Comparative Study on Blowfly-Derived DNA and Camera Trapping in Assessing Mammalian Diversity in Subtropical Forests
Abstract
:1. Introduction
2. Materials and Methods
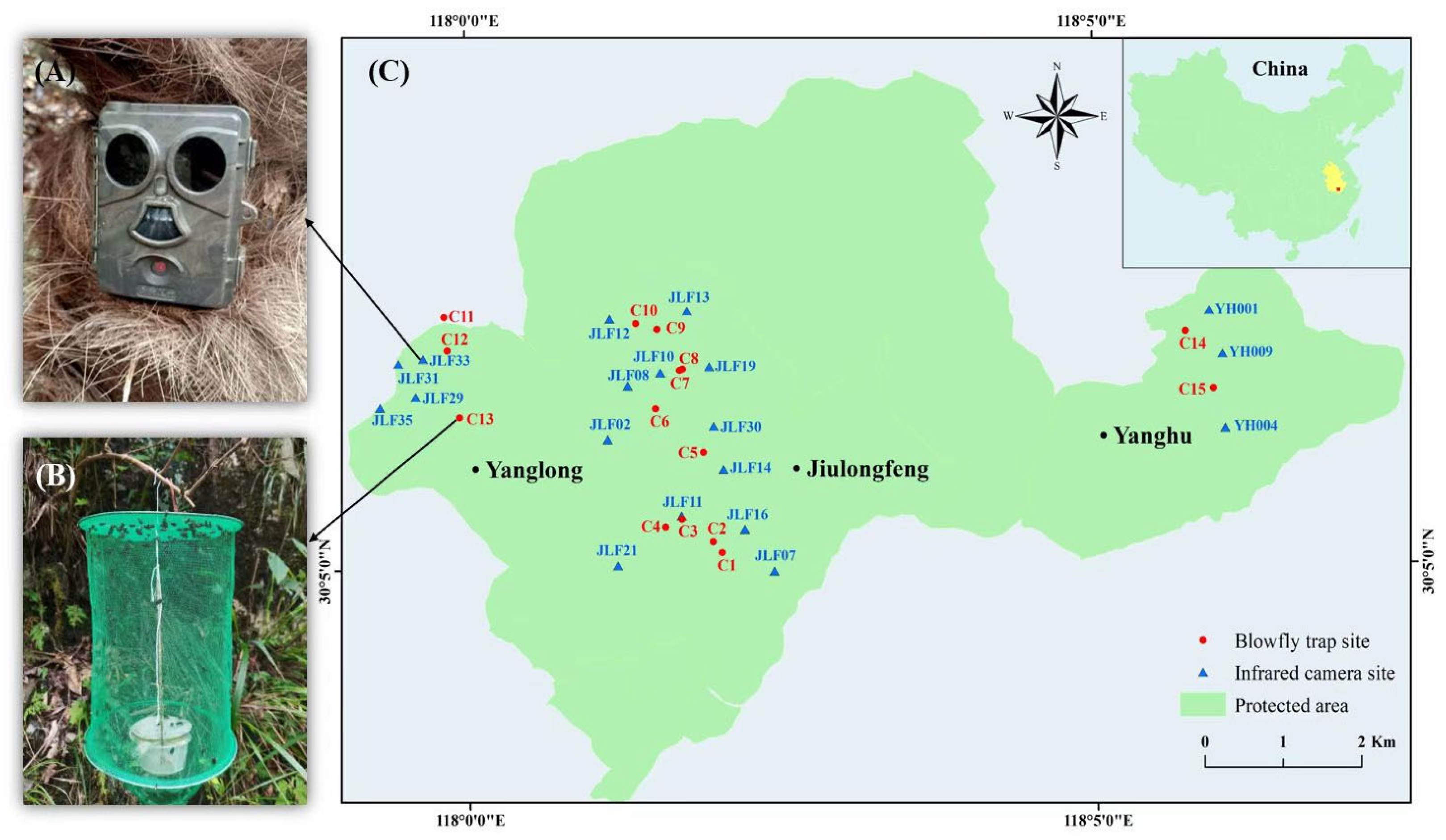
2.1. Study Sites
2.2. Field Survey with Infrared Camera
2.2.1. Infrared Camera Trapping
2.2.2. Blowfly Trapping and Identification
2.3. Identification and Analysis of Mammalian Species from the Blowfly-Derived DNA
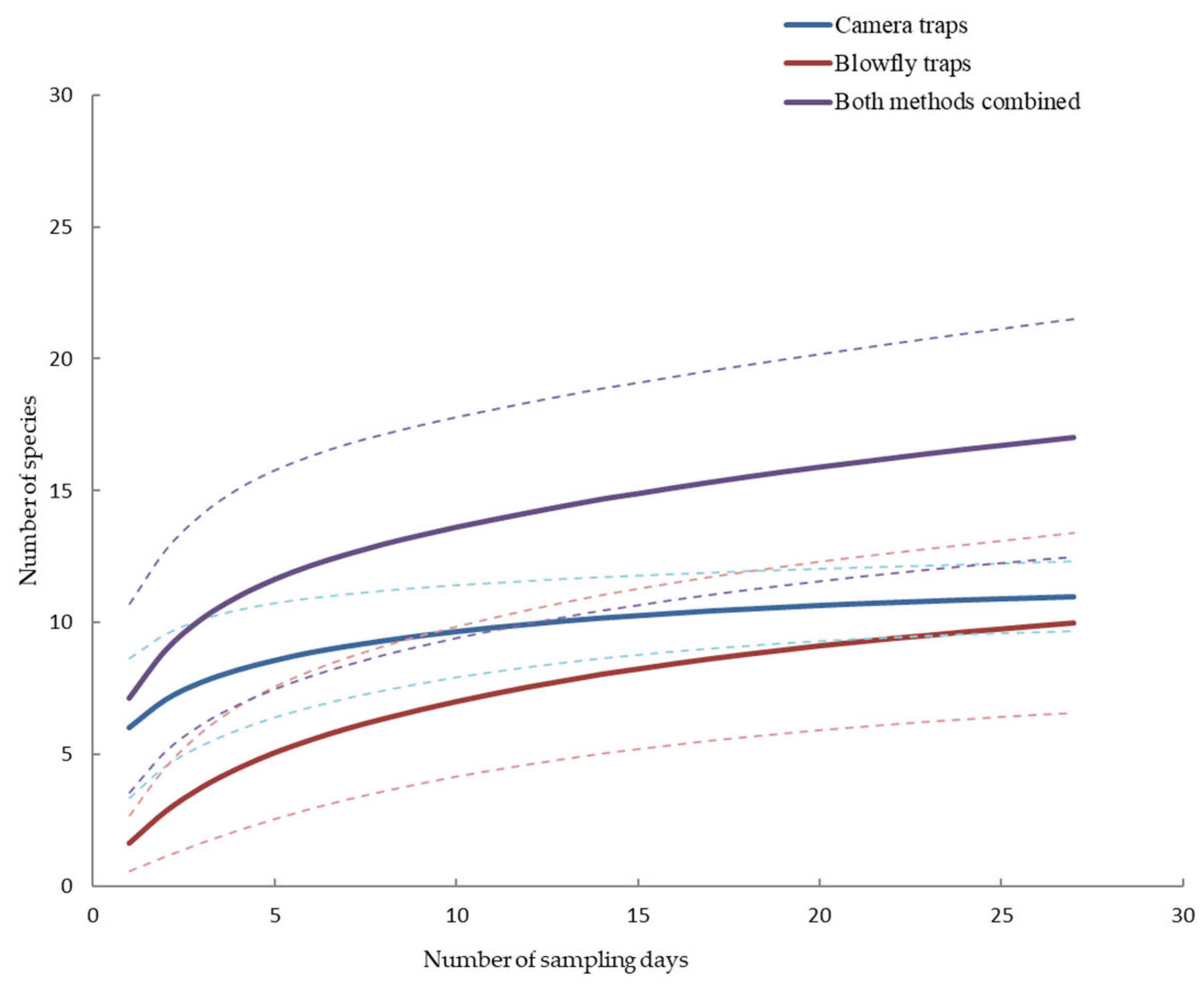
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, C.; Liu, Z.; Tian, J.; Ma, Q. Urban expansion dynamics and natural habitat loss in China: A multiscale landscape perspective. Glob. Chang. Biol. 2014, 20, 2886–2902. [Google Scholar] [CrossRef]
- Wilson, M.C.; Chen, X.Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jiang, L. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Beaudrot, L.; Ahumada, J.A.; O’Brien, T.; Alvarez-Loayza, P.; Boekee, K.; Campos-Arceiz, A.; Eichberg, D.; Espinosa, S.; Fegraus, E.; Fletcher, C. Standardized Assessment of Biodiversity Trends in Tropical Forest Protected Areas: The End Is Not in Sight. PLoS Biol. 2016, 14, e1002357. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; WWF: Gland, Switzerland, 2020. [Google Scholar]
- Hawkins, B.A.; Diniz-Filho, J.A.F.; Jaramillo, C.A.; Soeller, S.A. Post-Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. J. Biogeogr. 2006, 33, 770–780. [Google Scholar] [CrossRef]
- Schipper, J.; Chanson, J.S.; Chiozza, F.; Cox, N.A.; Hoffmann, M.; Katariya, V.; Lamoreux, J.; Rodrigues, A.S.; Stuart, S.N.; Temple, H.J. The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science 2008, 322, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.F.; Hoerig, L.A.; Brozovic, R.; Axtner, J.; Crampton-Platt, A.; Mohamed, A.; Wong, S.T.; Sollmann, R.; Yu, D.W.; Wilting, A. Shifting up a gear with iDNA: From mammal detection events to standardized surveys. Cold Spring Harb. Lab. 2019, 56, 1637–1648. [Google Scholar] [CrossRef]
- Burton, A.C.; Neilson, E.; Moreira, D.; Ladle, A.; Steenweg, R.; Fisher, J.T.; Bayne, E.; Boutin, S.; Stephens, P. REVIEW: Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 2015, 52, 675–685. [Google Scholar] [CrossRef]
- Nichols, J.D.; O’Connell, A.F.; Karanth, K.U. Camera Traps in Animal Ecology and Conservation: What’s Next? Springer: Tokyo, Japan, 2011; pp. 253–263. [Google Scholar]
- Trolliet, F.; Huynen, M.C.; Vermeulen, C.; Hambuckers, A. Use of camera traps for wildlife studies. A review. Biotechnol. Agron. Soc. Et Environ. 2014, 18, 446–454. [Google Scholar]
- Liu, X.; Wu, P.; He, X.; Zhao, X. Application and data mining of infra-red camera in the monitoring of species. Biodivers. Sci. 2018, 26, 850–861. [Google Scholar] [CrossRef]
- Ahumada, J.A.; Silva, C.E.; Gajapersad, K.; Hallam, C.; Hurtado, J.; Martin, E.; McWilliam, A.; Mugerwa, B.; O’Brien, T.; Rovero, F. Community structure and diversity of tropical forest mammals: Data from a global camera trap network. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2703–2711. [Google Scholar] [CrossRef]
- Li, X.; Bleisch, W.V.; Jiang, X. Using large spatial scale camera trap data and hierarchical occupancy models to evaluate species richness and occupancy of rare and elusive wildlife communities in southwest China. Divers. Distrib. 2018, 24, 1560–1572. [Google Scholar] [CrossRef]
- Roberts, N.J. Investigation into survey techniques of large mammals: Surveyor competence and camera-trapping vs. transect-sampling. Biosci. Horiz. 2011, 4, 40–49. [Google Scholar] [CrossRef]
- Guschanski, K.; Vigilant, L.; McNeilage, A.; Gray, M.; Kagoda, E.; Robbins, M.M. Counting elusive animals: Comparing field and genetic census of the entire mountain gorilla population of Bwindi Impenetrable National Park, Uganda. Biol. Conserv. 2009, 142, 290–300. [Google Scholar] [CrossRef]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Calvignac-Spencer, S.; Merkel, K.; Kutzner, N.; Kühl, H.; Boesch, C.; Kappeler, P.M.; Metzger, S.; Schubert, G.; Leendertz, F.H. Carrion fly-derived DNA as a tool for comprehensive and cost-effective assessment of mammalian biodiversity. Mol. Ecol. 2013, 22, 915–924. [Google Scholar] [CrossRef]
- Drinkwater, R.; Jucker, T.; Potter, J.H.; Swinfield, T.; Coomes, D.A.; Slade, E.M.; Gilbert, M.T.P.; Lewis, O.T.; Bernard, H.; Struebig, M.J. Leech blood-meal invertebrate-derived DNA reveals differences in Bornean mammal diversity across habitats. Mol. Ecol. 2021, 30, 3299–3312. [Google Scholar] [CrossRef] [PubMed]
- Gogarten, J.F.; Hoffmann, C.; Arandjelovic, M.; Sachse, A.; Merkel, K.; Dieguez, P.; Agbor, A.; Angedakin, S.; Brazzola, G.; Jones, S. Fly-derived DNA and camera traps are complementary tools for assessing mammalian biodiversity. Environ. DNA 2020, 2, 63–76. [Google Scholar] [CrossRef]
- Kocher, A.; de Thoisy, B.; Catzeflis, F.; Valière, S.; Bañuls, A.L.; Murienne, J. iDNA screening: Disease vectors as vertebrate samplers. Mol. Ecol. 2017, 26, 6478–6486. [Google Scholar] [CrossRef]
- Lee, P.-S.; Gan, H.M.; Clements, G.R.; Wilson, J.-J. Field calibration of blowfly-derived DNA against traditional methods for assessing mammal diversity in tropical forests. Genome 2016, 59, 1008–1022. [Google Scholar] [CrossRef]
- Schnell, I.B.; Sollmann, R.; Calvignac-Spencer, S.; Siddall, M.E.; Yu, D.W.; Wilting, A.; Gilbert, M.T. iDNA from terrestrial haematophagous leeches as a wildlife surveying and monitoring tool-prospects, pitfalls and avenues to be developed. Front. Zool. 2015, 12, 1–14. [Google Scholar] [CrossRef]
- Schnell, I.B.; Thomsen, P.F.; Wilkinson, N.; Rasmussen, M.; Jensen, L.R.; Willerslev, E.; Bertelsen, M.F.; Gilbert, M.T.P. Screening mammal biodiversity using DNA from leeches. Curr. Biol. 2012, 22, 262–263. [Google Scholar] [CrossRef]
- Logue, K.; Keven, J.B.; Cannon, M.V.; Reimer, L.; Siba, P.; Walker, E.D.; Zimmerman, P.A.; Serre, D. Unbiased characterization of Anopheles mosquito blood meals by targeted high-throughput sequencing. PLoS Neglected Trop. Dis. 2016, 10, e0004512. [Google Scholar] [CrossRef] [PubMed]
- Gillett, C.P.; Johnson, A.J.; Barr, I.; Hulcr, J. Metagenomic sequencing of dung beetle intestinal contents directly detects and identifies mammalian fauna. BioRxiv 2016, 12, 074849. [Google Scholar]
- Calvignac-Spencer, S.; Leendertz, F.H.; Gilbert, M.T.P.; Schubert, G. An invertebrate stomach’s view on vertebrate ecology: Certain invertebrates could be used as “vertebrate samplers” and deliver DNA-based information on many aspects of vertebrate ecology. BioEssays 2013, 35, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-S.; Sing, K.-W.; Wilson, J.-J. Reading mammal diversity from flies: The persistence period of amplifiable mammal mtDNA in blowfly guts (Chrysomya megacephala) and a new DNA mini-barcode target. PLoS ONE 2015, 10, e0123871. [Google Scholar] [CrossRef] [PubMed]
- Schubert, G.; Stockhausen, M.; Hoffmann, C.; Merkel, K.; Vigilant, L.; Leendertz, F.H.; Calvignac-Spencer, S. Targeted detection of mammalian species using carrion fly-derived DNA. Mol. Ecol. Resour. 2015, 15, 285–294. [Google Scholar] [CrossRef]
- Carvalho, C.S.; De Oliveira, M.E.; Rodriguez-Castro, K.G.; Saranholi, B.H.; Galetti, P.M., Jr. Efficiency of eDNA and iDNA in assessing vertebrate diversity and its abundance. Mol. Ecol. Resour. 2022, 22, 1262–1273. [Google Scholar] [CrossRef]
- Rodgers, T.W.; Xu, C.C.; Giacalone, J.; Kapheim, K.M.; Saltonstall, K.; Vargas, M.; Yu, D.W.; Somervuo, P.; McMillan, W.O.; Jansen, P.A. Carrion fly-derived DNA metabarcoding is an effective tool for mammal surveys: Evidence from a known tropical mammal community. Mol. Ecol. Resour. 2017, 17, 133–145. [Google Scholar] [CrossRef]
- Ji, Y.; Baker, C.C.; Popescu, V.D.; Wang, J.; Wu, C.; Wang, Z.; Li, Y.; Wang, L.; Hua, C.; Yang, Z. Measuring protected-area effectiveness using vertebrate distributions from leech iDNA. Nat. Commun. 2022, 13, 1555. [Google Scholar] [CrossRef]
- Massey, A.L.; Bronzoni, R.V.d.M.; da Silva, D.J.F.; Allen, J.M.; de Lázari, P.R.; dos Santos-Filho, M.; Canale, G.R.; Bernardo, C.S.S.; Peres, C.A.; Levi, T. Invertebrates for vertebrate biodiversity monitoring: Comparisons using three insect taxa as iDNA samplers. Mol. Ecol. Resour. 2022, 22, 962–977. [Google Scholar] [CrossRef]
- Cutajar, T.P.; Pulsford, S.A. Incidental invertebrate-derived DNA detection of invasive and threatened species in temperate dry Southeast Australian forest. Austral Ecol. 2023, 48, 774–786. [Google Scholar] [CrossRef]
- Chen, L.; Wu, L.; Liu, Y.; Xu, H. Application of environmental DNA metabarcoding in ecology. Acta Ecol. Sin. 2016, 36, 4573–4582. [Google Scholar]
- Cheng, F.-Y.; Cheng, Q.-Q.; Cheng, L.-H.; Chen, M.-Y.; Cheng, H.-J.; Yan, Y.-Z. Age determination of Phoxinus oxycephalus from the Jiulong Peak of the Huangshan Mountain. Chin. J. Ecol. 2014, 33, 2108. [Google Scholar]
- Yearly Statistics in Huangshan. Available online: https://tjj.huangshan.gov.cn (accessed on 12 July 2023).
- Wu, X.B. Comprehensive Investigation Report of Jiulongfeng Provincial Nature Reserve, Huangshan District, Anhui Province; Forestry Department of Jiulongfeng Nature Reserve of the Huangshan Mountain: Wuhu, China, 2019. [Google Scholar]
- Wang, C.G.; Cao, X.H.; Cao, Q.P.; Yan, L.; Wu, X.B. Application of the passive infrared sensor camera technology in monitoring the vertebrate diversity—A case study of Anhui Jiulongfeng Provincial Nature Reserve. Anhui For. Sci. Technol. 2015, 41, 5–8. [Google Scholar]
- Harmsen, B.J.; Foster, R.J.; Silver, S.; Ostro, L.; Doncaster, C.P. Differential use of trails by forest mammals and the implications for camera-trap studies: A case study from Belize. Biotropica 2010, 42, 126–133. [Google Scholar] [CrossRef]
- Tanwar, K.S.; Sadhu, A.; Jhala, Y.V. Camera trap placement for evaluating species richness, abundance, and activity. Sci. Rep. 2021, 11, 23050. [Google Scholar] [CrossRef]
- Smith, A.T.; Xie, Y.; Hoffmann, R.S.; Lunde, D.; MacKinnon, J.; Wilson, D.E.; Wozencraft, W.C. A Guide to the Mammals of China; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Kurahashi, H. Blow flies (Insecta: Diptera: Calliphoridae) of Malaysia and Singapore. Raffles Bull. Zool. 1997, 5, 1–88. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Zeale, M.R.; Butlin, R.K.; Barker, G.L.; Lees, D.C.; Jones, G. Taxon-specific PCR for DNA barcoding arthropod prey in bat faeces. Mol. Ecol. Resour. 2011, 11, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9.1.0. Available online: http://purl.oclc.org/estimates (accessed on 10 December 2022).
- Soberón, J.M.; Llorente, J.B.; Oñate, L. The use of specimen-label databases for conservation purposes: An example using Mexican Papilionid and Pierid butterflies. Biodivers. Conserv. 2000, 9, 1441–1466. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Leite Pitman, R.; Mares, R.; Powell, G. An evaluation of camera traps for inventorying large-and medium-sized terrestrial rainforest mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Deagle, B.E.; Jarman, S.N.; Coissac, E.; Pompanon, F.; Taberlet, P. DNA metabarcoding and the cytochrome c oxidase subunit I marker: Not a perfect match. Biol. Lett. 2014, 10, 20140562. [Google Scholar] [CrossRef] [PubMed]
- Geier, A.R.; Best, L.B. Habitat selection by small mammals of riparian communities: Evaluating effects of habitat alterations. J. Wildl. Manag. 1980, 44, 16–24. [Google Scholar] [CrossRef]
- Goulart, F.V.B.; Cáceres, N.C.; Graipel, M.E.; Tortato, M.A.; Ghizoni, I.R., Jr.; Oliveira-Santos, L.G.R. Habitat selection by large mammals in a southern Brazilian Atlantic Forest. Mamm. Biol. 2009, 74, 182–190. [Google Scholar] [CrossRef]
- Lee, P.S.; Dong, M.H.; Yan, X.L.; He, T.Y.; Yu, S.F.; Wee, S.L.; Wilson, J.J. Blowfly-derived mammal DNA as mammal diversity assessment tool: Determination of dispersal activity and flight range of tropical blowflies. Biodivers. Data J. 2023, 11, e108438. [Google Scholar] [CrossRef] [PubMed]


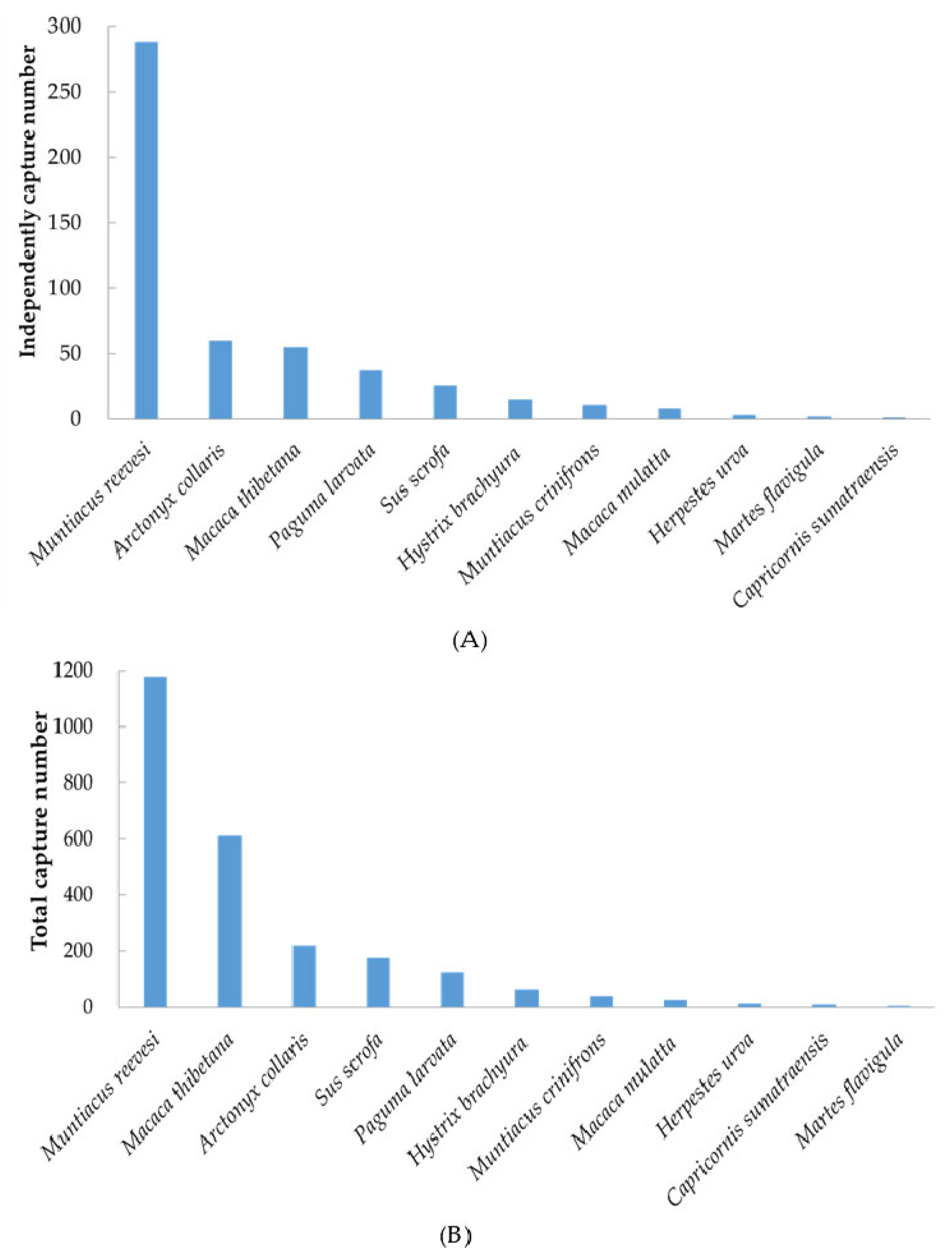

| Species | IUCN Species Red List | Key National Protection Level | CSRL Red List of Chinese Species | Number of Photos Captured | Camera Trap Detection | Blowfly-Derived DNA |
|---|---|---|---|---|---|---|
| ARTIODACTYLA | ||||||
| Bovidae | ||||||
| Capricornis sumatraensis | VU | II | VU | 10 | √ | X |
| Cervidae | ||||||
| Muntiacus crinifrons | VU | I | EN | 40 | √ | X |
| Muntiacus reevesi | LC | - | VU | 1179 | √ | X |
| Suidae | ||||||
| Sus scrofa | LC | - | LC | 176 | √ | √ |
| CARNIVORA Mustelidae | ||||||
| Arctonyx collaris | NT | - | NT | 189 | √ | X |
| Martes flavigula | LC | - | - | 5 | √ | X |
| Melogale moschata | LC | - | NT | X | √ | |
| Viverridae | ||||||
| Herpestes urva | LC | - | NT | 14 | √ | X |
| Paguma larvata | LC | - | NT | 125 | √ | √ |
| CHIROPTERA | ||||||
| Vespertilionidae | ||||||
| Eptesicus serotinus | LC | - | LC | X | √ | |
| INSECTIVORA | ||||||
| Erinaceidae | ||||||
| Mesechinus sp. | - | - | X | √ | ||
| Soricidae | ||||||
| Crocidura attenuata | LC | - | LC | X | √ | |
| PRIMATES | ||||||
| Cercopithecidae | ||||||
| Macaca mulatta | LC | II | LC | 27 | √ | √ |
| Macaca thibetana huangshanensis | NT | II | VU | 612 | √ | √ |
| RODENTIA | ||||||
| Hystricidae | ||||||
| Hystrix brachyura | LC | II | LC | 65 | √ | X |
| Muridae | 66 | √ | ||||
| Leopoldamys edwardsi | LC | - | LC | X | √ | |
| Niviventer niviventer | LC | - | - | X | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.; He, T.; Dong, M.; Huang, Q.; Zhou, X.; Liao, J.; Chen, X.; Wu, X.; Wee, S.-L.; Chen, J. Comparative Study on Blowfly-Derived DNA and Camera Trapping in Assessing Mammalian Diversity in Subtropical Forests. Forests 2023, 14, 2180. https://doi.org/10.3390/f14112180
Lee P, He T, Dong M, Huang Q, Zhou X, Liao J, Chen X, Wu X, Wee S-L, Chen J. Comparative Study on Blowfly-Derived DNA and Camera Trapping in Assessing Mammalian Diversity in Subtropical Forests. Forests. 2023; 14(11):2180. https://doi.org/10.3390/f14112180
Chicago/Turabian StyleLee, Pingshin, Tianyi He, Minhui Dong, Qiang Huang, Xiang Zhou, Jun Liao, Xiaochun Chen, Xiaobing Wu, Suk-Ling Wee, and Jinmin Chen. 2023. "Comparative Study on Blowfly-Derived DNA and Camera Trapping in Assessing Mammalian Diversity in Subtropical Forests" Forests 14, no. 11: 2180. https://doi.org/10.3390/f14112180
APA StyleLee, P., He, T., Dong, M., Huang, Q., Zhou, X., Liao, J., Chen, X., Wu, X., Wee, S.-L., & Chen, J. (2023). Comparative Study on Blowfly-Derived DNA and Camera Trapping in Assessing Mammalian Diversity in Subtropical Forests. Forests, 14(11), 2180. https://doi.org/10.3390/f14112180





