Study on the Extraction and Identification of DNA from Ten Dalbergia Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Pretreatment
2.1.1. Wood Powder Preparation
2.1.2. Rinsing Treatment
2.2. DNA Extraction
2.2.1. Modified Cetyltrimethylammonium Bromide (CTAB)
2.2.2. CTAB Plant Genome DNA Kit
2.2.3. DNeasy Plant Mini Kit
2.3. Statistical Analysis
2.4. PCR Amplification and Sequencing
2.5. ITS2 Secondary Structure Prediction and Phylogenetic Analysis
3. Results
3.1. Effect of Ammonium Acetate Gradient Concentrations on the Purity and Yield of Total Wood DNA
3.2. Effect of Rinse Frequency on the Purity and Yield of DNA from Wood
3.3. Effects of Extraction Methods on PCR Amplification
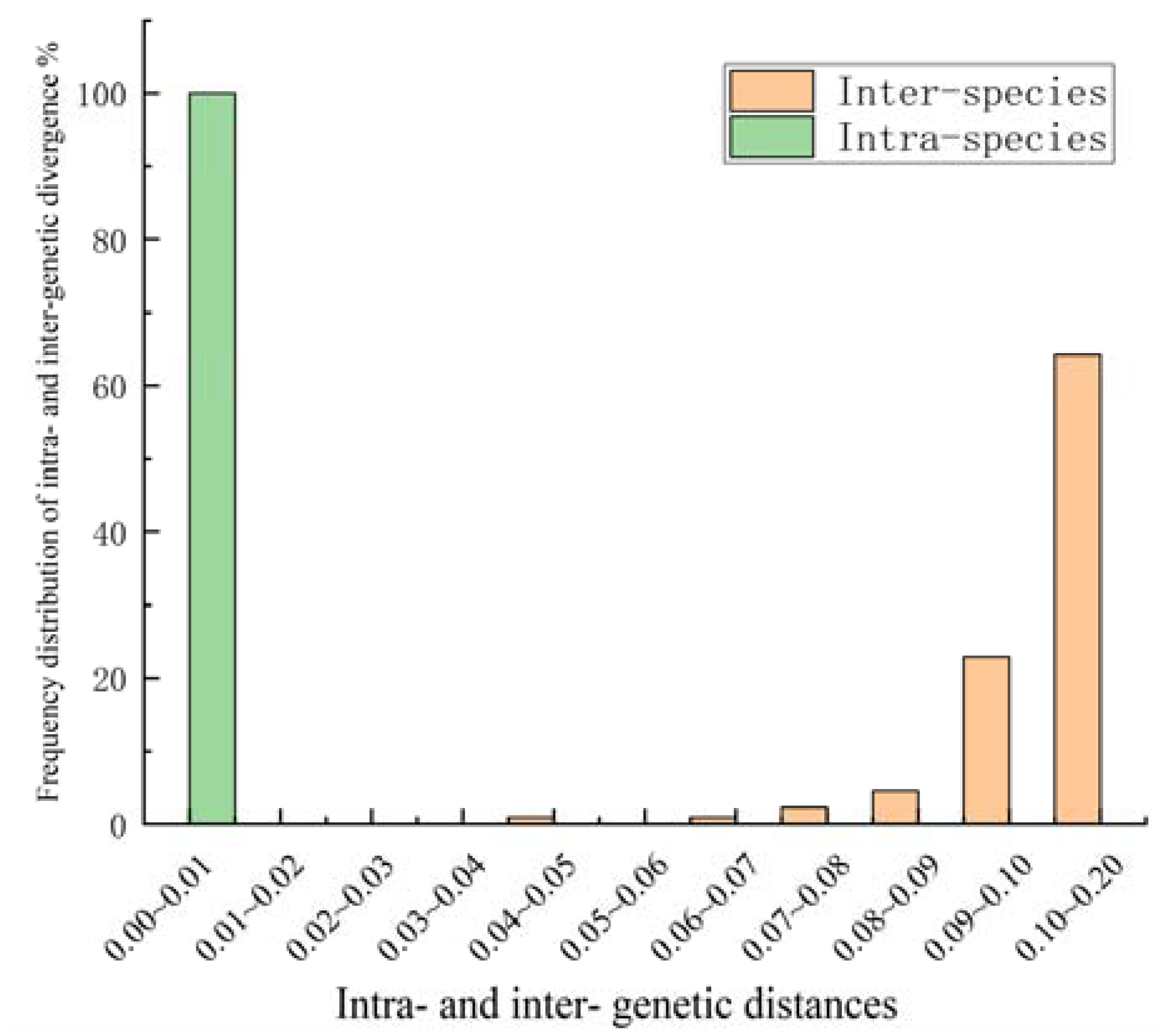
3.4. Sequence Length and Intra- and Inter-Species K2P Genetic Distance Analysis
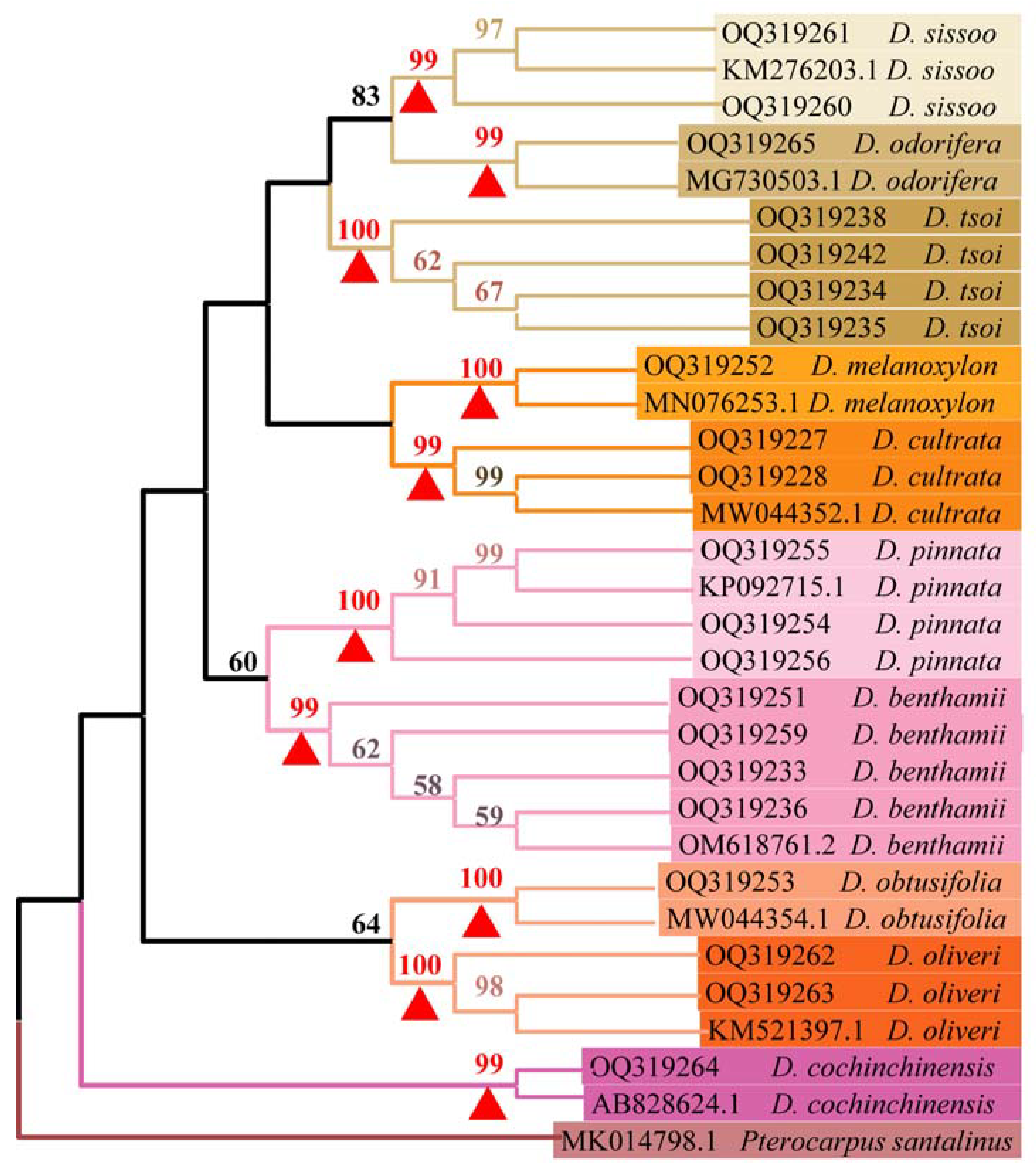
3.5. Phylogenetic Tree Analysis
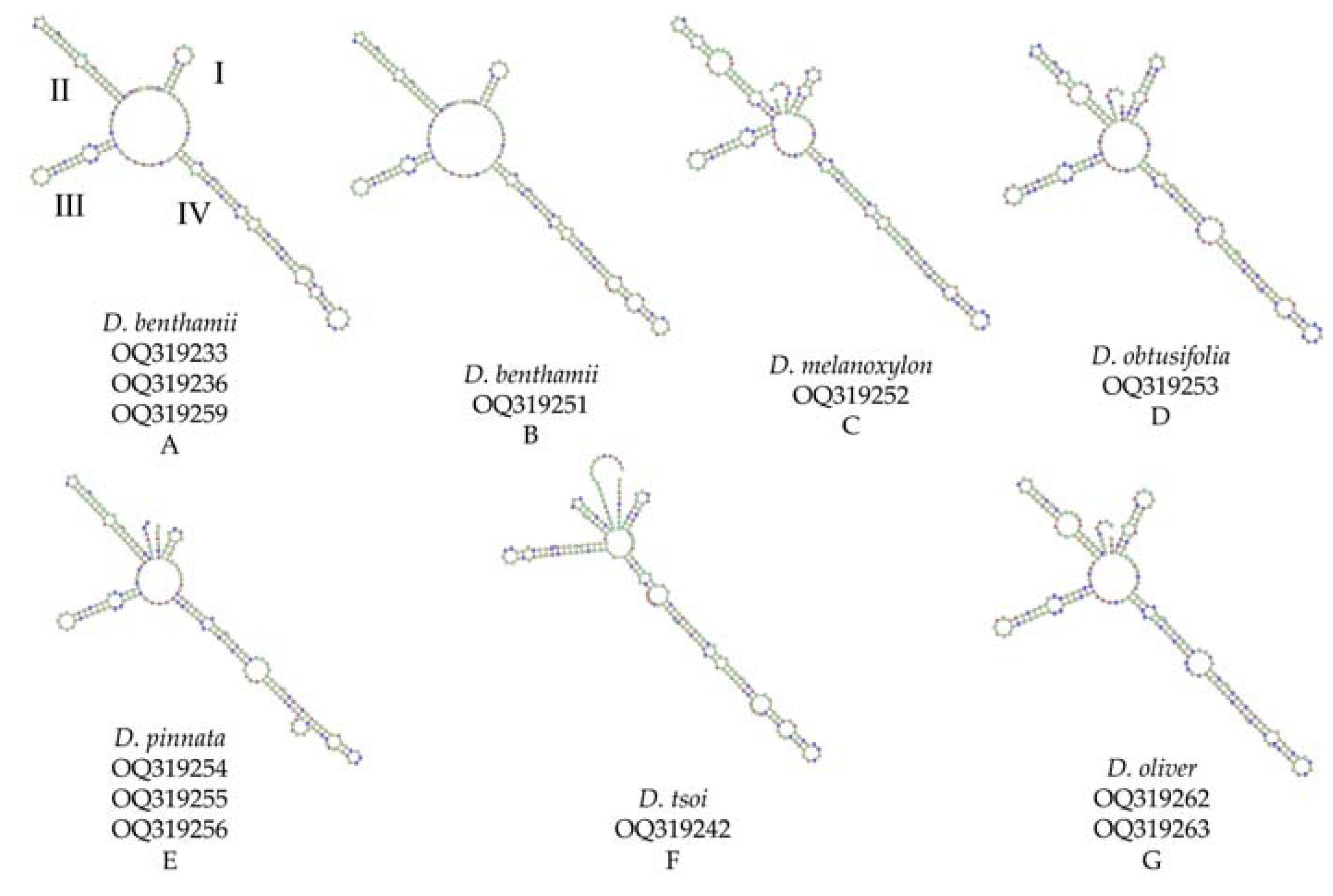
3.6. Secondary Structure Analysis
4. Discussion
4.1. Optimization of DNA Extraction Methods for Wood
4.2. Effect of Storage Time on the Quality of DNA Extracted from Wood
4.3. Effect of ITS2 Barcode Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassold, S.; Lowry, P.P.; Bauert, M.R.; Razafintsalama, A.; Ramamonjisoa, L.; Widmer, A. DNA barcoding of Malagasy rosewoods: Towards a molecular identification of CITES-listed Dalbergia species. PLoS ONE 2016, 11, e0157881. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Wong, K.H.; Kong, B.L.H.; Siu, T.Y.; But, G.W.C.; Tsang, S.S.K.; Lau, D.T.W.; Shaw, P.C. Comparative analysis of chloroplast genomes of Dalbergia species for identification and phylogenetic analysis. Plants 2022, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; He, W.; Liu, X.; Tembrock, L.R.; Wu, Z.; Xu, D. Comparative Analyses of 35 Complete Chloroplast Genomes from the Genus Dalbergia (Fabaceae) and the Identification of DNA Barcodes for Tracking Illegal Logging and Counterfeit Rosewood. Forests 2022, 13, 626. [Google Scholar] [CrossRef]
- Vasudeva, N.; Vats, M.; Sharma, S.; Sardana, S. Chemistry and biological activities of the genus Dalbergia—A review. Pharmacogn. Rev. 2009, 3, 307–319. [Google Scholar]
- Garzon Arbelaez, M. Rosewood Trade: A Case Study of the Imports into the United States. Master’s Thesis, University of Washington, Seattle, WA, USA, 2022. [Google Scholar]
- Crameri, S.; Wilding, N.; Phillipson, P.B.; Rakotonirina, N.; Randrianaivo, R.I.; Ravaomanalina, H. An integrative phylogenomic approach to species delimitation in Dalbergia precious woods. In Proceedings of the AETFAT Conference, Livingstone, Zambia, 27 June–1 July 2022. [Google Scholar]
- Deguilloux, M.F.; Pemonge, M.H.; Petit, R.J. Novel perspectives in wood certification and forensics: Dry wood as a source of DNA. Proc. Biol. Sci. 2002, 269, 1039–1046. [Google Scholar] [CrossRef]
- Lowe, A.J.; Cross, H.B. The application of DNA methods to timber tracking and origin verification. IAWA J. 2011, 32, 251–262. [Google Scholar] [CrossRef]
- Jiao, L.; Yin, Y.; Xiao, F.; Sun, Q.; Song, K. Comparative analysis of two DNA extraction protocols from fresh and dried wood of Cunninghamia lanceolata (Taxodiaceae). IAWA J. 2012, 33, 441–456. [Google Scholar] [CrossRef]
- Dormontt, E.E.; Boner, M.; Braun, B.; Breulmann, G.; Degen, B.; Espinoza, E. Forensic timber identification: It’s time to integrate disciplines to combat illegal logging. Biol. Conserv. 2015, 191, 790–798. [Google Scholar] [CrossRef]
- Nithaniyal, S.; Newmaster, S.G.; Ragupathy, S.; Krishnamoorthy, D.; Vassou, S.L.; Parani, M. DNA barcode authentication of wood samples of threatened and commercial timber trees within the tropical dry evergreen forest of India. PLoS ONE 2014, 9, e107669. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, M.; Wiedenhoeft, A.C.; He, T.; Li, J.; Liu, B. DNA Barcode Authentication and library development for the wood of six commercial pterocarpus species: The critical role of Xylarium specimens. Sci. Rep. 2018, 8, 1945. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Lu, Y.; He, T.; Guo, J.; Yin, Y. DNA barcoding for wood identification: Global review of the last decade and future perspective. IAWA J. 2020, 41, 620–643. [Google Scholar] [CrossRef]
- Murillo-Sánchez, I.E.; López-Albarrán, P.; Santoyo-Pizano, G.; Martínez-Pacheco, M.M.; Velázquez-Becerra, C. Molecular identification of timber species from sawn timber and roundwood. Conserv. Genet. Resour. 2021, 13, 191–200. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Cowan, R.S.; Chase, M.W.; Kress, W.J.; Savolainen, V. 300,000 Species to Identify: Problems, Progress, and Prospects in DNA Barcoding of Land Plants. Taxon 2006, 55, 611–616. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Little, D.P. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar]
- Chase, M.W.; Cowan, R.S.; Hollingsworth, P.M.; Berg, C.V.D.; Wilkinson, M.J. A proposal for a standardised protocol to barcode all land plants. Taxon 2007, 56, 295–299. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef]
- Li, Q.; Yan, H.; Lin, D.; Wang, Y.; He, M.; Zhang, W.; Gao, X.; Zhu, S. Molecular Identification of Three Aquilaria (Thymelaeaceae) Species through DNA Barcoding. Biol. Pharm. Bull. 2018, 41, 967. [Google Scholar] [CrossRef]
- Fazekas, A.J.; Burgess, K.S.; Kesanakurti, P.R.; Graham, S.W.; Newmaster, S.G.; Husband, B.C.; Percy, D.M.; Hajibabaei, M.; Barrett, S.C. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS ONE 2008, 3, e2802. [Google Scholar] [CrossRef]
- Li, D.Z.; Gao, L.M.; Li, H.T.; Wang, H.; Ge, X.J.; Liu, J.Q. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc. Natl. Acad. Sci. USA 2011, 108, 19641–19646. [Google Scholar]
- Yu, J.; Wu, X.; Liu, C.; Newmaster, S.; Ragupathy, S.; Kress, W.J. Progress in the use of DNA barcodes in the identification and classification of medicinal plants. Ecotoxicol. Environ. Saf. 2021, 208, 111691. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- Kalivas, A.; Ganopoulos, I.; Xanthopoulou, A.; Chatzopoulou, P.; Tsaftaris, A.; Madesis, P. DNA barcode ITS2 coupled with high resolution melting (HRM) analysis for taxonomic identification of Sideritis species growing in Greece. Mol. Biol. Rep. 2014, 41, 5147–5155. [Google Scholar] [CrossRef]
- Hartvig, I.; Czako, M.; Kjær, E.D.; Nielsen, L.R.; Theilade, I. The use of DNA barcoding in identification and conservation of rosewood (Dalbergia spp.). PLoS ONE 2015, 10, e0138231. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Ci, X.Q.; Liu, Z.F.; Dormontt, E.E.; Conran, J.G.; Lowe, A.J.; Li, J. Assessing candidate DNA barcodes for Chinese and internationally traded timber species. Mol. Ecol. Resour. 2022, 22, 1478–1492. [Google Scholar] [CrossRef]
- Semagn, K. Leaf Tissue Sampling and DNA Extraction Protocols. In Molecular Plant Taxonomy; Humana Press: Totowa, NJ, USA, 2014; Volume 1115, pp. 53–67. [Google Scholar]
- Kenzo, T.; Yoneda, R.; Tanaka-Oda, A.; Azani, M.A. Growth performance and leaf ecophysiological traits in three Aquilaria species in Malaysia. New For. 2018, 50, 699–715. [Google Scholar] [CrossRef]
- Rachmayanti, Y.; Leinemann, L.; Gailing, O.; Finkeldey, R. DNA from processed and unprocessed wood: Factors influencing the isolation success. Forensic Sci. Int. Genet. 2009, 3, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Verbylaite, R.; Beiys, P.; Rimas, V.; Kuusiene, S.J.B.F. Comparison of Ten DNA Extraction Protocols from Wood of European Aspen (Populus tremula L.). Balt. For. 2010, 16, 35–42. [Google Scholar]
- Yu, M.; Liu, K.; Zhou, L.; Zhao, L.; Liu, S. Testing three proposed DNA barcodes for the wood identification of Dalbergia odorifera T. Chen and Dalbergia tonkinensis Prain. Holzforschung 2016, 70, 127–136. [Google Scholar] [CrossRef]
- Lu, Y.; Jiao, L.; He, T.; Zhang, Y.; Jiang, X.; Yin, Y. An optimized DNA extraction protocol for wood DNA barcoding of Pterocarpus erinaceus. IAWA J. 2020, 41, 644–659. [Google Scholar] [CrossRef]
- Miller, D.; Bryant, J.; Madsen, E.; Ghiorse, W. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 1999, 65, 4715–4724. [Google Scholar] [CrossRef]
- Ihase, O.L.; Horn, R.; Anoliefo, G.O.; Eke, C.R.; Afolabi, A.S.; Asemota, O. Development of a method for DNA extraction from oil palm leaves and the effects of pH and ionic strength on nucleic acid quantification. J. Biol. Methods 2016, 3, e37. [Google Scholar] [CrossRef]
- Mehta, D.; Sivan, P.; Shah, D. Statistical in vitro model for upscaling biofilm of Chroococcidiopsis cubana by media optimization and its protocol for DNA extraction. Biosci. Biotechnol. Res. Commun. 2020, 13, 1962–1966. [Google Scholar] [CrossRef]
- Yu, N.; Wei, Y.L.; Zhang, X.; Zhu, N.; Wang, Y.L.; Zhu, Y.; Zhang, H.P.; Li, F.M.; Yang, L.; Sun, J.Q.; et al. Barcode ITS2: A useful tool for identifying Trachelospermum jasminoides and a good monitor for medicine market. Sci. Rep. 2017, 7, 5037. [Google Scholar] [CrossRef]
- Ya-Na, L.V.; Chun-Yong, Y.A.N.G.; Lin-Chun, S.H.I.; Zhang, Z.L.; An-Shun, X.U.; Zhang, L.X.; Xue-Lan, L.I.; Hai-Tao, L.I. Identification of medicinal plants within the Apocynaceae family using ITS2 and psbA-trnH barcodes. Chin. J. Nat. Med. 2020, 18, 594–605. [Google Scholar]
- Keller, A.; Schleicher, T.; Schultz, J.; Müller, T.; Dandekar, T.; Wolf, M. 5.8 S-28S rRNA interaction and HMM-based ITS2 annotation. Gene 2009, 430, 50–57. [Google Scholar] [CrossRef]
- Koetschan, C.; Förster, F.; Keller, A.; Schleicher, T.; Ruderisch, B.; Schwarz, R.; Müller, T.; Wolf, M.; Schultz, J. The ITS2 Database III—Sequences and structures for phylogeny. Nucleic Acids Res. 2010, 38 (Suppl. S1), D275–D279. [Google Scholar] [CrossRef]
- Čandek, K.; Kuntner, M. DNA barcoding gap: Reliable species identification over morphological and geographical scales. Mol. Ecol. Resour. 2015, 15, 268–277. [Google Scholar] [CrossRef]
- Maxam, A.M.; Gilbert, W. A new method for sequencing DNA. Proc. Natl. Acad. Sci. USA 1977, 74, 560–564. [Google Scholar] [CrossRef]
- Lev, O.; Ramil, V.; Aram, G.; Vladislav, S.; Sergey, K.; Anastasia, R. Prospects for DNA authentication in wine production monitoring. Foods Raw Mater. 2018, 6, 438–448. [Google Scholar]
- Konermann, L. Addressing a common misconception: Ammonium acetate as neutral pH “buffer” for native electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef]
- Frostegård, Å.S.A.; Courtois, S.; Ramisse, V.; Clerc, S.; Bernillon, D.; Le Gall, F.; Jeannin, P.; Nesme, X.; Simonet, P. Quantification of bias related to the extraction of DNA directly from soils. Appl. Environ. Microbiol. 1999, 65, 5409–5420. [Google Scholar] [CrossRef]
- Shi, S.R.; Cote, R.J.; Wu, L.; Liu, C.; Datar, R.; Shi, Y.; Liu, D.; Lim, H.; Taylor, C.R. DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: Heating under the influence of pH. J. Histochem. Cytochem. 2002, 50, 1005–1011. [Google Scholar] [CrossRef]
- Young, J.M.; Rawlence, N.J.; Weyrich, L.S.; Cooper, A. Limitations and recommendations for successful DNA extraction from forensic soil samples: A review. Sci. Justice 2014, 54, 238–244. [Google Scholar] [CrossRef]
- Dumolin-Lapègue, S.; Pemonge, M.-H.; Gielly, L.; Taberlet, P.; Petit, R.J. Amplification of oak DNA from ancient and modern wood. Mol. Ecol. 1999, 8, 2137–2140. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Lagane, F.; Seguin-Orlando, A.; Schubert, M.; Leroy, T.; Guichoux, E.; Chancerel, E.; Bech-Hebelstrup, I.; Bernard, V.; Billard, C.; et al. High-Throughput DNA sequencing of ancient wood. Mol. Ecol. 2018, 27, 1138–1154. [Google Scholar] [CrossRef]
- Qin, M.; Zhu, C.J.; Yang, J.B.; Vatanparast, M.; Schley, R.; Lai, Q.; Zhang, D.Y.; Tu, T.Y.; Klitgård, B.B.; Li, S.J.; et al. Comparative analysis of complete plastid genome reveals powerful barcode regions for identifying wood of Dalbergia odorifera and D. tonkinensis (Leguminosae). J. Syst. Evol. 2022, 60, 73–84. [Google Scholar] [CrossRef]

| Taxon | Sample ID | Location | Position | Total | Age (Years) |
|---|---|---|---|---|---|
| Dalbergia odorifera | HS | Hainan, China | sapwood | 3 | 1 |
| Dalbergia odorifera | HH | Hainan, China | heartwood | 33 | 2 |
| Dalbergia sissoo | BS | Yunnan, China | sapwood | 3 | 5 |
| Dalbergia sissoo | BH | Yunnan, China | heartwood | 3 | 5 |
| Dalbergia sissoo | YS | Yunnan, China | transition | 33 | 5 |
| Dalbergia oliveri | ES | Southeast Asia | sapwood | 3 | 5 |
| Dalbergia oliveri | EH | Southeast Asia | heartwood | 3 | 5 |
| Dalbergia cochinchinensis | JS | Southeast Asia | sapwood | 3 | 5 |
| Dalbergia cochinchinensis | JH | Southeast Asia | heartwood | 3 | 5 |
| Dalbergia cultrata | CS | Yunnan, China | sapwood | 3 | 5 |
| Dalbergia cultrata | CH | Yunnan, China | heartwood | 3 | 5 |
| Dalbergia benthamii | LH | Hainan, China | sapwood | 3 | 2 |
| Dalbergia benthamii | LS | Hainan, China | heartwood | 48 | 1 |
| Dalbergia benthamii | LA | Hainan, China | heartwood | 3 | 2 |
| Dalbergia benthamii | LB | Guangdong, China | heartwood | 3 | 2 |
| Dalbergia pinnata | PS | Hainan, China | sapwood | 3 | 2 |
| Dalbergia pinnata | PH | Hainan, China | heartwood | 3 | 2 |
| Dalbergia pinnata | PA | Hainan, China | heartwood | 3 | 2 |
| Dalbergia tsoi | TS | Hainan, China | sapwood | 3 | 2 |
| Dalbergia tsoi | TH | Hainan, China | heartwood | 3 | 2 |
| Dalbergia tsoi | TA | Hainan, China | heartwood | 3 | 2 |
| Dalbergia tsoi | TB | Hainan, China | heartwood | 3 | 2 |
| Dalbergia melanoxylon | MS | Africa | sapwood | 3 | 5 |
| Dalbergia melanoxylon | MH | Africa | heartwood | 3 | 5 |
| Dalbergia obtusifolia | IS | Guangxi, China | sapwood | 3 | 1 |
| DNA Extraction Method | OD260/280 | Yield/ (ng·mg−1) | ||||
|---|---|---|---|---|---|---|
| D. benthamii | D. odorifera | D. sissoo | D. benthamii | D. odorifera | D. sissoo | |
| DNeasy Plant Mini Kit | 1.65 (0.10) | 1.63 (0.15) | 1.21 (0.01) | 9.26 (2.47) | 17.16(1.12) | 7.33 (0.12) |
| CTAB Kit | 1.88 (0.02) | 1.81 (0.10) | 1.74 (0.04) | 29.36 (5.67) | 25.15 (10.54) | 17.95 (0.77) |
| Modified CTAB (0.25 M ammonium acetate) | 2.07(0.02) | 1.81(0.04) | 1.77(0.02) | 64.67 A (9.92) | 22.14 A (1.76) | 18.73 A (0.24) |
| Modified CTAB (0.5 M ammonium acetate) | 1.86 (0.08) | 1.89 (0.02) | 1.79 (0.08) | 15.11 B (2.67) | 20.36 AB (0.20) | 13.15 B (0.11) |
| Modified CTAB (1 M ammonium acetate) | 1.74 (0.24) | 1.88 (0.01) | 1.73 (0.02) | 5.34 C (1.05) | 19.29 B (1.40) | 11.63 C (0.12) |
| Modified CTAB (2 M ammonium acetate) | 1.85 (0.14) | 1.82 (0.11) | 1.73 (0.15) | 8.64 C (2.22) | 20.39 AB (0.81) | 12.25 C (0.84) |
| DNA Extraction Method | OD260/280 | Yield/ (ng·mg−1) | ||||
|---|---|---|---|---|---|---|
| D. benthamii | D. odorifera | D. sissoo | D. benthamii | D. odorifera | D. sissoo | |
| Modified CTAB (No rinsing treatment) | 1.66 (0.05) | 1.61 (0.05) | 1.50 (0.05) | 92.87 A (5.14) | 50.48 A (4.70) | 56.37 A (5.63) |
| Modified CTAB (Rinsing treatment 1 time) | 2.07 (0.03) | 1.80 (0.02) | 1.71 (0.04) | 75.29 B (5.16) | 26.34 B (4.04) | 19.83 B (3.90) |
| Modified CTAB (Rinsing treatment 2 times) | 2.06 (0.02) | 1.81 (0.04) | 1.77 (0.02) | 71.56 B (8.68) | 22.14 BC (1.77) | 18.97 B (0.46) |
| Modified CTAB (Rinsing treatment 3 times) | 2.05 (0.02) | 1.88 (0.02) | 1.83 (0.08) | 66.63 B (3.44) | 16.60 C (0.35) | 17.32 B (0.14) |
| Modified CTAB (Rinsing treatment 4 times) | 2.04 (0.03) | 1.80 (0.11) | 1.71 (0.12) | 62.92 B (8.69) | 17.60 C (0.69) | 15.09 B (2.27) |
| Type of Samples | Amplification Efficiency (%) | Sequencing Efficiency (%) |
|---|---|---|
| Sapwood | 100 | 100 |
| Heartwood | 80 | 80 |
| Heartwood (under 2 years) | 100 | 100 |
| Heartwood (more than 5 years) | 40 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, C.; He, H.; Qiu, J. Study on the Extraction and Identification of DNA from Ten Dalbergia Species. Forests 2023, 14, 2318. https://doi.org/10.3390/f14122318
Gan C, He H, Qiu J. Study on the Extraction and Identification of DNA from Ten Dalbergia Species. Forests. 2023; 14(12):2318. https://doi.org/10.3390/f14122318
Chicago/Turabian StyleGan, Changtao, Haishan He, and Jian Qiu. 2023. "Study on the Extraction and Identification of DNA from Ten Dalbergia Species" Forests 14, no. 12: 2318. https://doi.org/10.3390/f14122318




