. [Google Scholar]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Sahu, B.; Naithani, S.C. Role of reactive oxygen species and antioxidative enzymes in the loss and re-establishment of desiccation tolerance in germinated pea seeds. South Afr. J. Bot. 2022, 163, 75–86. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Wang, L.; Wu, R.; Phillips, J.; Deng, X. LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco. Plant Sci. 2009, 176, 90–98. [Google Scholar] [CrossRef]
- Eisvand, H.; Moori, S.; Ismaili, A.; Sasani, S. Effects of late-season drought stress on physiology of wheat seed deterioration: Changes in antioxidant enzymes and compounds. Seed Sci. Technol. 2016, 44, 327–341. [Google Scholar] [CrossRef]


| Reaction Reagent | Measuring Tube | Light Pair Tube | Dark Pair Tube | ||
|---|---|---|---|---|---|
| Test tube number | 1 | 2 | 3 | 4 | 5 |
| 50 mmol/L Phosphate buffer (mL) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| 130 mmol/L Met solution (mL) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| 750 umol/L NBT solution (mL) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| 100 umol/L EDTA-Na2 (mL) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| 20 umol/L Riboflavin solution (mL) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| enzyme extract solution (mL) | 0.05 | 0.05 | 0.05 | 0 | 0 |
| Distilled water (mL) | 0.5 | 0.5 | 0.5 | 0.55 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Wang, M.; Wu, Y.; Hua, Q.; Shen, Y. Study on Desiccation Tolerance and Biochemical Changes of Sassafras tzumu (Hemsl.) Hemsl. Seeds. Forests 2023, 14, 2183. https://doi.org/10.3390/f14112183
Peng C, Wang M, Wu Y, Hua Q, Shen Y. Study on Desiccation Tolerance and Biochemical Changes of Sassafras tzumu (Hemsl.) Hemsl. Seeds. Forests. 2023; 14(11):2183. https://doi.org/10.3390/f14112183
Chicago/Turabian StylePeng, Chenyin, Mingzhu Wang, Yu Wu, Qilong Hua, and Yongbao Shen. 2023. "Study on Desiccation Tolerance and Biochemical Changes of Sassafras tzumu (Hemsl.) Hemsl. Seeds" Forests 14, no. 11: 2183. https://doi.org/10.3390/f14112183





