Abstract
The tea plant is a vital strategic forest resource in China. Dark tea produced from its leaves is an indispensable health-promoting product in western China due to its unique lipid-lowering function. Eurotium cristatum is the dominant strain in Fuzhuan brick tea (a variety of Anhua dark tea) and could produce many functional components, including lovastatin, a lipid-lowering compound. In this study, the lovastatin yield of dark tea was improved by breeding Eurotium cristatum using the protoplast fusion method. The experiments were carried out by inducing a fusion between inactivated Eurotium cristatum JH1205 and Monascus CICC5031. Among the 92 fusants screened the HPLC method, four strains (A4, A36, A54, and A76) with higher lovastatin production (more than three times as high) were obtained. The A76 strain had the highest lovastatin yield, which was 23.93 μg/mL. The location of the tea forest strongly influenced the lovastatin yield of loose dark tea. The strain bred in this study improved the lovastatin yield of loose dark tea by more than three times when compared to wild Eurotium cristatum. These results are promising for the development of tea forest resources.
1. Introduction
The beverage known as tea holds a prominent position in Chinese culture. Tea consumption in China dates back approximately 5000 years, and today, China stands as the world’s foremost tea producer. Originally, tea was crafted from the leaves of the Camellia sinensis L. plant species [1,2,3]. People make tea by steeping tea leaves in hot water. In addition to Camellia sinensis L., Chinese people also drink water with pieces of leaves from other plants (Eucommia ulmoides Oliver, Morus alba), flowers (honeysuckle, Rosa spp., chrysanthemum), and fruit (lemon, Matrimony vine, sterculia scaphigera), which is known as specialty tea, scented tea, and fruit tea, respectively [4,5,6]. In China, vast forests harbor a variety of plant species. One essential forest product is tea, which can be produced by harvesting leaves, flowers, and fruit from various plants grown in these forests. The economic benefits of tea production using forest plants far surpass those of forest logging. Tea, specifically Camellia sinensis L., has become a prominent forest crop with a significant economic role in China. According to statistics from the International Tea Commission, the global tea output in 2020 reached 6.269 million tons, with China contributing a substantial 2.986 million tons, constituting 47.6% of the global total. Also, tea products are an important part of the forestry industry in poor mountainous areas of China.
The Camellia sinensis L. plant is a significant forest economic resource due to the high-value tea products made from its leaves [7,8]. There are six categories of tea in China, based on their manufacturing processes and special characteristics, namely green tea, white tea, yellow tea, oolong tea, black tea, and dark tea. Dark tea is a post-fermented tea with increasing popularity due to its potential health benefits. Its health benefits include antioxidant activity, antiobesity activity, antidiabetic activity, cardiovascular protective activity, and so on. In addition, researchers have found that in diet-induced obese animals, body weight gain and fat accumulation can be prevented by feeding them dark tea extracts (pu-erh and Fuzhuan brick tea) or components [9,10,11,12,13]. Moreover, extracts from Eurotium cristatum have been found to exhibit hypolipidemic effects [14,15]. However, the potential health benefits of dark tea, including its lipid-lowering effect, are strongly influenced by microorganism species [16,17]. Hence, an investigation of the effect of fungi on the quality of dark tea could provide insights into the development of new dark teas with various health benefits and lay the foundation for the efficient use of tea forest resources in China [18,19].
Chinese Fuzhuan brick tea is one of the most significant and unique varieties of dark tea due to its manufacturing process and dominant fungus. Eurotium cristatum is the dominant fungus involved in the production of Chinese Fuzhuan brick tea [20,21,22,23,24,25]. The sensory properties and health benefits of Fuzhuan brick tea mainly depend on the transformation of the tea leaf constituents by their dominant fungus. Hence, the content and metabolites of Eurotium cristatum are essential for evaluating the quality of Fuzhuan brick tea. The lipid-lowering effect and antiobesity activity of dark tea are related to its polysaccharides, polyphenols, amino acids, and some active small-molecule substances, such as lovastatin (<20 μg/g) [6]. Lovastatin, a widely used lipid-lowering drug [26,27,28], has been detected in the fermentation broth of Eurotium cristatum. Lovastatin is the principal active substance in Monascus spp. and Aspergillus terreus fermentation products [29,30]. As a result, the biosynthetic pathway and gene cluster composition of lovastatin have been thoroughly studied in Monascus spp. and Aspergillus terreus. Also, other aspergillus species, such as Eurotium cristatum, Aspergillus niger, Aspergillus terreus, Aspergillus flavus, etc., can produce lovastatin [31,32]. However, the yield of lovastatin in Eurotium cristatum is very low [33,34,35,36]. For instance, a small quantity of lovastatin (<10 μg/mL) is produced by wild Eurotium cristatum [12]. Therefore, the breeding of Eurotium cristatum is needed to enhance the yield of lovastatin, thereby increasing the hypolipidemic effects of Fuzhuan brick tea.
Protoplast fusion is an effective way to construct genetically engineered strains [37]. It is also frequently used in microorganism breeding for the large-scale enhancement of metabolite yields. After protoplast fusion, a high strain recombination frequency commonly occurs due to the two genomes coming from their parents [38]. In order to improve the success rate of recombinant separation, parental protoplasts are inactivated through physical or chemical methods [39,40]. A higher inactivation rate could also increase the recombination frequency. For instance, a two- to ten-fold rate increase can be obtained when the survival rate of parental protoplasts is 0.1%.
Therefore, this study induced a protoplast fusion between Eurotium cristatum JH1205 and Monascus CICC5031 (lovastatin production > 100 μg/mL) to produce breeding recombinants in order to improve the yield of lovastatin in Eurotium cristatum strains and increase the antiobesity activity of loose dark tea. A 99% inactivation ratio of parental protoplasts was used for screening for potential fusants. The influence of the fermentation conditions on lovastatin production and that of the source of tea samples on the lovastatin concentration in loose dark tea were also investigated. Furthermore, the organs responsible for lovastatin production in four fusants and Eurotium cristatum JH1205 were studied.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
Eurotium cristatum JH1205 was separated from dark tea and saved at the Hunan Provincial Key Laboratory of Dark Tea and Jin-hua (Yiyang, China). Monascus CICC5031 was obtained from the China General Microbiological Culture Collection Center (Beijing, China).
Eurotium cristatum JH1205 and Monascus CICC5031 were maintained on a potato dextrose agar (PDA) medium. The seed medium is a PDA medium containing an additional 10% sucrose, while the lovastatin production medium is a PDA medium containing an additional 5% sucrose. The rotation speed and temperature were 120 r/min and 28 °C, respectively.
2.2. Production of Protoplasts from Eurotium cristatum and Monascus spp.
The inoculum of Eurotium cristatum JH1205 and Monascus CICC5031 was prepared by separating the spores from a PDA solid medium. Then, 1 mL of spore (the concentration was 107/mL) was added to 100 mL of a PDA fluid medium and cultured at 28 °C with a rotation speed of 100 rpm. After 6 days, the fermentation broth was centrifuged for 20 min at 3000 rpm and 4 °C. The liquid supernatant was discarded, and the sediment was washed three times with 0.1 M of phosphate buffer (pH 6.6). The washed wet mycelia was collected by passage through 4 layers of degreased gauze. The collected mycelia (3 g) were treated with 2.0% lywallzyme and 0.5% snailase in 0.1 M of phosphate buffer and incubated in a water bath shaker (100 rpm) at room temperature. After 3 h, all treated cells were filtered through sterile cotton, washed with phosphate buffer, and centrifuged at 500 rpm. The obtained protoplasts were washed three times with phosphate buffer and immediately resuspended in the osmotically stabilized buffer. The age of mycelia (4 to 12 days) and the concentration of lywallzyme and snailase (Table 1) were optimized for protoplast production. The concentration of the protoplasts was determined using a blood-counting chamber. The protoplasts were observed through a microscope (×400).

Table 1.
Concentration of enzyme.
2.3. Protoplast Fusion and Regeneration
The fusogen solution was polyethylene glycol (PEG, whose molecular weight is 6000, Sangon Biotech Co., LTD, Shanghai, China). PEG was prepared at 30% (v/v) in phosphate buffer (pH 6.6). The protoplasts were incubated in a 60 °C water bath to inhibit their regeneration ability. The protoplasts of Eurotium cristatum and Monascus spp. were inactivated through heating in a 60 °C water bath for 35 and 30 min, obtaining a survival rate lower than 0.1%. The survival rate of protoplasts was determined via the agar plate dilution method. The following formula was used to determine the inactivation ratio of protoplasts:
%Inactivation ratio = (1 − (No. of growing colonies on PDA)/(No. of initial protoplasts)) × 100%
Then, 1 mL of inactivated parent protoplasts (105 mL−1) was added to 1 mL of fusogen solution and incubated at 28 °C for 20 min. The fused protoplasts were added to a PDA fluid medium with 1.2 M of sorbitol for regeneration. The regeneration was carried out after incubation at 28 °C for 24 h. The regenerated cells were spread on a solid PDA medium and then incubated at 28 °C for 3–7 days. The potential fusants’ strains were screened and labeled based on their colonial morphology. The fusants were stored on a PDA agar plate at 4 °C.
2.4. Production of Ascocarp, Ascospore, and Conidium from Eurotium cristatum
The tea sample (20 g) was sterilized at 121 °C for 20 min and inoculated with 2 mL of the Eurotium cristatum JH1205 strain or fusants. The inoculated tea sample was then incubated at 28 °C for 10 days. The fermented loose dark tea was stored in a ventilated and dry environment. The ascocarp was separated from the loose dark tea using degreased gauze. The ascospore was separated from the ascocarp and broken via ultrasonics at 600 W for 10 min. The conidium was formed in a hyperosmotic solution. A 2 mL seed solution was added to 100 mL of the PDA medium containing 15% sucrose and 10% sodium chloride, followed by stationary culture at 28 °C for 8 days. After fermentation, the conidium was separated from the mycoderm using degreased gauze and dried in a baking oven.
2.5. Lovastatin Production by Eurotium cristatum JH1205, Monascus CICC5031 and Fusants
Eurotium cristatum, Monascus CICC5031, and fused mutants were first cultured in a seed medium for fermentation. After incubation at 28 °C for 5 days, 2 mL of seed solution was added to 100 mL of sterilized fermentation medium and inoculated at 32 °C and 120 rpm. Except for one instance, lovastatin production was detected after 8 days.
The fermentation time, carbon source, rotation speeds, and temperature were optimized for lovastatin production by Eurotium cristatum JH1205 and four strains (A4, A36, A54, and A76). The lovastatin concentration in the fermentation broth was detected at 4, 6, 8, 10, and 12 days. The additional 5% sucrose in the lovastatin production medium was replaced by glucose, maltose, lactose, and soluble starch, respectively. The concentration of soluble starch was changed from 2 to 12 g/100 mL. The rotation speed and temperature were 0 to 160 r/min and 24 to 40 °C, respectively. The lovastatin concentration in the fermentation broth was detected after 8 days.
2.6. Loose Dark Tea Produced by Breeded Eurotium cristatum
2.6.1. Tea Leaf Collection Area in the Anhua Tea Forest
Tea leaves were collected from Anhua County (110°–112° E, 27°–29° N), with abundant tea forest resources. The original tea samples from four tea production areas were selected to produce loose dark tea. The four selected tea forests are representative tea production areas in Anhua County due to their climatic conditions, geological conditions, tea varieties, fresh leaf harvesting method, and commercial value. These areas are Yuntai Mountain (111°02′ E, 28°37′ N), Furong Mountain (111°45′ E, 28°10′ N), Gaojia Mountain (111°45′ E, 28°10′ N), and Wulong Mountain, with a corresponding maximum elevation of 1.3 km, 1.4 km, 1.2 km, and 0.8 km.
2.6.2. Loose Dark Tea Production
The tea sample (20 g) was sterilized at 121 °C for 20 min and inoculated with 2 mL of fungal suspension (107 CFU/mL). Four fusants (A4, A36, A54, and A76) and wild Eurotium cristatum were used to ferment the tea at 28 °C for 10 days. The humidity during fermentation was maintained at 80%. After fermentation, the fermented loose dark tea was dried at 40 °C for 2 h and stored in a ventilated and dry environment.
2.7. Extraction of Lovastatin
The fermented broth was centrifuged at 3000 rpm, and the mycelium (2 g) was treated with 10 mL of 95% ethanol for 2 h. Then, 5 mL of the mixture was added to 5 mL of 95% ethanol for 2 h of extraction. Also, 2 g of ascocarp was suspended in 5 mL of water and broken via ultrasonics at 600 W for 10 min. The solution of broken ascocarp was centrifuged at 2500 rpm. The liquid supernatant was added to 5 mL of ethanol (95%) for 2 h of extraction. The sediment was resuspended in 5 mL of water and filtered through 4 layers of gauze. The filtrates were dried in a baking oven to obtain the ascospores. Ascospore or conidium (5 mL, 108/mL) was broken using the glass bead-beating method and extracted with 5 mL of ethanol (95%) for 2 h. Then, 10 mL of 95% ethanol was added to 2 g of dark tea powder, which was then extracted at 100 r/min for 30 min.
2.8. Determination of Lovastatin
The lovastatin extracts were filtered through a 0.2 μm filter membrane and then measured using an HPLC system at 238 nm. The column type is EC-C18 chromatography (2.1 × 50 mm, impacted PEEK-lined stainless steel columns) purchased from Agilent Technologies Co., Ltd. (Santa Clara, CA, USA). An isocratic mobile phase of methanol and water in an 85:15 (v/v) ratio was used. The flow rate was 0.8 mL/min at 25 °C. Compared with standard lovastatin, the error in the retention time of lovastatin in the fermentation broth should be lower than 5%. All the above experiments were repeated three times, and the results were averaged.
2.9. Statistical Analysis
For all experiments, samples were selected randomly and repeated at least three times. All statistical analyses were performed using SPSS (Statistical Product and Service Solutions) 22.0 statistical software (IBM Corp., Armonk, NY, USA). Values were presented as the mean ± standard deviation. For multi-group comparisons, p values were derived from a one-way analysis of variance (ANOVA). The p values < 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Preparation, Fusion, and Regeneration of the Parent Protoplasts
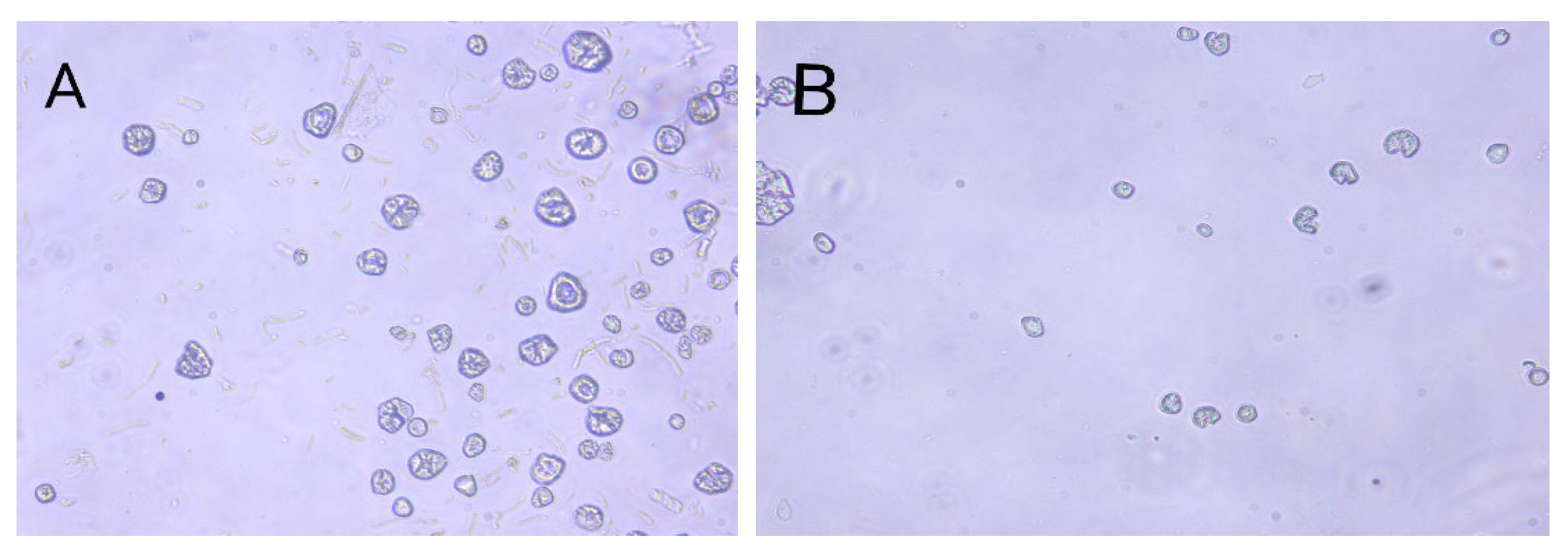
The maximum number of protoplasts in Eurotium cristatum and Monascus was obtained by considering several conditions, such as the concentrations of lywallzyme and snailase, which are the enzymes used in breaking down the cytoderm. It was found that the optimal concentrations of lywallzyme and snailase were 2.0% and 0.5%, and the protoplast concentrations of Eurotium cristatum and Monascus spp. were 5.3 × 105 and 8.5 × 105, respectively (Table 2). The age of Mycelia was also tested, resulting in different optimal ages for the preparation of the Eurotium cristatum and Monascus protoplasts. With 6 d of mycelia, the maximum number of protoplasts released from Monascus spp. mycelia was 8.5 × 105, whereas 8 d of mycelia resulted in Eurotium cristatum protoplasts of 1.2 × 106. The two parents’ protoplasts were spheres and pigment-free (Figure 1), with Eurotium cristatum protoplasts having a larger diameter than those released from Monascus spp.

Table 2.
Conditions for protoplast production.
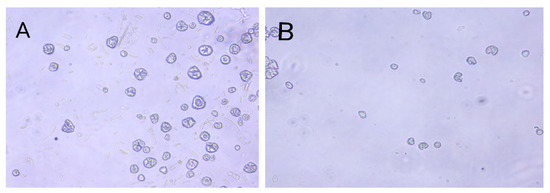
Figure 1.
Protoplasts of Eurotium cristatum JH1205 (A) and Monascus CICC 5031 (B). (×400).
Potential fusants were selected by inactivating protoplasts, preventing the protoplast cells from regenerating. Meanwhile, the potential fusants possess abilities because they contain two genomes from Eurotium cristatum and Monascus spp. To easily select potential fusants, the protoplast inactivation ratio should be above 99.9%. In this research, protoplasts were inactivated in a 60 °C water bath. As shown in Table 3, after incubating at 60 °C, the optimal inactivation time of the Eurotium cristatum JH1205 and Monascus CICC 5031 protoplasts was 35 and 30 min, respectively.

Table 3.
Inactivation ratio of protoplasts treated with 60 °C water bath.
Fusion between the inactivated protoplasts of Eurotium cristatum and Monascus spp. was carried out to improve the production of lovastatin in Eurotium cristatum. The fusion between the protoplasts occurred in the PEG fusogen solution. After inactivation and fusion, the protoplast solution was incubated for 5 d at 28 °C. The obtained fusants presented various colony morphologies in the PDA medium. Some of those had similar morphologies to Eurotium cristatum but did not produce red pigment. Based on the different morphologies, putative recombinant strains were collected for further cultivation, of which 92 fusants were selected to analyze lovastatin production in their fermentation broth using HPLC.
3.2. Comparison of the Lovastatin Yield of Fusant with the Parent Strains
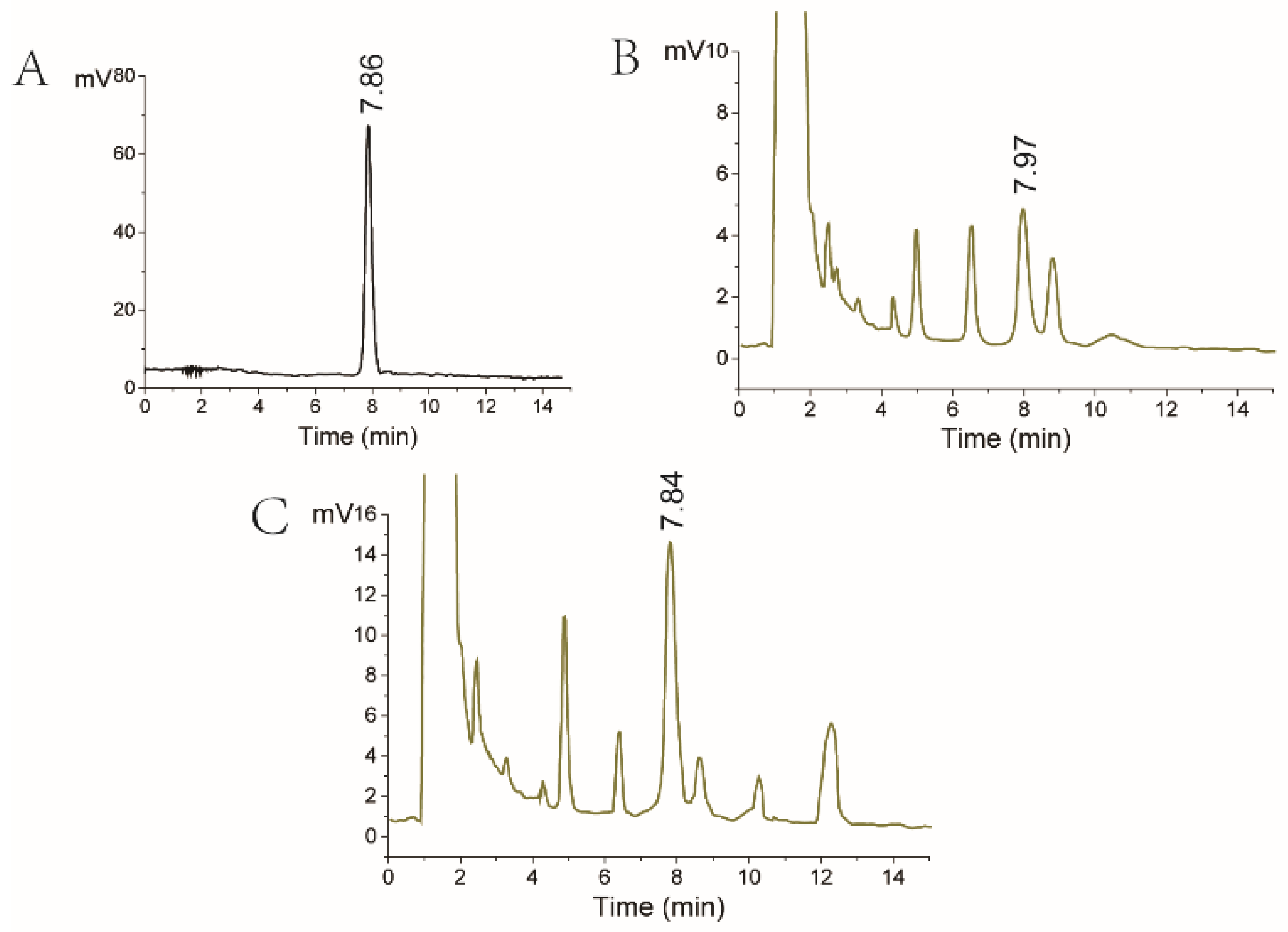
All strains were cultivated in a PDA medium. After 8 days of cultivation at 28 °C, lovastatin production by Eurotium cristatum, Monascus spp., and fusant was analyzed using HPLC. As shown in Figure 2, the A76 fusant and Eurotium cristatum JH1205 produced a component with a similar retention time of 7.85 min compared with the standard lovastatin solution. This indicates that the two strains could produce lovastatin and that the lovastatin yields of the A76 fusant were enhanced compared to those of the wild Eurotium cristatum strain JH1205. Moreover, variation was found in some metabolites in the A76 fusant due to its diploid chromosome. For instance, the yield of the metabolites at retention times of 4.5 and 10.3 min increased. In addition, a new metabolite could be detected at a retention time of 12.3 min.
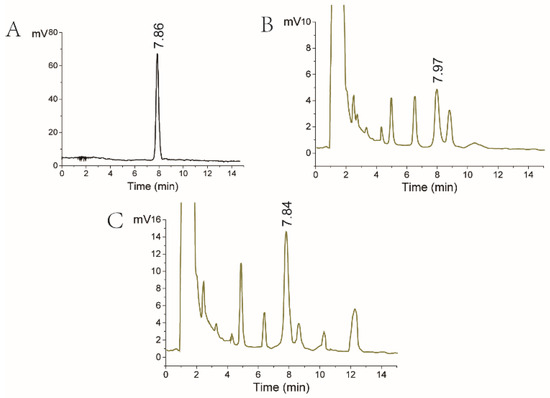
Figure 2.
HPLC analysis of antibiotic production. (A) HPLC chromatogram of lovastatin standard substance. (B) HPLC chromatogram of lovastatin fermented by Eurotium cristatum JH1205. (C) HPLC chromatogram of lovastatin fermented by the A76 fusant. The lovastatin concentration was detected in the fermentation broth.
The selected 92 fusants produced lovastatin with different yields. Compared to Eurotium cristatum JH1205, more than 41% of the fusants exhibited higher lovastatin production (Table 4). Furthermore, the lovastatin yield of the four fusants (A4, A36, A54, and A76) was thrice that of Eurotium cristatum JH1205. Eurotium cristatum and Monascus spp. could produce yellow and red pigments, respectively. Most of the fusants had a yellow colony morphology. However, the fusants with red pigment produced higher lovastatin production. Among the four fusants with the highest increased level of lovastatin production, two fusants had a yellow colony morphology (A54 and A76), similar to Eurotium cristatum.

Table 4.
Increased level of lovastatin production in fusants compared to Eurotium cristatum JH1205.
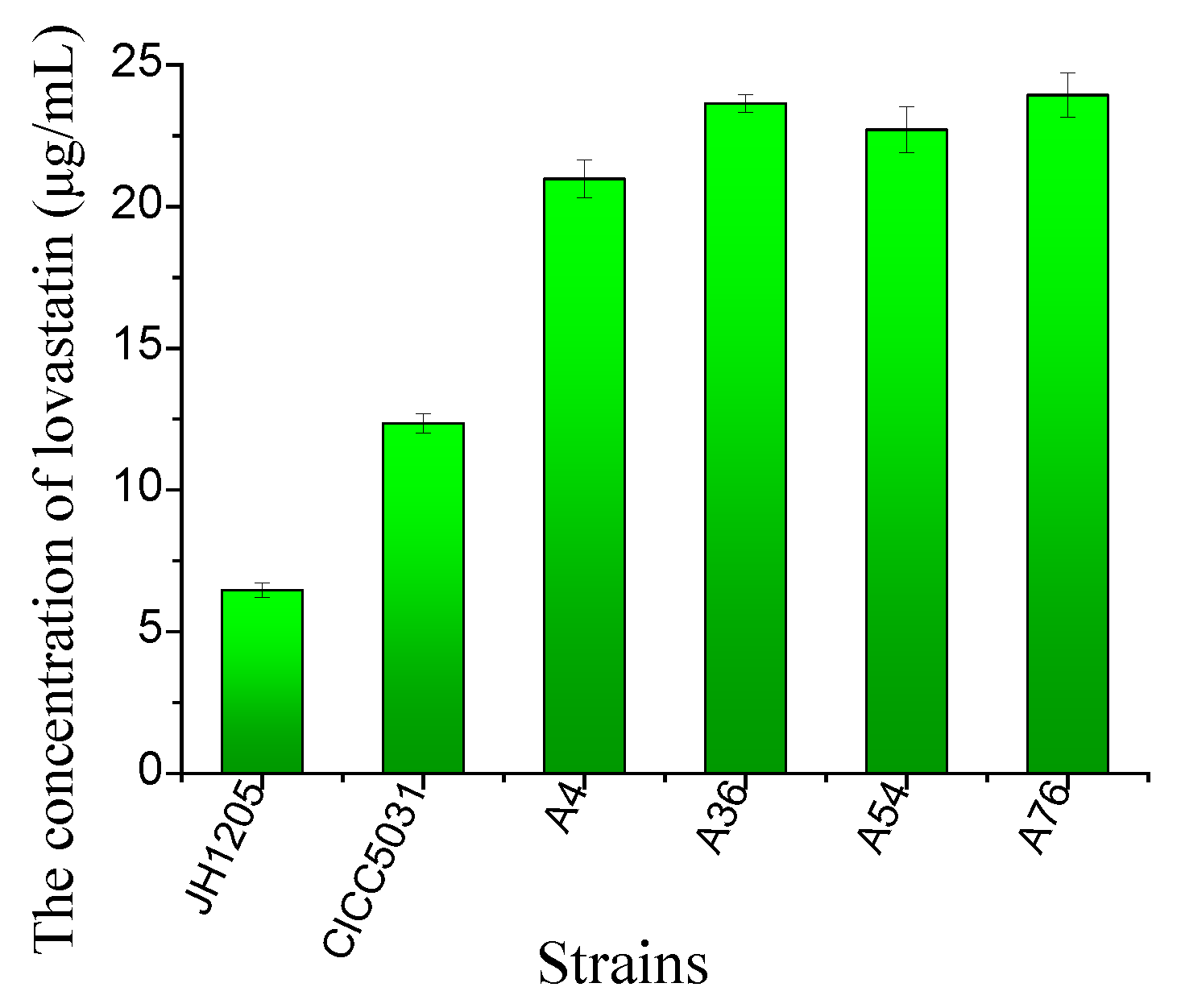
The four fusants and their parents were cultivated in a production medium. The results showed that all the fusants were able to produce more lovastatin compared to their parents. The strains A4, A36, A54, and A76 increased the lovastatin yield by 324.3%, 365.24%, 351.03%, and 369.87% compared to that of the wild-type Eurotium cristatum strains, respectively (Figure 3). The A76 fusant produced the highest lovastatin yield of 23.93 μg/mL.
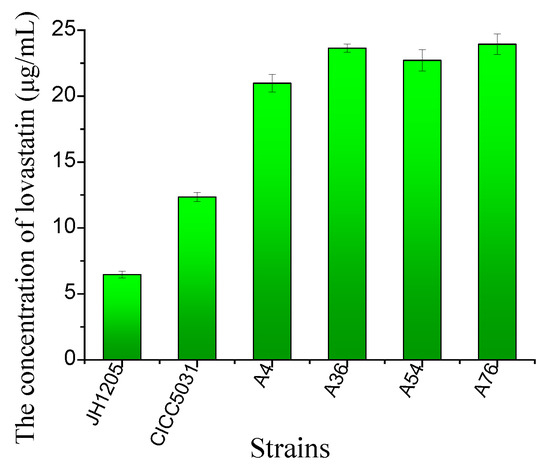
Figure 3.
Lovastatin yield of four fusants and their parental strains after cultivation in PDA medium for 8 days at 28 °C. The lovastatin concentration was detected in the fermentation broth. The error bars are standard deviations.
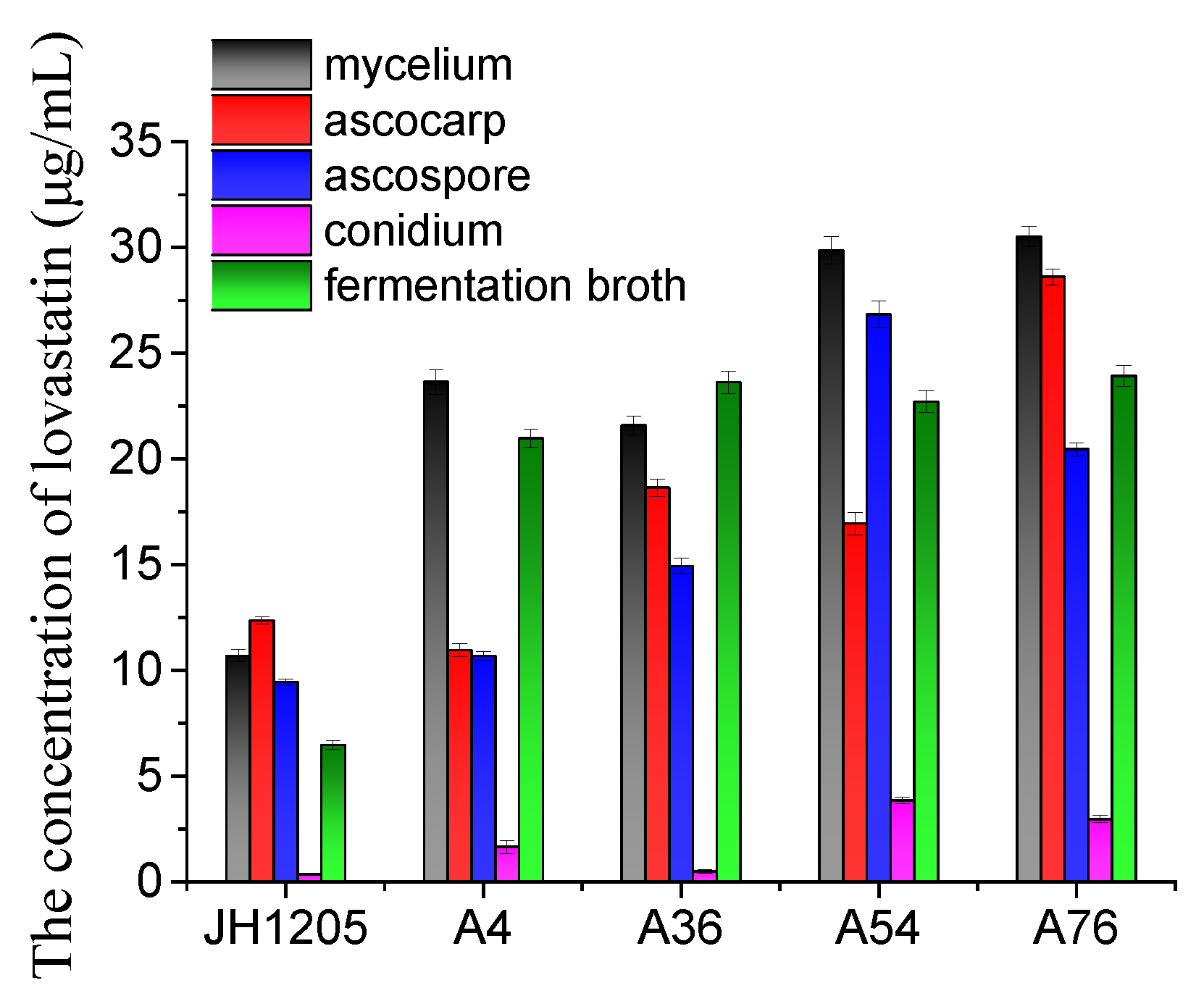
The organ for lovastatin production using fusants (A4, A36, A54, and A76) and Eurotium cristatum JH1205 was investigated. As shown in Figure 4, the yields of lovastatin produced by the fusants in mycelium, ascospore, ascocarp, and the fermentation broth were higher than those of wild Eurotium cristatum JH1205. However, the level of increase varies between organs, depending on the strain type. For instance, the lovastatin yields in mycelium, conidium, and the fermentation broth for the A4 fusant were enhanced by more than twice that of the ascocarp and ascospore media. In conidium, the lovastatin produced by the A36 fusant was the same as that produced by wild Eurotium cristatum JH1205, while that of the A54 fusant was ten times higher. Interestingly, the A76 fusant could produce more lovastatin in all the media used; hence, it was chosen for further studies.
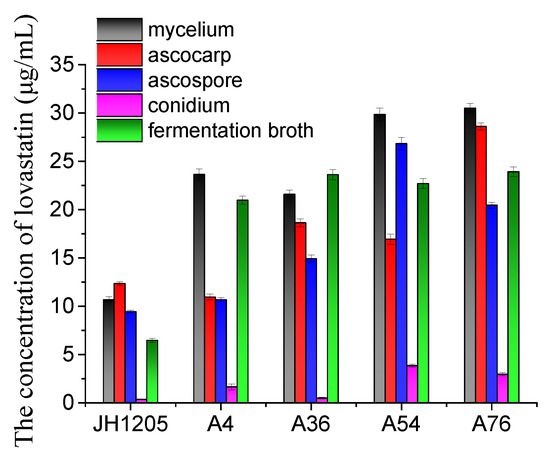
Figure 4.
Lovastatin production by JH1205 and four fusants in different organs. Strains were fermented at 28 °C for 8 days. The propagule includes sexual propagule (ascospore and ascocarp) and asexual propagule (conidium). The error bars are standard deviations.
3.3. Influences of Fermentation Parameters on Lovastatin Production by JH1205 Strain and Four Fusants
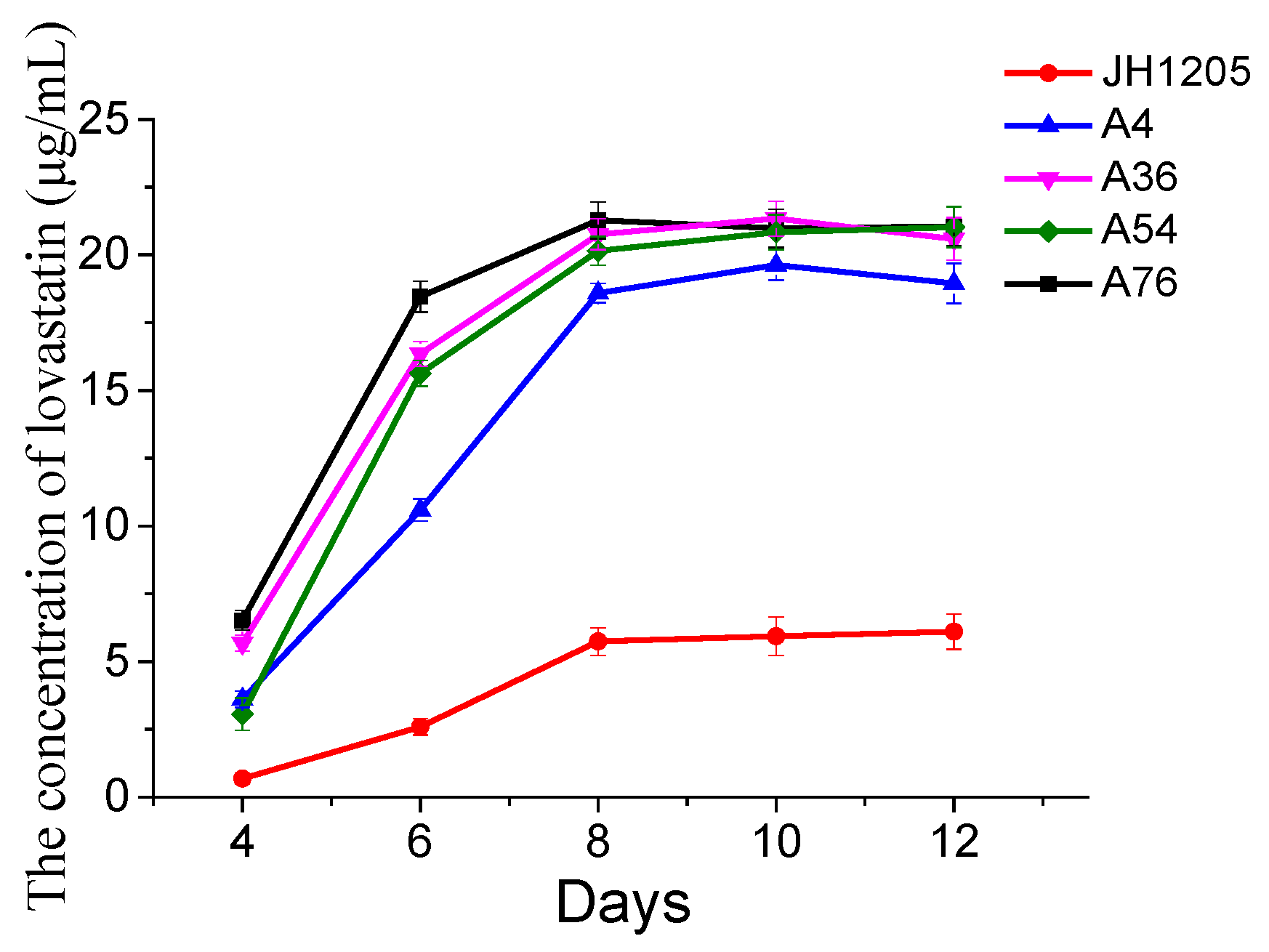
A detailed fermentation parameter study of lovastatin production was performed on the JH1205 strain and four fusants. Initially, the influence of the fermentation time on the lovastatin produced by Eurotium cristatum JH1205 and the four fusants was studied. The fermentation profiles for incubation time are presented in Figure 5, which also confirmed that the four fusant strains could produce thrice the amount of lovastatin compared to Eurotium cristatum JH1205. Like Eurotium cristatum JH1205, the lovastatin yield of the four fusants reached its maximum value at 8 days and plateaued. These results indicated that lovastatin was no longer produced in the late fermentation process due to the consumption of nutrients in the fermentation broth.
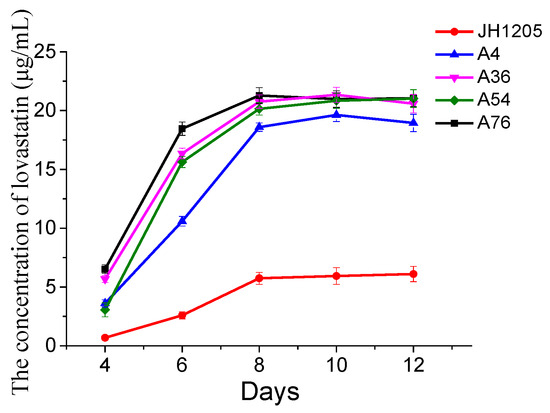
Figure 5.
Time profile of lovastatin production by the Eurotium cristatum JH1205 strain and four fusants on PDA fluid medium at 28 °C. The lovastatin concentration was detected in the fermentation broth. The error bars are standard deviations.
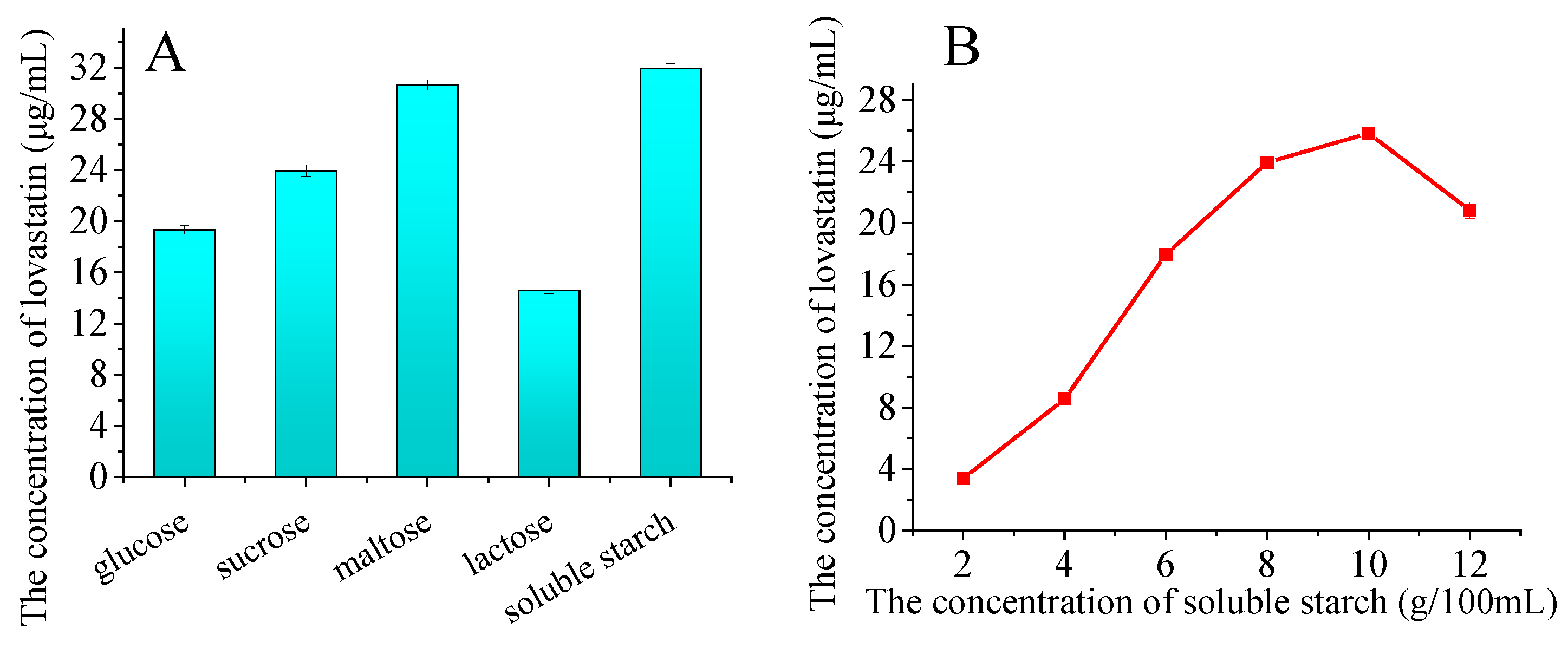
To investigate the effect of different carbohydrate sources on lovastatin production, 5% monosaccharides, disaccharides, and polysaccharides were added to the PDA medium. As shown in Figure 6A, the carbohydrate source strongly influenced the lovastatin yields. Maltose and soluble starch were the best for lovastatin production, with approximately twice the yield compared to lactose. The effect of soluble starch concentration on lovastatin production has also been studied. The yield of lovastatin improved with an increase in the soluble starch concentration below 8% (Figure 6B). A maximum of 23.93 μg/mL of lovastatin was produced by adding 8% soluble starch.
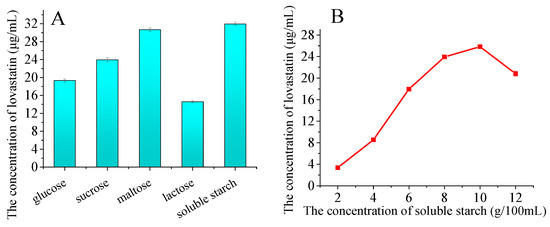
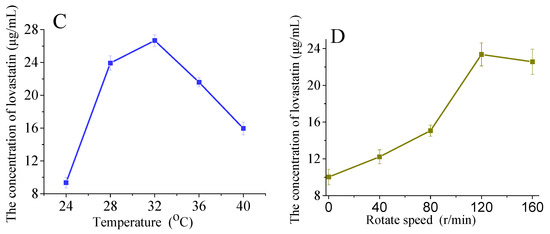
Figure 6.
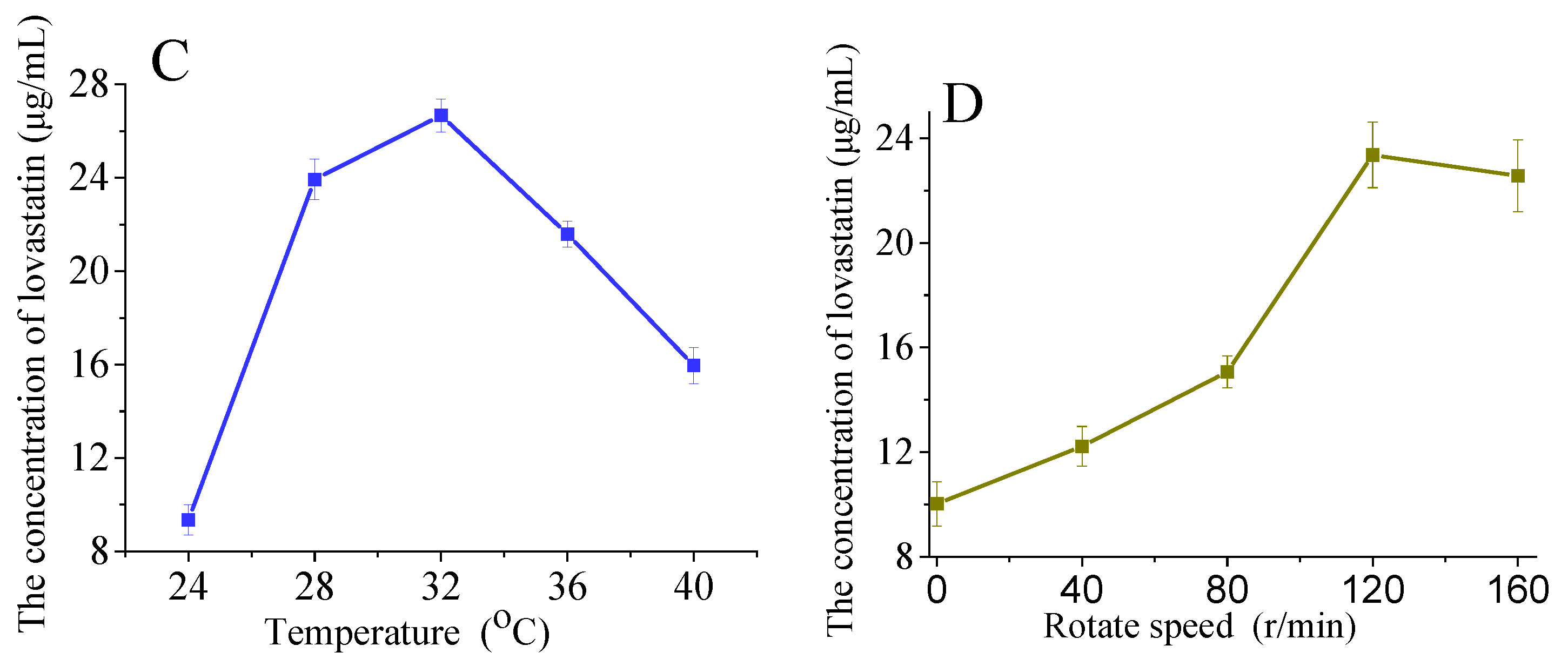
Influence of additional carbohydrate sources on lovastatin production (A,B). The A76 fusants were fermented at 28 °C for 8 days. Influence of temperature (C) and rotation speed (D) on lovastatin production. The A76 fusants were fermented for 8 days. The lovastatin concentration was detected in the fermentation broth. The error bars are standard deviations.
Temperature influences the enzyme activity of microorganisms, affecting their growth and secondary metabolite accumulation. The highest lovastatin yield (26.67 μg/mL) was achieved at 32 °C, and a further increase in temperature reduced the lovastatin yield (Figure 6C). Since Eurotium cristatum is an aerobic fungus, the rotation speed was adjusted to control the dissolved oxygen in the fermentation broth. As shown in Figure 6D, the highest lovastatin yield was achieved at 120 r/min.
3.4. Loose Dark Tea Produced by Bred Eurotium cristatum Using Tea Forest Resources from Different Locations
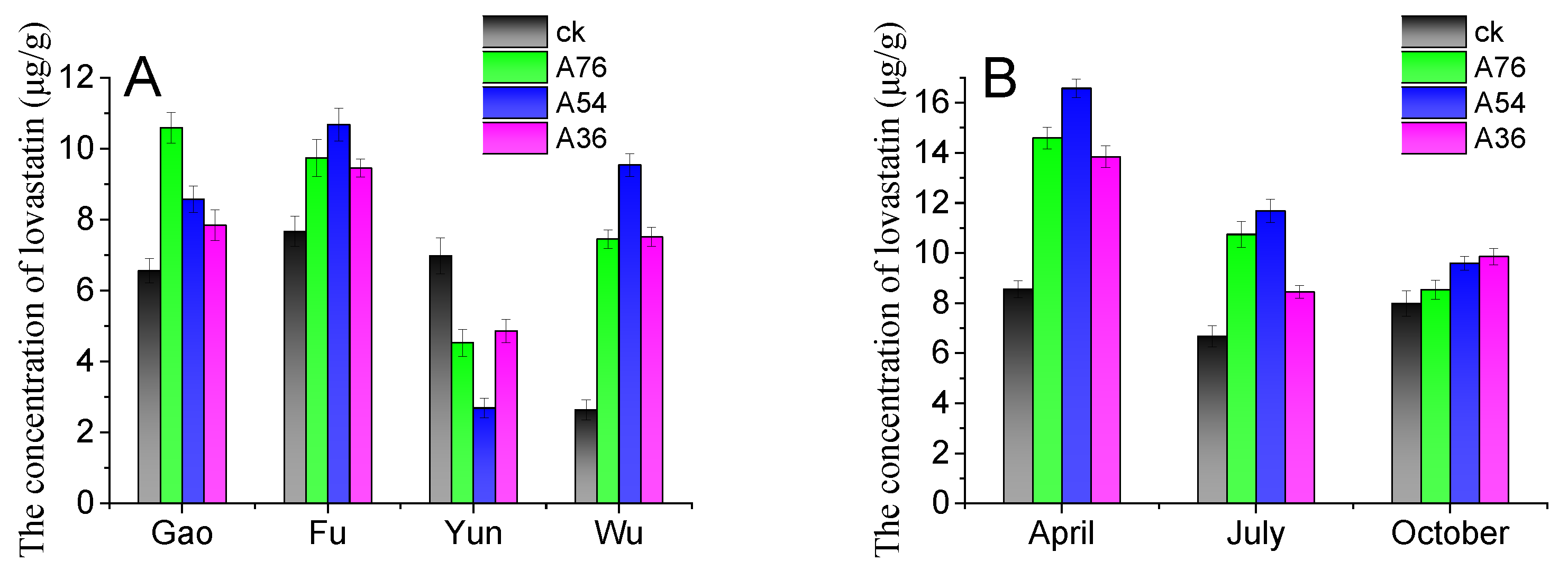
The obtained fusants were used to ferment tea samples in order to maximize the value of the tea forest resource and improve the yield of lovastatin in loose dark tea. The original tea samples were purchased from different tea production areas under different climatical and geological conditions in Anhua County. The four famous tea forests were Yuntai Mountain, Furong Mountain, Gaojia Mountain, and Wulong Mountain. As shown in Figure 7A, the tea sample location strongly affected the lovastatin yield in loose dark tea. Among the four tea production areas, the tea sample from Yuntai Mountain produced loose dark tea with a lower lovastatin concentration. The lovastatin concentration in loose dark tea produced by tea samples from Furong Mountain and Gaojia Mountain was over 10 μg/g. Compared with other tea production areas, Wulong Mountain loose dark tea had the lowest lovastatin yield when fermented by wild Eurotium cristatum. Also, the type of Eurotium cristatum strains significantly influenced the lovastatin yield in loose dark tea. Compared with wild Eurotium cristatum, the bred strain improved lovastatin yield in loose dark tea. The A54 and A76 strains could produce adequate lovastatin concentrations in most tea samples.
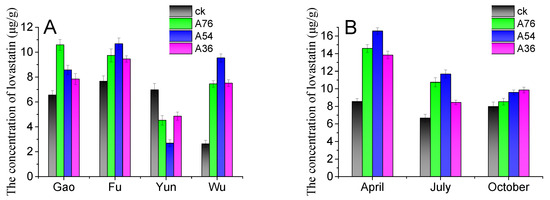
Figure 7.
Influence of tea tree location (A) and picking time (B) on lovastatin production in loose dark tea. The ck represents loose dark tea fermented by wild Eurotium cristatum. A76, A54, and A36 are loose dark teas fermented by the A76, A54, and A36 fusants, respectively. Gao, Fu, Yun, and Wu stand for tea samples collected from the Gaojia Mountain, Furong Mountain, Yuntai Mountain, and Wulong Mountain tea production areas. The error bars are standard deviations.
The biochemical content of tea leaves is subject to seasonal variations. In the spring, the biochemical content of tea leaves is richer because of moderate temperatures, abundant rainfall, and a lengthy break from winter. Due to the hot summer weather, the shoots of the tea plants grow rapidly, resulting in a decrease in amino acids and vitamins in the tea leaves. Whereas in the autumn, the mild climate with insufficient rainfall results in poor contents in the leaves after being picked in the spring and summer. To study the effect of the leaf-picking time on lovastatin production in loose dark tea, fresh leaves were picked in April, July, and October. As shown in Figure 7B, the leaf-picking time highly affected the lovastatin yield in loose dark tea. The tea samples picked in April produced loose dark tea with higher lovastatin concentrations. This result was attributed to the richer biochemical content of the tea leaves. The tea harvest in spring is generally lower. To obtain dark tea with higher lovastatin concentrations and a lower cost, a few spring-picked leaves can be added to summer-picked leaves. Furthermore, dark tea produced from blended tea leaves will have a distinct flavor.
4. Conclusions
The protoplast fusion technique was used to improve the lovastatin yield of wild-type Eurotium cristatum strains, with Eurotium cristatum JH1205 and Monascus CICC5031 being the parent strains. Several haploid progenies isolated from numerous recombinants exhibited higher lovastatin production by fermentation, accounting for more than 41% of the obtained fusants. In addition, 18% of the fusants had over a two-fold increase in lovastatin production. Compared to that of Eurotium cristatum JH1205, the strains A4, A36, A54, and A76 showed dramatic improvements in lovastatin production of 324.3%, 365.24%, 351.03%, and 369.87%, respectively. In addition, the strains A54 and A76 showed similar morphological properties to Eurotium cristatum JH1205 due to their yellow colony. The tea sample location and type of Eurotium cristatum strains strongly influenced the lovastatin yield in loose dark tea. A maximum lovastatin yield of over 10 μg/g was obtained using original tea samples from different tea production areas. Compared with wild Eurotium cristatum, our bred A54 strain had over a three-fold increase in lovastatin yield in loose dark tea produced from Wulong Mountain tea samples. This study demonstrated that protoplast fusion technology is a powerful tool for increasing lovastatin yields in Eurotium cristatum and its fermented loose dark tea. In the future, the lipid-lowering effects and safety of our fermented loose dark tea will be investigated through animal experiments.
Author Contributions
Conceptualization, T.L. and Z.L. (Zhonghua Liu); methodology, Z.L. (Zhanjun Liu) and P.L.; validation, J.L. and Y.Z.; formal analysis, T.L. and Z.L. (Zhanjun Liu); investigation, J.L. and T.L.; resources, P.L., Y.Z. and J.L.; writing—original draft preparation, T.L. and Z.L. (Zhanjun Liu); writing—review and editing, T.L., S.L. and Z.L. (Zhonghua Liu); visualization, J.L. and T.L.; project administration, Z.L. (Zhonghua Liu) and P.L.; project administration, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hunan Provincial Key Lab of Dark Tea and Jin-hua (2016TP1022), the National Tea Industry Technology System Project (CARS-19), the Research Foundation of Education Bureau of Hunan Province (22B0785), and the Natural Science Foundation’s regional joint fund project of Hunan Province (2023JJ50338).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Authors Taotao Li, Jun Li, Yajun Zheng, Peixue Ling were employed by the company Hunan Weichu Furuida Biotechnology Limited Liability Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Hunan Weichu Furuida Biotechnology Limited Liability Company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
References
- Li, Z.; Wu, Y.; Zhang, L.; Hasan, M.; Zhang, L.; Yan, P.; Fu, J.; Han, W.; Li, X. A Chemical Explanation for Variations in Antioxidant Capacity across Camellia sinensis L. Cultivars. Forests 2023, 14, e249. [Google Scholar] [CrossRef]
- Sharma, S.; Mukherjee, P. Improved cryopreservation protocol for tea (Camellia sinensis (L.) O. Kuntze) using preconditioning of shoot tip donor plants and V cryo-plate technique. In Vitro Cell Dev. Plant. 2023, 59, 285–297. [Google Scholar] [CrossRef]
- Li, F.; Lv, C.; Zou, Z.; Duan, Y.; Zhou, J.; Zhu, X.; Ma, Y.; Zhang, Z.; Fang, W. CsAAP7.2 is involved in the uptake of amino acids from soil and the long-distance transport of theanine in tea plants (Camellia sinensis L.). Tree Physiol. 2022, 42, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, H.; Feng, M.; Tian, C. Phenotypic Variation in Leaf, Fruit and Seed Traits in Natural Populations of Eucommia ulmoides, a Relict Chinese Endemic Tree. Forests 2023, 14, 462. [Google Scholar] [CrossRef]
- Fotis, A.; Flower, C.; Atkins, J.; Pinchot, C.; Rodewald, A.; Matthews, S. The short-term and long-term effects of honeysuckle removal on canopy structure and implications for urban forest management. Forest Ecol. Manag. 2022, 517, e120251. [Google Scholar] [CrossRef]
- Barghouthy, Y.; Somani, K. Role of Citrus Fruit Juices in Prevention of Kidney Stone Disease (KSD): A Narrative Review. Nutrients 2021, 13, e4117. [Google Scholar] [CrossRef]
- Gu, H.; Yan, J.; Liu, Y.; Yu, X.; Feng, Y.; Yang, X.; Lam, S.; Naushad, M.; Li, C.; Sonne, C. Autochthonous bioaugmentation accelerates phenanthrene degradation in acclimated soil. Environ. Res. 2023, 224, e115543. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, Y.; Dong, M.; Liu, S.; Hu, Z.; Zhong, X.; Xu, Z. Exploring the evolutionary characteristics between cultivated tea and its wild relatives using complete chloroplast genomes. BMC Ecol. Evol. 2021, 21, e71. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.; Wu, Y.; Zhang, J.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. Lwt Food Sci. Technol. 2020, 117, e108629. [Google Scholar] [CrossRef]
- Shadwell, N.; Villalobos, F.; Kern, M.; Hong, M. Blooming reduces the antioxidant capacity of dark chocolate in rats without lowering its capacity to improve lipid profiles. Nutr. Res. 2013, 33, 414–421. [Google Scholar] [CrossRef]
- Li, C.; Ye, H.; Ge, S.; Yao, Y.; Ashok, B.; Hariram, N.; Liu, H.; Tian, H.; He, Y.; Guo, G.; et al. Fabrication and properties of antimicrobial flexible nanocomposite polyurethane foams with in situ generated copper nanoparticles. J. Mater. Res. Technol. 2022, 19, 3603–3615. [Google Scholar] [CrossRef]
- Lin, F.; Wei, X.; Liu, H.; Li, H.; Xia, Y.; Wu, D.; Zhang, P.; Gandhi, G.; Li, H.; Gan, R. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Fu, D.; Ryan, E.; Huang, J.; Liu, Z.; Weir, T.; Snook, R.; Ryan, T. Fermented Camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Res. Int. 2011, 44, 2999–3005. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Wu, Y.; Zhong, K.; Gao, H. Structural characteristics and hypolipidemic activity of theabrownins from dark tea fermented by single species Eurotium cristatum PW-1. Biomolecules 2020, 10, e204. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, Z.; Huang, Y.; Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium cristatum and their lipid-lowering activity. LWT-Food Sci. Technol. 2018, 98, 204–211. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Fang, W.; Tang, Q.; Zhan, L.; Shi, Y.; Tang, M.; Liu, Z.; Zhang, S.; Liu, A. Research progress on the lipid-lowering and weight loss effects of tea and the mechanism of its functional components. J. Nutr. Biochem. 2023, 112, e109210. [Google Scholar] [CrossRef]
- Qu, J.; Ye, M.; Wen, C.; Cheng, X.; Zou, L.; Li, M.; Liu, X.; Liu, Z.; Wen, L.; Wang, J. Compound dark tea ameliorates obesity and hepatic steatosis and modulates the gut microbiota in mice. Front. Nutr. 2023, 10, e1082250. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Cheng, X.; Wang, Z.; Yuan, F.; Wu, W.; Liao, S. Evaluating the productivity of ancient Pu’er tea trees (Camellia sinensis var. assamica): A multivariate modeling approach. Plant Methods 2022, 18, e95. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Wang, S.; Wang, Y.; Wei, L.; Zheng, L.; Gu, H.; Lam, S.; Naushad, M.; Li, C.; et al. Bamboo-based magnetic activated carbon for efficient removal of sulfadiazine: Application and adsorption mechanism. Chemosphere 2023, 323, e138245. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.; Wu, Y.; Gao, H. Insight into effects of isolated Eurotium cristatum from Pingwu Fuzhuan brick tea on the fermentation process and quality characteristics of Fuzhuan brick tea. J. Sci. Food Agric. 2020, 100, 3598–3607. [Google Scholar] [CrossRef]
- Lu, X.; Jing, Y.; Zhang, N.; Cao, Y. Eurotium cristatum, a probiotic fungus from Fuzhuan brick tea, and its polysaccharides ameliorated DSS-induced ulcerative volitis in mice by modulating the gut microbiota. J. Agric. Food Chem. 2022, 70, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Y.; Wang, Y.; Yuan, X.; Zhang, B.; Wu, Z.; Tian, H. A novel environment-friendly adhesive based on recycling of broussonetia papyrifera leaf forestry waste protein. Forests 2022, 13, e291. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, Y.; Yu, L.; Huang, X.; Huang, J.; Wang, K.; Liu, Z. Protective effect of Eurotium cristatum fermented loose dark tea and Eurotium cristatum particle on MAPK and PXR/AhR signaling pathways induced by electronic cigarette exposure in Mice. Nutrients 2022, 14, e2843. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Zhu, M.; He, C.; Li, Z.; Wang, Y.; Liu, Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT-Food Sci. Technol. 2022, 169, e113925. [Google Scholar] [CrossRef]
- Yi, H.; Wu, M.; Zhang, Q.; Lu, L.; Yao, H.; Chen, S.; Li, Y.; Zheng, C.; He, G.; Deng, X. Reversal of HER2 negativity: An unexpected role for lovastatin in triple-negative breast cancer stem cells. J. Cancer 2020, 11, 3713–3716. [Google Scholar] [CrossRef]
- Shahrezaee, M.; Oryan, A.; Bastami, F.; Hosseinpour, S.; Shahrezaee, M.; Kamali, A. Comparative impact of systemic delivery of atorvastatin, simvastatin, and lovastatin on bone mineral density of the ovariectomized rats. Endocrine 2018, 60, 138–150. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An overview of the bioactivity of monacolin K/lovastatin. Food Chem. Toxicol. 2019, 131, e110585. [Google Scholar] [CrossRef]
- Suraiya, S.; Kim, J.; Tak, J.; Siddique, M.; Young, C.; Kim, J.; Kong, I. Influences of fermentation parameters on lovastatin production by Monascus purpureus using Saccharina japonica as solid fermented substrate. LWT-Food Sci. Technol. 2018, 92, 1–9. [Google Scholar] [CrossRef]
- Zhgun, A.; Nuraeva, G.; Dumina, M.; Voinova, T.; Dzhavakhiya, V.; Eldarov, M. 1,3-Diaminopropane and spermidine upregulate lovastatin production and expression of lovastatin biosynthetic genes in Aspergillus terreus via LaeA regulation. Appl. Biochem. Microbiol. 2019, 55, 243–254. [Google Scholar] [CrossRef]
- Butinar, L.; Zalar, P.; Frisvad, J.C.; Gunde-Cimerman, N. The genus Eurotium–members of indigenous fungal community in hypersaline waters of salterns. FEMS Microbiol. Ecol. 2005, 51, 155–166. [Google Scholar] [CrossRef]
- Moura, M.A.; Gomes, D.C.; Takahashi, J.A. Applications of fungi secondary metabolites in the food industry. Natural Secondary Metab. 2023, 22, 739–776. [Google Scholar]
- Qin, Y.; Yuan, Z.; Yang, F.; Yu, Y. Development of a new type of Anhua black tea and its application: Black tea wine. J. Food Process. Pres. 2022, 46, e15862. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, W.; Dong, Q.; Tang, C.; Cao, F.; Su, E. Submerged fermentation of Ginkgo bilobaseed powder using Eurotium cristatum for the development of ginkgo seeds fermented products. J. Sci. Food Agric. 2021, 101, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Balraj, J.; Jairaman, K.; Kalieswaran, V.; Jayaraman, A. Bioprospecting lovastatin production from a novel producer Cunninghamella blakesleeana. 3 Biotech 2018, 8, e59. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tan, Y.; Wang, K.; Cai, Y.; Li, J.; Sonne, C.; Li, C. Reviewing wood-based solar-driven interfacial evaporators for desalination. Water Res. 2022, 223, e119011. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, F.; Zhou, K.; Zhao, Q.; Sun, H.; Wang, S.; Zhao, Y.; Fu, J. Breeding of high protein Chlorella sorokiniana using protoplast fusion. Bioresour. Technol. 2020, 313, e123624. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, A.; Crowhurst, A.; Umaretiya, P.; Growns, D. Breeding and development of novel hybrids of waxflowers by protoplast fusion. Acta Hortic. 2015, 1097, 85–92. [Google Scholar] [CrossRef]
- Guo, J.; Gong, D.; Li, Z.; Zheng, Z. Construction of yeast strain capable of Co-fermenting pentose and hexose by protoplast fusion. Adv. Mater. Res. 2013, 781, 847. [Google Scholar]
- Zhang, D.; Wang, F.; Wang, C.; Dai, W. Construction of engineering bacteria degrading residual polyacrylamide in coal slime water by protoplast fusion technique. Int. J. Glob. Energy 2021, 43, 340–355. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).