Rapid Assessment of Land Use Legacy Effect on Forest Soils: A Case Study on Microarthropods Used as Indicators in Mediterranean Post-Agricultural Forests
Abstract
:1. Introduction
2. Materials and Methods
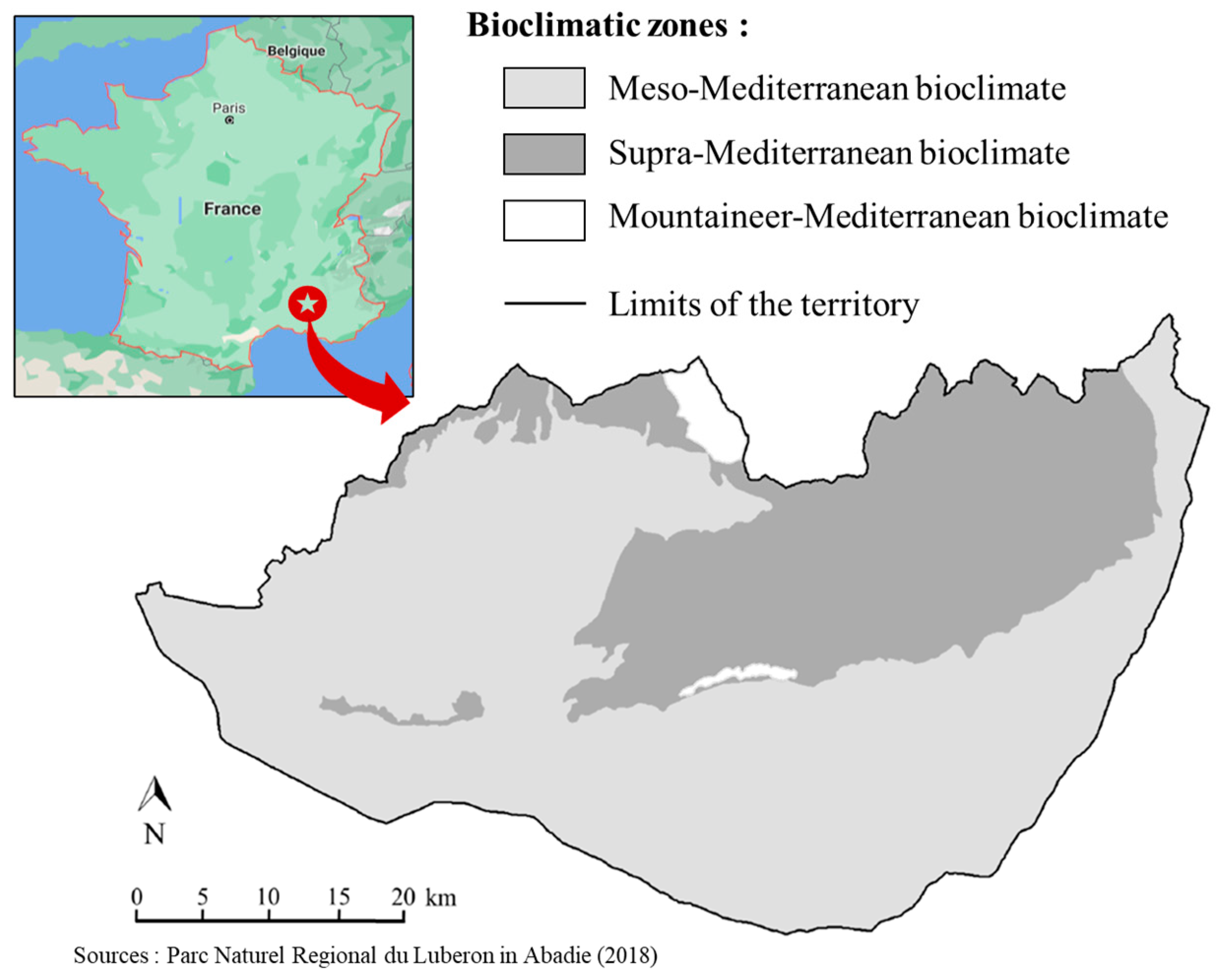
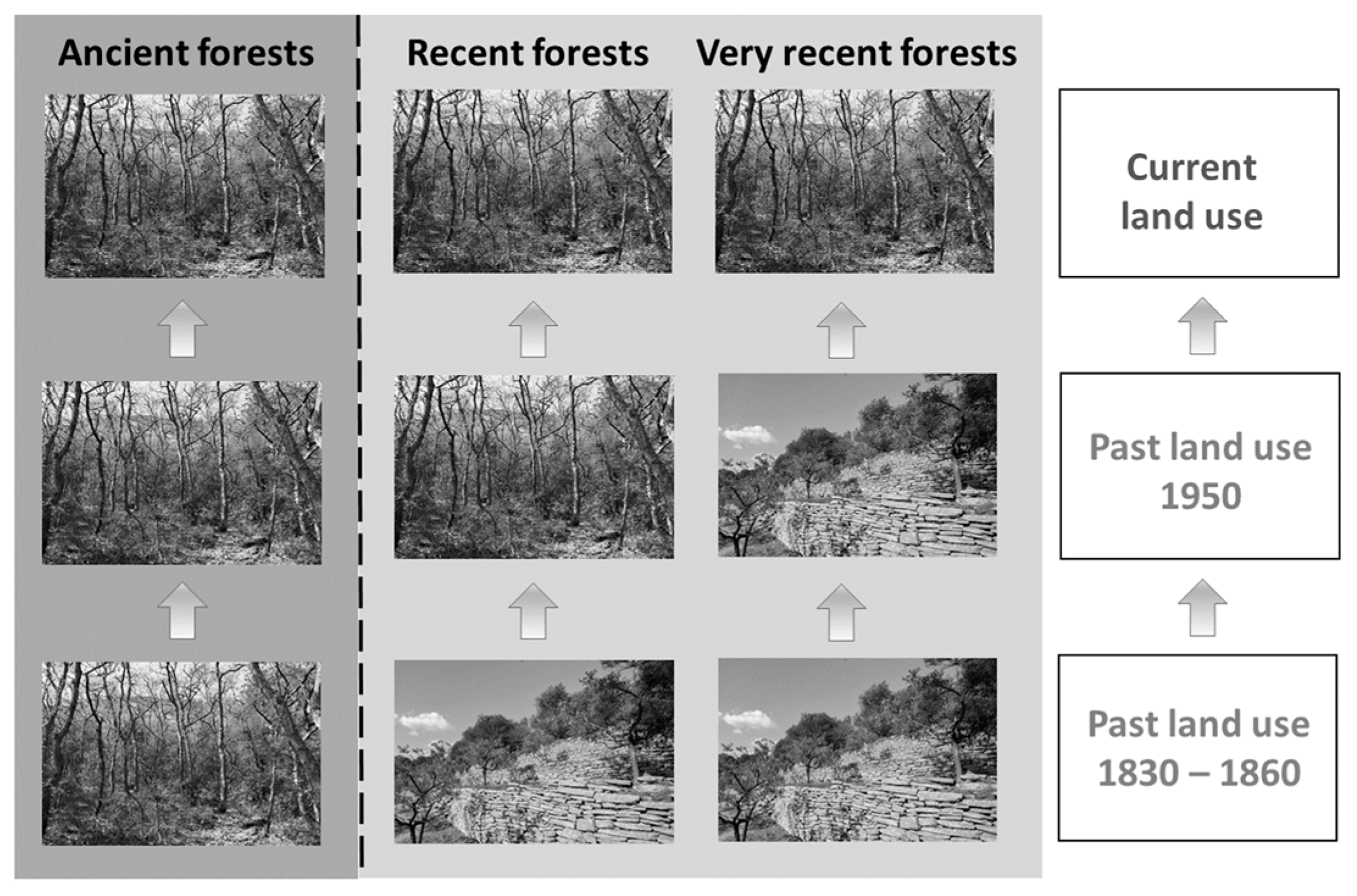
2.1. Site Description and Soil Sampling
- 1958: Historical aerial photographs taken between 1953 and 1958, photo-interpreted for each vegetation plot.
2.2. Microarthropods Extraction and Identification
2.3. Soil Physico-Chemical Properties
2.4. Statistical Analyses
3. Results
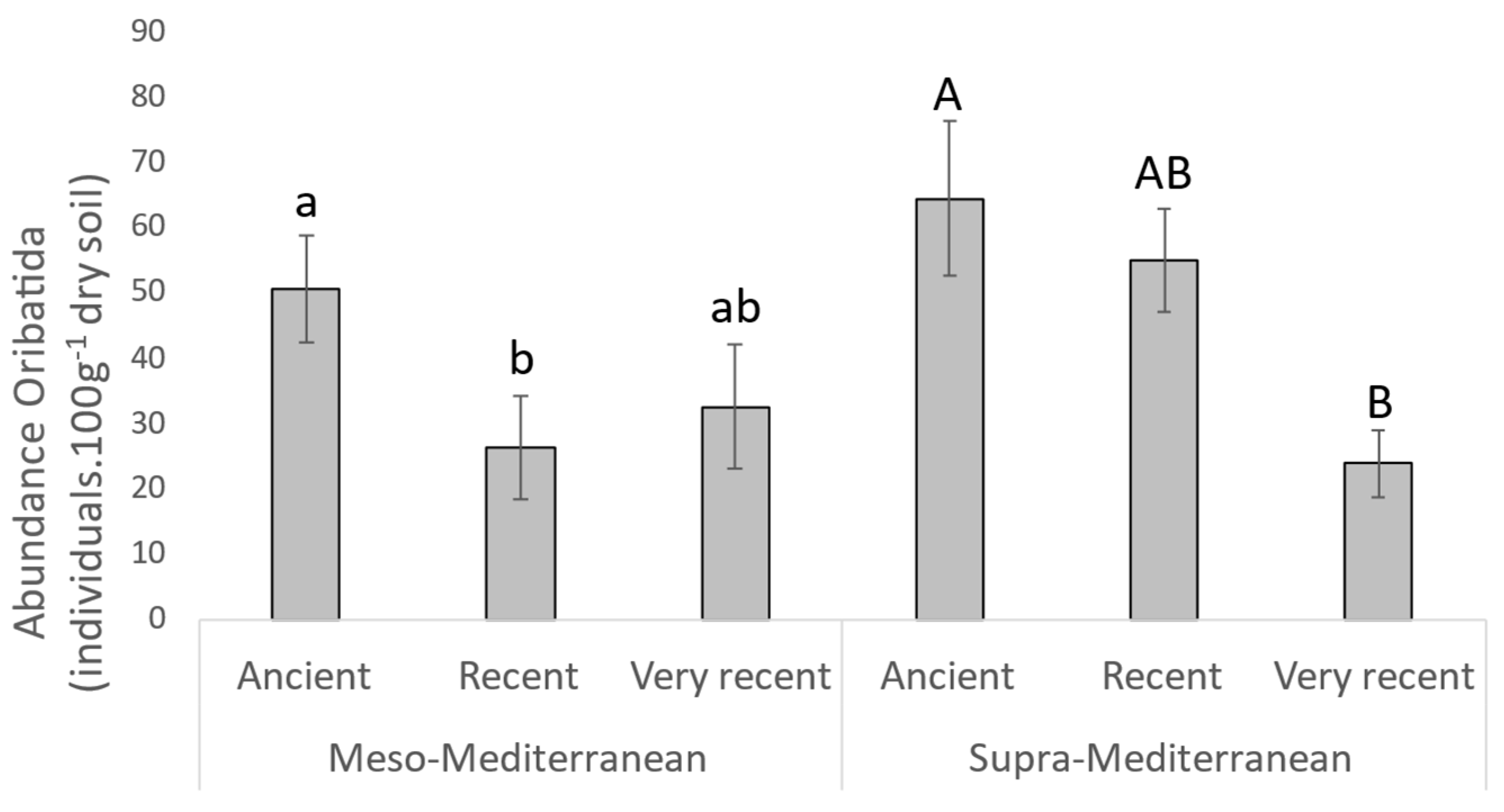
3.1. LUL Effects on Microarthropods Demographic Parameters
3.2. LUL Effects on Forest Structure and Soil Properties and Links with Microarthropod Indicators
4. Discussion
4.1. Past Agricultural Activities Negatively Affected Microarthropods
4.2. Pedoclimatic Conditions Influenced Forest Regeneration
4.3. Land Use Legacy and Soil Vulnerability in the Context of Global Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Hou, K.; Li, X.; Zhang, Y.; Chen, P. Review of land use and land cover change research progress. IOP Conf. Ser. Earth Environ. 2018, 113, 012087. [Google Scholar] [CrossRef]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Change Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Rébufa, C.; Dupuy, N.; Boukhdoud, N.; Brunel, C.; Abadie, J.; Giffard, I.; Farnet-Da Silva, A.M. Infrared spectroscopy as a useful tool to predict land use depending on Mediterranean contrasted climate conditions: A case study on soils from olive-orchards and forests. Sci. Total Environ. 2019, 686, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kanianska, R. Agriculture and its impact on land-use, environment, and ecosystem services. In Landscape Ecology—The Influences of Land Use and Anthropogenic Impacts of Landscape Creation; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Martí-Roura, M.; Hagedorn, F.; Rovira, P.; Romanyà, J. Effect of land use and carbonates on organic matter stabilization and microbial communities in Mediterranean soils. Geoderma 2019, 351, 103–115. [Google Scholar] [CrossRef]
- De Cárcer, P.S.; Sinaj, S.; Santonja, M.; Fossati, D.; Jeangros, B. Long-term effects of crop succession, soil tillage and climate on wheat yield and soil properties. Soil Tillage Res. 2019, 190, 209–219. [Google Scholar] [CrossRef]
- Tosi, M.; Chludil, H.D.; Correa, O.S.; Vogrig, J.A.; Montecchia, M.S. Long-term legacy of land-use change in soils from a subtropical rainforest: Relating microbiological and physicochemical parameters. Eur. J. Soil Sci. 2020, 72, 1054–1069. [Google Scholar] [CrossRef]
- De la Peña, E.; Baeten, L.; Steel, H.; Viaene, N.; Sutter, N.D.; Schrijver, A.D.; Verheyen, K. Beyond plant–soil feedbacks: Mechanisms driving plant community shifts due to land-use legacies in post-agricultural forests. Funct. Ecol. 2016, 30, 1073–1085. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Taranu, Z.E. The influence of time, soil characteristics, and land-use history on soil phosphorus legacies: A global meta-analysis. Glob. Change Biol. 2012, 18, 1904–1917. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.L.; Hernández, T.; García, C. The long-term effects of the management of a forest soil on its carbon content, microbial biomass and activity under a semi-arid climate. Appl. Soil Ecol. 2007, 37, 53–62. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Changes in soil microbial community structure following the abandonment of agricultural terraces in mountainous areas of Eastern Spain. Appl. Soil Ecol. 2009, 42, 315–323. [Google Scholar] [CrossRef]
- Delcourt, N.; Dupuy, N.; Rébufa, C.; Abadie, J.; Foli, L.; Farnet-Da Silva, A.-M. Microbial functioning in Mediterranean forest soils: Does land use legacy matter? Land Degrad. Dev. 2023, 34, 3932–3942. [Google Scholar] [CrossRef]
- Gobat, J.-M.; Aragno, M.; Matthey, W. Le Sol Vivant: Bases de Pédologie, Biologie des Sols, 3rd ed.; Presses Polytechniques et Universitaires Romandes: Lausanne, France, 2010; p. 848. [Google Scholar]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Cassani, M.T.; Sabatté, M.L.; González Arzac, A.; Massobrio, M.J. Mesofauna as an indicator of agroecosystem stability: Degree of artificialization effect on land uses in Azul district, Argentina. SN Appl. Sci. 2020, 2, 324. [Google Scholar] [CrossRef]
- George, P.B.L.; Keith, A.M.; Creer, S.; Barrett, G.L.; Lebron, I.; Emmett, B.A.; Robinson, D.A.; Jones, D.L. Evaluation of mesofauna communities as soil quality indicators in a national-level monitoring programme. Soil Biol. Biochem. 2017, 115, 537–546. [Google Scholar] [CrossRef]
- Lindberg, N.; Bengtsson, J. Population responses of oribatid mites and collembolans after drought. Appl. Soil Ecol. 2005, 28, 163–174. [Google Scholar] [CrossRef]
- González-Macé, O.; Scheu, S. Response of Collembola and Acari communities to summer flooding in a grassland plant diversity experiment. PLoS ONE 2018, 13, e0202862. [Google Scholar] [CrossRef] [PubMed]
- Quézel, P.; Médail, F. Écologie et Biogéographie des Forêts du Bassin Méditerranéen, Collection Environnement; Elsevier Masson: Paris, France, 2003; p. 573. [Google Scholar]
- Verheye, W.; de la Rosa, D. Mediterranean soils. In Land Use and Land Cover, from Encyclopedia of Life Support Systems (EOLSS); Developed under the Auspices of the UNESCO; Eolss Publishers: Oxford, UK, 2006; p. 26. Available online: https://www.eolss.net/Sample-Chapters/C12/E1-05-07-17.pdf (accessed on 10 November 2023).
- García-Ruiz, J.M.; Nadal-Romero, E.; Lana-Renault, N.; Beguería, S. Erosion in Mediterranean landscapes: Changes and future challenges. Geomorphology 2013, 198, 20–36. [Google Scholar] [CrossRef]
- Torrent, J. Mediterranean Soils. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 418–427. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Stanchi, S.; Freppaz, M.; Agnelli, A.; Reinsch, T.; Zanini, E. Properties, best management practices and conservation of terraced soils in Southern Europe (from Mediterranean areas to the Alps): A review. Quat. Int. 2012, 265, 90–100. [Google Scholar] [CrossRef]
- Abadie, J.; Dupouey, J.-L.; Avon, C.; Rochel, X.; Tatoni, T.; Bergès, L. Forest recovery since 1860 in a Mediterranean region: Drivers and implications for land use and land cover spatial distribution. Landsc. Ecol. 2017, 33, 289–305. [Google Scholar] [CrossRef]
- Serra, P.; Pons, X.; Saurí, D. Land-cover and land-use change in a Mediterranean landscape: A spatial analysis of driving forces integrating biophysical and human factors. Appl. Geogr. 2008, 28, 189–209. [Google Scholar] [CrossRef]
- Abadie, J.; Avon, C.; Dupouey, J.-L.; Lopez, J.-M.; Tatoni, T.; Bergès, L. Land use legacies on forest understory vegetation and soils in the Mediterranean region: Should we use historical maps or in situ land use remnants? For. Ecol. Manag. 2018, 427, 17–25. [Google Scholar] [CrossRef]
- Abadie, J.; Dupouey, J.; Salvaudon, A.; Gachet, S.; Videau, N.; Avon, C.; Dumont, J.; Tatoni, T.; Bergès, L. Historical ecology of Mediterranean forests: Land use legacies on current understory plants differ with time since abandonment and former agricultural use. J. Veg. Sci. 2021, 32, e12860. [Google Scholar] [CrossRef]
- Salvaudon, A.; Hamel, A.; Grel, A.; Rossi, M.; Vallauri, D. Notice de la Carte des Forêts Anciennes du Parc Naturel Régional du Lubéron (1:40000) avec Référence aux Autres Usages du sol; OATAO: Toulouse, France, 2012; p. 18. [Google Scholar]
- Favre, C.; Grel, A.; Granier, E.; Cosserat-Mangeot, R.; Bachacou, J.; Dupouey, J.L. Digitalisation des Cartes Anciennes. Manuel Pour la Vectorisation de L’usage des Sols et le Géoréférencement des Minutes 1:40 000 de la Carte d’Etat-Major (v. 12.7.3); INRA: Nancy, France, 2013; p. 54. [Google Scholar]
- De Réparaz, A. Les campagnes de l’ancienne Haute-Provence vues par les géographes du passé: 1880–1950. Les Alpes Lumière 2000, 136, 180. [Google Scholar]
- Berlese, A. Apparecchio per Raccogliere Presto ed in Gran Numero Piccoli Arthropodi; Redia 2; Kessinger Publishing, LLC: Whitefish, MN, USA, 1905; pp. 85–89. [Google Scholar]
- Gisin, H. Collembolenfauna Europas; Museum d’Histoire Naturelle: Geneva, Switzerland, 1960. [Google Scholar]
- Hopkins, S.P. The Biology of Springtails (Insects: Collembolan); Oxford University Press: New York, NY, USA, 1997; p. 340. [Google Scholar]
- Coleman, D.C.; Crossley, J.D.A.; Hendrix, P.F. Fundamentals of Soil Ecology; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Donoso, D.A.; Johnston, M.K.; Clay, N.A.; Kaspari, M.E. Trees as templates for trophic structure of tropical litter arthropod fauna. Soil Biol. Biochem. 2013, 61, 45–51. [Google Scholar] [CrossRef]
- Santonja, M.; Milcu, A.; Fromin, N.; Rancon, A.; Shihan, A.; Fernandez, C.; Baldy, V.; Hättenschwiler, S. Temporal shifts in plant diversity effects on carbon and nitrogen dynamics during litter decomposition in a Mediterranean shrubland exposed to reduced precipitation. Ecosystems 2018, 22, 939–954. [Google Scholar] [CrossRef]
- Feng, W.; Mariotte, P.; Xu, L.; Buttler, A.; Bragazza, L.; Jiang, J.; Santonja, M. Seasonal variability of groundwater level effects on the growth of Carex cinerascens in lake wetlands. Ecol. Evol. 2019, 10, 517–526. [Google Scholar] [CrossRef]
- Aupic-Samain, A.; Baldy, V.; Delcourt, N.; Krogh, P.H.; Gauquelin, T.; Fernandez, C.; Santonja, M. Water availability rather than temperature control soil fauna community structure and prey–predator interactions. Funct. Ecol. 2021, 35, 1550–1559. [Google Scholar] [CrossRef]
- Schneider, K.; Maraun, M. Top-down control of soil microarthropods—Evidence from a laboratory experiment. Soil Biol. Biochem. 2009, 41, 170–175. [Google Scholar] [CrossRef]
- Boulaine, J. Quatre siècles de fertilisation, seconde partie. Etude Gest. Sols 1995, 2, 219–226. [Google Scholar]
- Casas, J.J.; Bonachela, S.; Moyano, F.J.; Fenoy, E.; Hernández, J. Chapter 3—Agricultural Practices in the Mediterranean: A Case Study in Southern Spain. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 23–36. [Google Scholar] [CrossRef]
- Aupic-Samain, A.; Santonja, M.; Chomel, M.; Pereira, S.; Quer, E.; Lecareux, C.; Limousin, J.-M.; Ourcival, J.-M.; Simioni, G.; Gauquelin, T.; et al. Soil biota response to experimental rainfall reduction depends on the dominant tree species in mature northern Mediterranean forests. Soil Biol. Biochem. 2021, 154, 108122. [Google Scholar] [CrossRef]
- Thakur, M.P.; Künne, T.; Griffin, J.N.; Eisenhauer, N. Warming magnifies predation and reduces prey coexistence in a model litter arthropod system. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162570. [Google Scholar] [CrossRef] [PubMed]
- Santonja, M.; Fernandez, C.; Proffit, M.; Gers, C.; Gauquelin, T.; Reiter, I.M.; Cramer, W.; Baldy, V. Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a Mediterranean oak forest. J. Ecol. 2017, 105, 801–815. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Feketeová, Z.; Mangová, B.; Čierniková, M. The soil chemical properties influencing the oribatid mite (Acari; Oribatida) abundance and diversity in Coal Ash Basin Vicinage. Appl. Sci. 2021, 11, 3537. [Google Scholar] [CrossRef]
- Gergócs, V.; Rétháti, G.; Hufnagel, L. Litter quality indirectly influences community composition, reproductive mode and trophic structure of oribatid mite communities: A microcosm experiment. Exp. Appl. Acarol. 2015, 67, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Wolters, V. Responses of oribatid mite communities to summer drought: The influence of litter type and quality. Soil Biol. Biochem. 2005, 37, 2117–2130. [Google Scholar] [CrossRef]
- Lesschen, J.P.; Cammeraat, L.H.; Nieman, T. Erosion and terrace failure due to agricultural land abandonment in a semi-arid environment. Earth Surf. Process. Landf. 2008, 33, 1574–1584. [Google Scholar] [CrossRef]
- Ackermann, O.; Zhevelev, H.M.; Svoray, T. Agricultural systems and terrace pattern distribution and preservation along climatic gradient: From sub-humid mediterranean to arid conditions. Quat. Int. 2019, 502, 319–326. [Google Scholar] [CrossRef]
- Delcourt, N.; Farnet-Da Silva, A.-M.; Rébufa, C.; Foli, L.; Dupuy, N. Land use legacy footprint in Mediterranean forest soils: An infrared spectroscopy approach. Geoderma 2023, 430, 116299. [Google Scholar] [CrossRef]
- Culliney, T.W. Role of Arthropods in Maintaining Soil Fertility. Agriculture 2013, 3, 629–659. [Google Scholar] [CrossRef]
- Renker, C.; Otto, P.; Schneider, K.; Zimdars, B.; Maraun, M.; Buscot, F. Oribatid mites as potential vectors for soil microfungi: Study of mite-associated fungal species. Microb. Ecol. 2005, 50, 518. [Google Scholar] [CrossRef]
- Wolters, V. Invertebrate control of soil organic matter stability. Biol. Fertil. Soils 2000, 31, 1–19. [Google Scholar] [CrossRef]
- Kosmas, C.; Gerontidis, S.; Marathianou, M. The effect of land use change on soils and vegetation over various lithological formations on Lesvos (Greece). Catena 2000, 40, 51–68. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, G.; Liu, Y.; Nie, X.; Li, Z.; Liu, J.; Zhu, D. Advantages and disadvantages of terracing: A comprehensive review. ISWCR 2021, 9, 344–359. [Google Scholar] [CrossRef]
- Moreno-de-las-Heras, M.; Lindenberger, F.; Latron, J.; Lana-Renault, N.; Llorens, P.; Arnáez, J.; Romero-Díaz, A.; Gallart, F. Hydro-geomorphological consequences of the abandonment of agricultural terraces in the Mediterranean region: Key controlling factors and landscape stability patterns. Geomorphology 2019, 333, 73–91. [Google Scholar] [CrossRef]



| Land Use Legacy (LUL) | Climate (C) | Season (S) | LUL × C | LUL × S | S × C | LUL × S × C | |||
|---|---|---|---|---|---|---|---|---|---|
| df | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Mesofauna (individuals 100 g−1 dry soil) | Total abundance | χ2 | 10.65 | 4.36 | 0.07 | 2.13 | 0.45 | 5.03 | 3.92 |
| p-Value | ** | * | ns | ns | ns | * | ns | ||
| Microbi-detritivorous abundance | χ2 | 7.43 | 5.53 | 12.21 | 0.10 | 0.39 | 2.54 | 0.71 | |
| p-Value | * | * | *** | ns | ns | ns | ns | ||
| Predator abundance | χ2 | 8.75 | 2.61 | 0.03 | 3.83 | 0.36 | 5.18 | 3.75 | |
| p-Value | * | ns | ns | ns | ns | * | ns | ||
| Acari abundance | χ2 | 7.92 | 2.18 | 0.37 | 5.08 | 0.19 | 5.23 | 2.12 | |
| p-Value | * | ns | ns | ns | ns | * | ns | ||
| Collembola abundance | χ2 | 5.73 | 4.15 | 0.13 | 3.28 | 2.33 | 0.05 | 4.23 | |
| p-Value | ns | * | ns | ns | ns | ns | ns | ||
| Oribatida abundance | χ2 | 6.00 | 0.68 | 0.20 | 6.09 | 0.84 | 6.21 | 1.91 | |
| p-Value | * | ns | ns | * | ns | * | ns | ||
| Physico-chemical properties | Ctot (%) | χ2 | 0.55 | 0.68 | 1.98 | 2.83 | 0.80 | 7.04 | 1.64 |
| p-Value | ns | ns | ns | ns | ns | ** | ns | ||
| Ntot (%) | χ2 | 0.10 | 0.82 | 1.04 | 4.21 | 2.05 | 8.32 | 3.40 | |
| p-Value | ns | ns | ns | ns | ns | ** | ns | ||
| Ctot/Ntot | χ2 | 1.27 | 0.05 | 1.11 | 0.20 | 1.76 | 0.20 | 2.79 | |
| p-Value | ns | ns | ns | ns | ns | ns | ns | ||
| Corg (%) | χ2 | 5.91 | 1.00 | 0.73 | 13.92 | 6.04 | 3.99 | 4.43 | |
| p-Value | ns | ns | ns | *** | * | * | ns | ||
| CaCO3 (%) | χ2 | 31.43 | 2.78 | 0.15 | 8.27 | 0.36 | 0.79 | 0.54 | |
| p-Value | *** | ns | ns | * | ns | ns | ns | ||
| pH | χ2 | 12.36 | 11.17 | 7.43 | 2.08 | 5.51 | 2.05 | 0.84 | |
| p-Value | ** | *** | ** | ns | ns | ns | ns | ||
| Conductivity (μS cm−1) | χ2 | 1.63 | 0.08 | 0.37 | 0.77 | 0.69 | 1.07 | 0.54 | |
| p-Value | ns | ns | ns | ns | ns | ns | ns | ||
| Environmental data | Number of Q. pubescens stems /ha | χ2 | 21.90 | 2.20 | NA | 3.31 | NA | NA | NA |
| p-Value | *** | ns | ns | ||||||
| Understory vegetation (%) | χ2 | 1.81 | 2.20 | NA | 1.74 | NA | NA | NA | |
| p-Value | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcourt, N.; Dupuy, N.; Rébufa, C.; Aupic-Samain, A.; Foli, L.; Farnet-Da Silva, A.-M. Rapid Assessment of Land Use Legacy Effect on Forest Soils: A Case Study on Microarthropods Used as Indicators in Mediterranean Post-Agricultural Forests. Forests 2023, 14, 2223. https://doi.org/10.3390/f14112223
Delcourt N, Dupuy N, Rébufa C, Aupic-Samain A, Foli L, Farnet-Da Silva A-M. Rapid Assessment of Land Use Legacy Effect on Forest Soils: A Case Study on Microarthropods Used as Indicators in Mediterranean Post-Agricultural Forests. Forests. 2023; 14(11):2223. https://doi.org/10.3390/f14112223
Chicago/Turabian StyleDelcourt, Ninon, Nathalie Dupuy, Catherine Rébufa, Adriane Aupic-Samain, Lisa Foli, and Anne-Marie Farnet-Da Silva. 2023. "Rapid Assessment of Land Use Legacy Effect on Forest Soils: A Case Study on Microarthropods Used as Indicators in Mediterranean Post-Agricultural Forests" Forests 14, no. 11: 2223. https://doi.org/10.3390/f14112223
APA StyleDelcourt, N., Dupuy, N., Rébufa, C., Aupic-Samain, A., Foli, L., & Farnet-Da Silva, A.-M. (2023). Rapid Assessment of Land Use Legacy Effect on Forest Soils: A Case Study on Microarthropods Used as Indicators in Mediterranean Post-Agricultural Forests. Forests, 14(11), 2223. https://doi.org/10.3390/f14112223





