Effects of Afforestation Patterns on Soil Nutrient and Microbial Community Diversity in Rocky Desertification Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sample Collection
2.3. Analysis of Soil Physical and Chemical Properties
2.4. Illumina MiSeq High-Throughput Sequencing
2.5. Statistical Analyses
3. Results
3.1. Physicochemical Properties of Soil in Different Afforestation Patterns
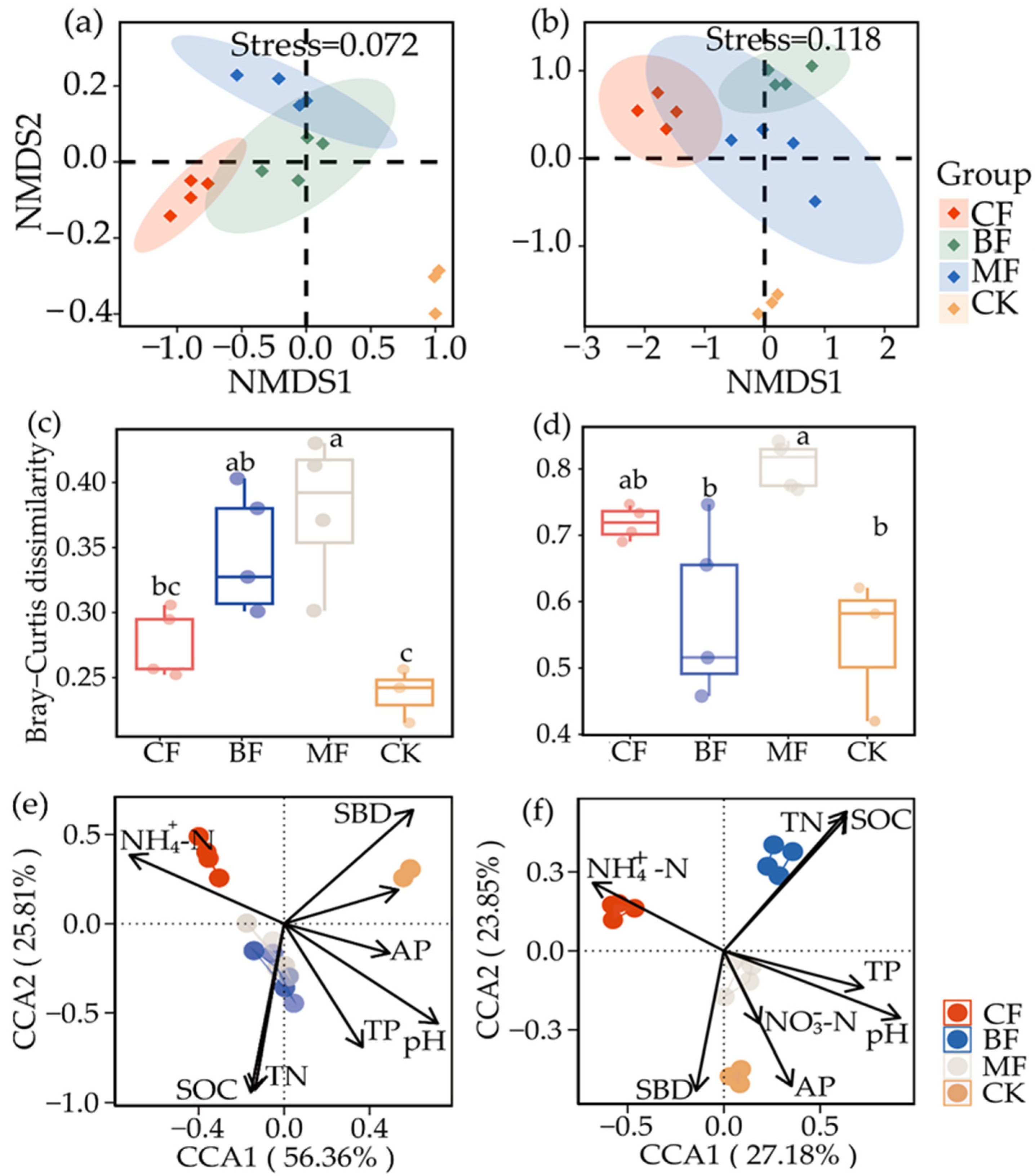
3.2. Soil Microbial Community Structure among Different Afforestation Patterns
3.3. Co-Occurrence Patterns of Soil Microbial in Different Afforestation Patterns
4. Discussion
4.1. Physicochemical Properties of Soil at Different Afforestation Patterns
4.2. Variety of Microbial Community Composition and Structure in Different Afforestation Patterns
4.3. Correlation between Environmental Factors and Microbial Communities in Different Forest Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.-X.; Wang, P.; Ou, H.-B.; Wei, S.-X.; Wu, L.-C.; Jiang, Y.; Wang, R.-J.; Liu, X.-S.; Wang, Z.-H.; Chen, L.-J.; et al. Effects of different vegetation restoration on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. For. Ecol. Manag. 2022, 508, 120002. [Google Scholar] [CrossRef]
- Wang, S.J. Concept deduction and ITS connotation of Karst rocky desertification. China Karst 2002, 21, 101–105. [Google Scholar]
- Li, Y.B.; Shao, J.A.; Yang, H.; Xiong, Y.B. The relations between land use and karst rocky desertification in a typical karst area, China. Environ. Earth Sci. 2009, 57, 621–627. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Liao, X. Soil nutrients, enzyme activities, and bacterial communities in varied plant communities in karst rocky desertification regions in Wu shan County, Southwest China. Front. Microbiol. 2023, 14, 1180562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, Y.; Long, M.; Gao, M.; Zhang, Y.; Lin, J.; Li, X. Moss biocrusts buffer the negative effects of karst rocky desertification on soil properties and soil microbial richness. Plant Soil 2020, 475, 153–168. [Google Scholar] [CrossRef]
- Chai, Y.; Cao, Y.; Yue, M.; Tian, T.; Yin, Q.; Dang, H.; Quan, J.; Zhang, R.; Wang, M. Soil abiotic properties and plant functional traits mediate associations between soil microbial and plant communities during a secondary forest succession on the Loess plateau. Front. Microbiol. 2019, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.Y.; Wang, Y.X.; Wang, Y.J. Effects of Different Vegetation Control Models on Soil Quality in Karst Rocky Desertification Areas. West. For. Sci. 2020, 49, 41–47. [Google Scholar]
- Wang, J.; Liu, Z.Q.; Bao, E.M. Effects of Forest and Grass Restoration on Soil Aggregates and Its Organic Carbon in Karst Rocky Desertification Areas. J. Soil Water Conserv. 2019, 33, 249–256. [Google Scholar]
- Zhang, Y.; Cong, J.; Lu, H.; Yang, C.; Yang, Y.; Zhou, J.; Li, D. An Integrated Study to Analyze Soil Microbial Community Structure and Metabolic Potential in Two Forest Types. PLoS ONE 2014, 9, e93773. [Google Scholar] [CrossRef]
- Fu, Y.; Zhuang, L.; Wang, Z.K. On the physical chemical and soil microbial properties of soils in the habitat of wild Ferula in Xinjiang. Acta Ecol. Sin. 2012, 32, 3279–3287. [Google Scholar]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C. Microbial regulation of the soil carbon cycle: Evidence from gene-enzyme rela-tionships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Lin, S.; Zhuang, J.-Q.; Chen, T.; Zhang, A.-J.; Zhou, M.-M.; Lin, W.-X. Analysis of nutrient and microbial Biolog function diversity in tea soils with different planting years in Fujian Anxi. Chin. J. Eco-Agric. 2012, 20, 1471–1477. [Google Scholar] [CrossRef]
- Song, S.; Xiong, K.; Chi, Y.; He, C.; Fang, J.; He, S. Effect of cultivated pastures on soil bacterial communities in the karst rocky desertification area. Front. Microbiol. 2022, 13, 922989. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Xie, G.; Chen, Y. Present Situation and Dynamic Variation of Rocky Desertification in Karst Area of Guizhou. China Soil Water Conserv. 2020, 53–56+7. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, W.; Rao, C.; Wang, E.; Yan, W. Soil Quality Assessment and Management in Karst Rocky Desertification Ecosystem of Southwest China. Forests 2022, 13, 1513. [Google Scholar] [CrossRef]
- Wang, X.F.; Huang, X.F.; Hu, J.W. Relationship among Soil Organic Carbon and Small Environment and Lithology in the Rocky Desertification Process in Different Karst Landforms. J. Soil Water Conserv. 2020, 34, 295–303. [Google Scholar]
- Ma, T.; Deng, X.; Chen, L.; Xiang, W. The soil properties and their effects on plant diversity in different degrees of rocky desertification. Sci. Total. Environ. 2020, 736, 139667. [Google Scholar] [CrossRef]
- Sheng, M.; Xiong, K.; Wang, L.; Li, X.; Li, R.; Tian, X. Response of soil physical and chemical properties to Rocky desertification succession in South China Karst. Carbonates Evaporites 2016, 33, 15–28. [Google Scholar] [CrossRef]
- Stokdyk, J.P.; Herrman, K.S. Effects of Frangula alnus on soil microbial communities and biogeochemical processes in Wisconsin forests. Plant Soil 2016, 409, 65–75. [Google Scholar] [CrossRef]
- Institute of Soil Science; Chinese Academy of Sciences. Soil Physical Properties Determination Method; Science Press: Beijing, China, 1978; Chapters 2, 4, 6. [Google Scholar]
- LY/T 1237-1999; National Forestry Bureau. Forestry Industry Standards of P. R. China. Determination of Organic Matter in Forest Soil and Calculation Carbon-Nitrogen Ratio Chinese Standards Press: Beijing, China, 1999.
- Edga, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Joshi, D.R.; Zhang, Y.; Zhang, H.; Gao, Y.; Yang, M. Characteristics of microbial community functional structure of a biological coking wastewater treatment system. J. Environ. Sci. 2018, 63, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Evolution of Species Diversity in Land Communities. Evol. Biol. 1977, 10, 1–67. [Google Scholar] [CrossRef]
- Wu, G.L.; Du, G.Z.; Liu, Z.H. Effect of grazing exclusion and grazing on a Kobresia-dominated meadow in the Qinghai–Tibetan Plateau. Plant Soil 2009, 319, 115–126. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Catherine, L.; Rob, K. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar]
- Oksanen, J. Multivariate analysis of ecological communities in R: Vegan tutorial. R Package Version 2011, 1, 1–43. [Google Scholar]
- Guan, H.; Fan, J. Effects of vegetation restoration on soil quality in fragile karst ecosystems of southwest China. PeerJ 2020, 8, e9456. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Fromin, N.; Milcu, A.; Buatois, B.; Pontoizeau, C.; Hättenschwiler, S. Higher tree diversity increases soil microbial resistance to drought. Commun. Biol. 2020, 3, 377. [Google Scholar] [CrossRef]
- Long, H.F.; Su, W.C.; Xia, C. Relationship between Soil Organic Matter and Number of Microorganisms of Different Cropping Patterns in Desertification Region. Environ. Sci. Technol. 2013, 36, 57–62. [Google Scholar]
- Xiao, W.; Feng, S.; Liu, Z.; Su, Y.; Zhang, Y.; He, X. Interactions of soil particulate organic matter chemistry and microbial community composition mediating carbon mineralization in karst soils. Soil Biol. Biochem. 2017, 107, 85–93. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, G.Y.; Tian, Y.Y. Differential analysis of soil bacteria diversity in different mixed forests of Dalbergia odorifera. Acta Ecol. Sin. 2015, 35, 8117–8127. [Google Scholar]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Tang, Y.; Yu, M.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Glob. Ecol. Conserv. 2021, 26, e01478. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, Y.; Wang, L.; Wang, S. Responses of soil nutrients to vegetation restoration in China. Reg. Environ. Chang. 2020, 20, 82. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, Y.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 10, 21271. [Google Scholar] [CrossRef]
- Wu, Y.T.; Gutknecht, J.; Nadrowski, K.; Geißler, C.; Kühn, P.; Scholten, T.; Both, S.; Erfmeier, A.; Böhnke, M.; Bruelheide, H.; et al. Relationships between soil microorganisms, plant communities, and soil characteristics in Chinese subtropical forests. Ecosystems 2012, 15, 624–636. [Google Scholar] [CrossRef]
- Tian, W.; Xiang, X.; Ma, L.; Evers, S.; Wang, R.; Qiu, X.; Wang, H. Rare species shift the structure of bacterial communities across Sphagnum compartments in a subalpine Peatland. Front. Microbiol. 2020, 10, 3138. [Google Scholar] [CrossRef]
- Cheng, X.; Yun, Y.; Wang, H.; Ma, L.; Tian, W.; Man, B.; Liu, C. Contrasting bacterial communities and their assembly processes in karst soils under different land use. Sci. Total. Environ. 2021, 751, 142263. [Google Scholar] [CrossRef]
- Yang, Q.Y. Soil Microbial Community and Functional Response to Vegetation Succession under Rocky Desertification Conditions in Different Plant Types; Nanjing University of Information Science and Technology: Nanjing, China, 2022. [Google Scholar]
- Chen, L.; Song, T.Q.; Wang, H. Soil bacterial diversity and optimal sampling number in a karst evergreen and deciduous broad-leaved mixed forest. Acta Ecol. Sin. 2019, 39, 3287–3296. [Google Scholar]
- Wang, R.; Zhang, H.; Sun, L.; Qi, G.; Chen, S.; Zhao, X. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, L.B.; Pugnaire, F.I. Arbuscular mycorrhizal fungi host preference and site effects in two plant species in a semiarid environment. Appl. Soil Ecol. 2011, 48, 313–317. [Google Scholar] [CrossRef]
- Si, B.; Yao, X.H.; Ren, H.D.; Li, S.; He, B.H. Species composition and diversity in the process of natural succession of karst vegetation in central Guizhou: Case study of puding country in Guizhou. For. Res. 2008, 21, 669–674. [Google Scholar]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Wang, Z.; Chen, Y.; Gang, C.; An, R.; Li, J. Vegetation dynamics and its driving forces from climate change and human activities in the Three-River Source Region, China from 1982 to 2012. Sci. Total. Environ. 2016, 563–564, 210–220. [Google Scholar] [CrossRef]
- Liu, B.; Chen, C.; Lian, Y.; Chen, J.; Chen, X. Long-term change of wet and dry climatic conditions in the southwest karst area of China. Glob. Planet. Chang. 2015, 127, 1–11. [Google Scholar] [CrossRef]
- Peura, S.; Bertilsson, S.; Jones, R.I.; Eiler, A. Resistant microbial cooccurrence patterns inferred by network topology. Appl. Environ. Microbiol. 2015, 81, 2090–2097. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in ag-ricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Tan, W.; Di, H.; Xu, J.; Li, Y. High manure load reduces bacterial diversity and network complexity in a paddy soil under crop rotations. Soil Ecol. Lett. 2020, 2, 104–119. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Zhu, Q. Response of deep soil moisture to different vegetation types in the Loess Plateau of northern Shannxi, China. Sci. Rep. 2021, 11, 15098. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Vallicrosa, H.; Zuccarini, P.; Farré-Armengol, G.; Fernández-Martínez, M.; Peguero, G.; Gargallo-Garriga, A.; Ciais, P.; Janssens, I.A.; Obersteiner, M.; et al. Empirical support for the biogeochemical niche hypothesis in forest trees. Nat. Ecol. Evol. 2021, 5, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Erb, K.-H.; Fetzel, T.; Plutzar, C.; Kastner, T.; Lauk, C.; Mayer, A.; Niedertscheider, M.; Körner, C.; Haberl, H. Biomass turnover time in terrestrial ecosystems halved by land use. Nat. Geosci. 2016, 9, 674–678. [Google Scholar] [CrossRef]
- Deltedesco, E.; Keiblinger, K.M.; Piepho, H.P.; Antonielli, L.; Poetsch, E.M.; Zechmeister-Boltenstern, S.; Gorfer, M. Soil mi-crobial community structure and function mainly respond to indirect effects in a multifactorial climate manipulation experiment. Soil Biol. Biochem. 2020, 142, 107704. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Silva, L.C.R.; Lambers, H. Soil-plant-atmosphere interactions: Structure, function, and predictive scaling for climate change mitigation. Plant Soil 2020, 461, 5–27. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; De Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH determines microbial diversity and composition in the park grass experiment. Microb. Ecol. 2014, 69, 395–406. [Google Scholar] [CrossRef]
- Devi, A.S. Influence of trees and associated variables on soil organic carbon: A review. J. Ecol. Environ. 2021, 45, 5. [Google Scholar] [CrossRef]




| Dominant Species | Elevation (m) | Slope (°) | Average Tree Height (m) | Mean DBH (cm) | Canopy Density | Density (plant/hm2) |
|---|---|---|---|---|---|---|
| CF | 497 | 15 | 17.1 | 16.7 | 0.93 | 1250 |
| BF | 540 | 21 | 16.9 | 13.8 | 0.93 | 1316 |
| MF | 452 | 18 | 16.6 | 21.8 | 0.77 | 1169 |
| CK | 700 | 13 | 0 | 0 | 0 | 0 |
| Different Afforestation Modes | Soil Saturated Water Holding Capacity (%) | Soil Capillary Water Holding Capacity (%) | Soil Field Water Holding Capacity (%) | Soil Bulk Density (g/cm3) |
|---|---|---|---|---|
| CF | 10.73 ± 0.24 c | 10.15 ± 0.24 c | 9.60 ± 0.35 a | 1.52 ± 0.01 b |
| BF | 14.20 ± 0.15 b | 13.43 ± 0.39 b | 12.28 ± 0.14 a | 1.35 ± 0.01 c |
| MF | 12.42 ± 0.23 b | 11.23 ± 0.32 bc | 10.73 ± 0.21 a | 1.31 ± 0.03 c |
| CK | 28.34 ± 1.91 a | 23.58 ± 1.04 a | 11.85 ± 1.94 a | 1.70 ± 0.04 a |
| Different Afforestation Modes | SOC (g/kg) | TP (g/kg) | TN (g/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | AP (mg/kg) | pH |
|---|---|---|---|---|---|---|---|
| CF | 23.57 ± 0.69 bc | 0.22 ± 0.00 b | 1.26 ± 0.06 bc | 34.98 ± 3.21 b | 10.08 ± 0.58 b | 0.64 ± 0.14 b | 5.48 ± 0.04 b |
| BF | 43.90 ± 1.68 a | 0.30 ± 0.01 a | 2.07 ± 0.05 a | 38.96 ± 2.33 a | 10.28 ± 0.13 b | 0.78 ± 0.01 ab | 5.73 ± 0.03 a |
| MF | 43.83 ± 1.64 a | 0.31 ± 0.01 a | 2.05 ± 0.13 a | 41.51 ± 0.95 a | 9.59 ± 1.33 b | 0.82 ± 0.07 a | 5.72 ± 0.06 a |
| CK | 20.40 ± 0.42 c | 0.27 ± 0.01 a | 1.13 ± 0.02 c | 27.15 ± 3.51 b | 12.93 ± 0.52 a | 0.88 ± 0.02 a | 5.78 ± 0.06 a |
| Bacteria | Fungi | |||
|---|---|---|---|---|
| Soil Properties | R2 | p-Value | R2 | p-Value |
| TP (g/kg) | 0.592 | 0.005 | 0.531 | 0.019 |
| SOC (g/kg) | 0.891 | 0.001 | 0.666 | 0.004 |
| NH4+-N (mg/kg) | 0.639 | 0.001 | 0.517 | 0.019 |
| NO3−N (mg/kg) | 0.307 | 0.104 | 0.106 | 0.517 |
| TN (g/kg) | 0.859 | 0.001 | 0.642 | 0.005 |
| AP (mg/kg) | 0.257 | 0.148 | 0.373 | 0.060 |
| pH | 0.792 | 0.002 | 0.883 | 0.001 |
| SBD (g/cm3) | 0.746 | 0.001 | 0.289 | 0.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; He, T.; Zhu, N.; Peng, Y.; Gao, X.; Liu, Z.; Dang, P. Effects of Afforestation Patterns on Soil Nutrient and Microbial Community Diversity in Rocky Desertification Areas. Forests 2023, 14, 2370. https://doi.org/10.3390/f14122370
Liu L, He T, Zhu N, Peng Y, Gao X, Liu Z, Dang P. Effects of Afforestation Patterns on Soil Nutrient and Microbial Community Diversity in Rocky Desertification Areas. Forests. 2023; 14(12):2370. https://doi.org/10.3390/f14122370
Chicago/Turabian StyleLiu, Liling, Ting He, Ninghua Zhu, Yuanying Peng, Xiaoqian Gao, Zongxin Liu, and Peng Dang. 2023. "Effects of Afforestation Patterns on Soil Nutrient and Microbial Community Diversity in Rocky Desertification Areas" Forests 14, no. 12: 2370. https://doi.org/10.3390/f14122370





